Introduction
Colon cancer cells, which invade the blood
circulation, readily form metastases in the liver (1). The current available treatments for the
hepatic metastasis of colonic carcinoma mainly focus on surgical
removal of the metastasis; however, such treatments do not yield
satisfactory results (2,3). Hepatic metastasis is considered the
primary reason for the failure of colon cancer treatment in clinic,
affecting patient prognosis and long-term survival (survival rate
10–20%) (4). The growth hormone
receptor (GHR) genes are located on the fifth chromosome and
GHR is widely distributed in human organs and tissues. Findings of
a preliminary study showed that GHR is highly expressed in human
colorectal carcinoma (5,6). Growth hormone (GH) induces cell
differentiation and maturation by combining its receptor GHR with
191 amino acid monomeric peptides that are secreted from the
eosinocyte anterior pituitary, initiating the anabolism inside the
cells and promoting cell proliferation (7,8). GH/GHR
plays an essential role in the occurrence of colon cancer and the
development of hepatic metastases. By utilizing the technology of
macromolecule interfering, we constructed a plasmid of small
interfering RNA (siRNA) to interfere with GHR expression under
diseased conditions involving hepatic metastases in colon
cancer.
Materials and methods
Animals and cell lines
Thirty-six 8-week-old BALB/c mice with a body mass
of 20–22 g were randomly selected from the Vital River Lab Animal
Technology Co., Ltd. (Beijing, China). The mice were fed in a
specific pathogen-free environment. The SW480 colorectal cancer
cell line was purchased from the Cell Resource Center of the
Shanghai Institutes for Biological Sciences at the Chinese Academy
of Sciences (Shanghai, China). We have obtained the approval of the
study by the Ethics Committee of Xiangyang Hospital Affiliated to
Hubei University of Medicine and the humane treatment of the mice
was ensured.
Cell suspension preparation
The human SW480 colonic cancer cells were cultivated
in RPMI-1640 nutrient solution (Sigma, St. Louis, MO, USA)
containing 10% fetal bovine serum (Thermo Fisher Scientific,
Waltham, MA, USA), penicillin (100×103 U/l) and
streptomycin (100 mg/l), and then placed in an incubator at 37°C,
containing 5% CO2. The cells were collected at the
exponential growth stage with 0.25% trypsinase and subsequently
mechanically isolated to obtain cell suspension at a centrifugation
of 200 × g for 5 min. The supernatant was discarded and moderate
normal saline (NS) was added to adjust the cell concentration to
1×107/ml. The cell viability was measured using trypan
blue 95% (Chongqing Chemicals Co., Chongqing, China).
Introduction of cancer cells via
animal surgery
Mice were weighed prior to anesthesia. Confirmation
that animals were anesthetized was indicated by a decrease in limb
tension, corneal reflex showing no response and disappearance of
skin pain. Aseptically, an oblique incision was performed in the
left rear of the animals of 0.5–1.0 cm under the juncture of the
left posterior axillary line and costal margin. The abdominal
cavity was subsequently exposed and obliquely punctured using a
size five needle along the length of the spleen into the membranes
below. By forwarding the needle approximately 0.5 cm under the
membrane, human SW480 colonic cancer cells were injected into the
spleen. Each mouse was injected for 3 min with 0.1 ml cell
suspension (or 1×106/mouse). When the spleen membranes
swelled and became white, the needle was withdrawn and pressure was
applied to the site using cotton for disinfection, avoiding the
exposure of cancer cells and bleeding. The spleen was then returned
and the abdominal cavity was secured. After the mice recovered, the
animals were fed a regular diet.
Construction of siRNA synthesis and
eukaryotic expression vector
The mRNA sequence of the human GHR gene was
obtained from the GenBank database, and the full-length genomic
sequence was 4,414 bp (access no.: X06562, GI: 31737). According to
the design principle of siRNA (http://bioinfo.clontech.com/rnaidesigner), the siRNA
was designed to the genetic locus of hGHR online which is 1602–1622
bp: TGGTCTCACTCTGCCAAGAAA. The restriction sites for BamHI
and HindIII were inserted in the siRNA with enzyme digestion
into the eukaryotic expression vector pcDNATM6.2- GW/EmGFPmiRmiRNA,
which was designated as pcDNATM6.2-GW/EmGFPmiRmiRNA-GHR-4 (G4).
Interfering particle
The bacterial solution containing the interfering
particle was added into the lysozyme nutrient solution and
centrifuged at 220 × g overnight at 37°C. An effective and
efficient plasmid with high purity was extracted using Plasmid
Midiprep kit (high-quality; Sigma) to prepare the plasmid according
to the manufacturer's instructions.
Experiment reagents and treatment
TRIzol reagent was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA), and the one-step RT-PCR kit and
Qiagen plasmid mini kit were purchased from Qiagen (Hilden,
Germany). BamHI and HindIII were produced by Promega
Corp. (Madison, WI, USA), 5-fluorouracil (5-FU) was produced in the
Hubei Yuancheng Pharmaceutical Co., Ltd. (Wuhan, China) and
recombinant human growth hormone (rhGH) was produced by Merck
Serono [(Schweiz) AG (Zug, Switzerland)].
Inoculation of animals
The mice were injected on the first day of
inoculation with tumor cells. Based on the delivery and dose of
treatment, the animals were divided as follows: i) NS, each mouse
was injected with 10 µl NS in the abdominal cavity. ii) Plasmid G4,
where animals were injected subcutaneously with eukaryotic
expression plasmid as 10 µg/mouse. iii) GH, each mouse was injected
subcutaneously with rhGH 2 IU/kg. iv) 5-FU, the intraperitoneal
injection was applied at a dose of 20 mg of 5-FU/kg. v) FU+G4,
intraperitoneal injection at a dose of 20 mg 5-FU/kg and 10 µg
G4/mouse. vi) FU+G4+GH, intraperitoneal injection performed as 20
mg of 5-FU/kg, 10 µg of G4/mouse and rhGH 2 IU/kg. The mice in the
abovementioned groups were injected once every 3 days and
subsequently continually injected 10-fold.
The observation index
Body mass, volume of drinking water, food intake,
mental and activity condition were observed on the 1st, 5th, 10th,
15th, 20th, 25th and 30th day piror to and following inoculation.
On the 30th day of inoculation, the animals were sacrificed to
collect liver and spleen and fixed in 4% of formaldehyde solution.
Sections (4 µm) were obtained and paraffin-embedded, followed by
hematoxylin and eosin (H&E) staining for histology. The animals
were sacrificed by cervical dislocation.
Statistical analysis
Data were shown as mean ± standard deviation, and
one-way analysis of variance was used for multi-groups. For
comparison between two groups, the q-value was used for analysis.
P<0.05 was considered to indicate a statistically significant
result.
Results
Animal weight
BALB/c mice inoculated with human SW480 colon cancer
cells survived. Following surgery, the mice were weighed every 5
days (Table I). On the first day, the
body mass of mice in each group appeared to decrease, with a lack
of activeness and a reduction in the consumption of water and food.
This lack of activity may be explained by the anesthesia and
subsequent surgical procedure. By the 15th day, the mice of the GH
group regained their body mass (21.87±0.74) to that prior to the
operation (21.93±0.58). By the 30th day, the body mass of the mice
(22.00±0.46) increased slightly as compared to that prior to the
operation. In the FU+GH group, the weight of the mice was recovered
at the 4th week. For the remaining four groups, the body mass of
the mice was decreased as compared to that prior to the operation
(NS, 21.69±0.37 vs. 20.18±0.35; G4, 21.78±0.6 vs. 20.01±0.49; FU,
21.98±0.53 vs. 19.35±0.42; FU+G4, 21.47±0.68 vs. 19.27±0.57),
particularly the FU+G4 group. The decrease for these groups was
statistically significant (Table
I).
 | Table I.The variation of mice body mass in
each group of liver metastases. |
Table I.
The variation of mice body mass in
each group of liver metastases.
|
| Mean body mass for
mice from each group (mean ± standard deviation) |
|---|
|
|
|
|---|
| Time | NS | G4 | GH | FU | FU+G4 | FU+G4+GH |
|---|
| Prior to surgery | 21.69±0.37 | 21.78±0.6 | 21.93±0.58 | 21.98±0.53 | 21.47±0.68 | 21.75±0.38 |
| 1 day after
surgery | 20.15±0.56 | 20.33±0.42 | 20.89±0.67 | 20.43±0.31 | 20.52±0.59 | 20.41±0.48 |
| 5 days after
surgery | 20.02±0.45 | 20.14±0.78 |
21.14±0.70a | 20.10±0.56 |
19.69±0.64a | 20.75±1.63 |
| 10 days after
surgery | 19.96±0.68 | 19.87±0.68 |
21.22±0.92a | 19.58±0.66 | 19.78±0.98 |
20.98±0.62a,b |
| 15 days after
surgery | 19.81±0.38 | 19.85±0.74 |
21.87±0.74a | 19.71±0.45 |
18.87±0.57a |
21.13±0.72a,b |
| 20 days after
surgery | 20.01±0.65 |
19.78±0.62a |
21.62±0.73a |
19.65±0.61a |
18.99±0.78a |
21.21±0.63a,b |
| 25 days after
surgery | 19.91±0.44 | 19.92±0.23 |
21.80±0.55a | 19.38±0.69 | 19.14±0.65 |
21.13±0.65a,b |
| 30 days after
surgery | 20.18±0.35 | 20.01±0.49 |
22.00±0.46a |
19.35±0.42a |
19.27±0.57a |
21.29±0.37a,b |
Morphology of liver metastases
Metastatic tumors were identified on the surface of
the liver of the BALB/c mice, indicating a 100% increase of th
eliver metastatic rate. Liver volume became smaller and the texture
appeared as crisp and hard. The metastasis tumor foci of the liver
surface were mainly identified in the lobe margin and lobe visceral
surface, especially the right lobe. The liver micrometastases for
26 animals showed diffused distribution with hoary appearance. Some
tumor surface ulceration was observed, while the hepatic tissue was
destroyed (Table II). Different
numbers of liver metastases for mice in each group were observed
under the microscope. The numbers of liver metastases in the NS
group (10.17±1.94) and GH group (10.50±1.38) were significantly
higher than those in the G4 group (2.67±137), FU group (3.17±0.98),
G4+FU group (2.33±1.03) and G4+GH+FU group (2.17±0.75) (P<0.05).
There were no significant differences between the NS and GH groups
(10.17±1.94 vs. 10.50±1.38; P>0.5). A low number of liver
metastasis was evident in the G4+GH+FU group (2.17±0.75). There
were significant differences compared to those of the G4 group
(2.67±137), FU group (3.17±0.98) and G4+FU group (2.33±1.03)
(P<0.5).
 | Table II.Comparison of number of liver
metastases for mice in each group. |
Table II.
Comparison of number of liver
metastases for mice in each group.
|
| Number of liver
metastases |
|---|
|
|
|
|---|
| Groups | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Mean ± SD |
|---|
| NS | 9 | 8 | 10 | 9 | 12 | 13 | 10.17±1.94 |
| G4 | 3 | 2 | 2 | 3 | 5 | 1 |
2.67±1.37a |
| GH | 10 | 9 | 11 | 12 | 9 | 12 |
10.50±1.38b |
| FU | 3 | 3 | 3 | 2 | 5 | 3 |
3.17±0.98a |
| FU+G4 | 2 | 2 | 4 | 1 | 3 | 2 |
2.33±1.03a |
| FU+G4+GH | 3 | 1 | 2 | 2 | 3 | 2 |
2.17±0.75a |
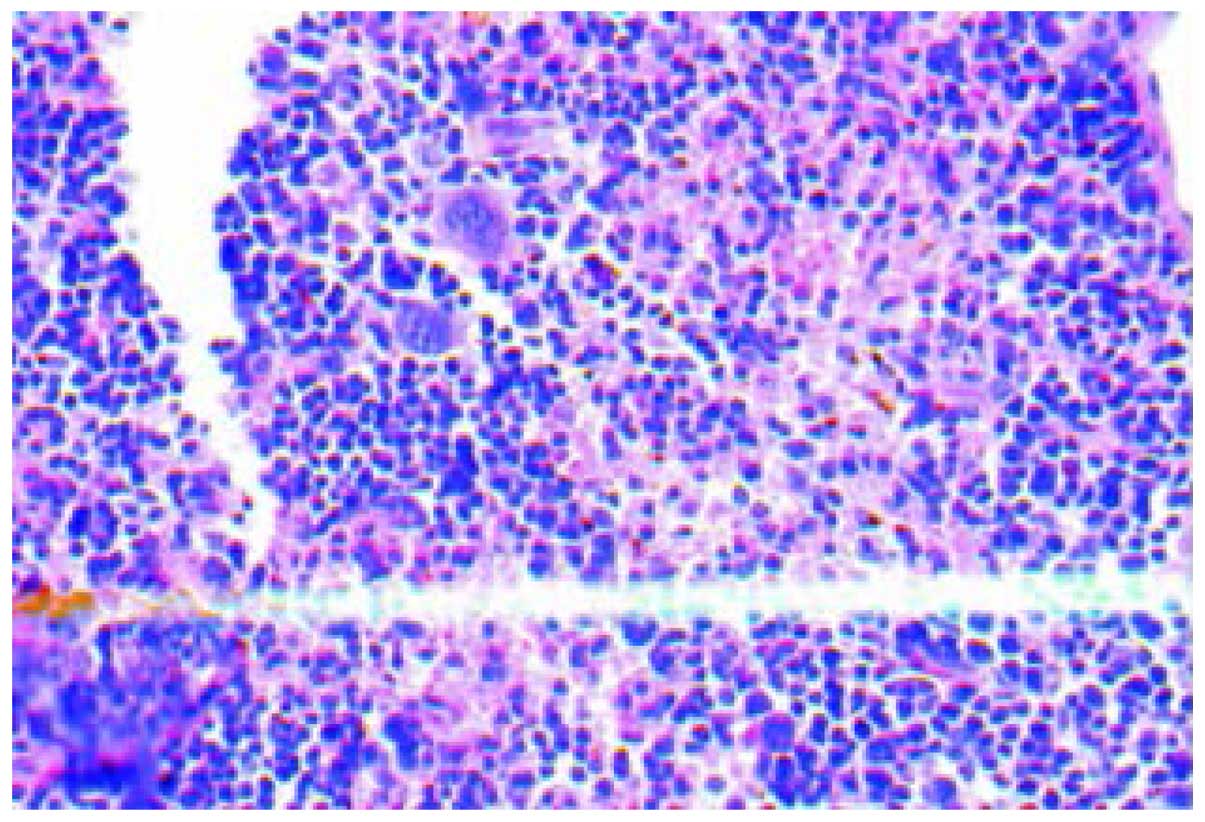
Histology
The H&E staining of tissues showed that inner
liver metastatic tumors were clustered. The normal liver lobule
structure was eradicated, cell volume was reduced, the cancer cell
differentiation was poor with obvious atypia and the cytoplasm,
karyopyknosis, karyorrhexis, dissolution and mitosis increased
(Fig. 1). Various hoary nodes, with a
diameter of 0.2–3 mm, were evident in part of the inoculated
spleen. The tumor formation rate of orthotopic inoculation was
100%. The cancer cells of the spleen orthotopic inoculation were
mainly distributed near the splenic sinusoids with obvious atypia,
concentrated in the nucleus and cytoplasm, with the chromosomal
becoming hard, and more intense staining (Fig. 2). The morphological structure of the
liver metastases was similar to that of the tumor nodules of the
spleen orthotopic inoculation, conforming to the structural
features of colon low-differentiated adenocarcinoma.
Discussion
Hepatic metastases occur in almost 500,000 colon
cancer patients during disease progression annually (9). The liver is the primary target organ of
hematogenous gastric and colorectal metastasis (10), which constitutes the highest hepatic
metastatic rate of colorectal cancer in alimentary cancer.
Currently, the treatment for hepatic metastasis of colon cancer is
mainly focused on hepatectomy while combining the adjuvant therapy
of chemo- and radiotherapy. However, surgery may not be a viable
treatment option for advanced stage patients. Additionally,
post-surgery tumor cells are not sensitive to chemotherapy leading
to treatment failure. The patient survival rate following surgery
is between 50 and 70% (11–13). Previous studies have focused on the
molecular mechanism of hepatic metastases of colorectal cancer to
select appropriate genes associated with tumor as therapeutic
target in order to identify the relevant therapy (1). Previous findings have shown that
organizing the specificity of 5-fluorocytosine/cytosine deaminase
to identify a thermochemotherapy system can effectively obtain the
targeting and inhibitory effect of hepatic metastases in colon
cancer of nude mice (14).
RNAi technology is associated with double-stranded
RNA, which is complementary with endogenous mRNA in cells, leading
to the specific degradation of mRNA and resulting in mRNA encoding
genes not expressing the result of gene silencing. The emergence of
RNAi has been beneficial in the study of the function of genes and
identification of the target of gene therapy (15). The rhGH is widely used in the surgical
field of opsonizing the metabolism, enhancing the immune system,
relieving postoperative fatigue, promoting wound healing,
maintaining the intestinal mucosal barrier, and reducing bacterial
translocation. The rhGH binds to its receptor (GHR) on the cell
surface and via the GH-GHR-insulin-like growth factors (IGFs) axis
triggers a series of biological effects (16). In recent years, investigators have
identified that the GH-GHR-IGFs axis markedly contributes to the
occurrence, development and metastasis of maligant tumors (17,18). The
expression of colon cancer tissues in GHR is high and rhGH can
promote the proliferation, differentiation, and metastasis of tumor
cells of postoperative residuals. Previous findings have suggested
that rhGH should be employed with care in patients with a high
expression of colon cancer (19–25).
Considering the role of GHR in tumor metastasis, the aim of the
present study was to investigate the GHR response by constructing
GHR siRNA. GRH is stimulated following colon surgery, in which the
cancer cells flow backward into the vein and then to the liver with
blood dissemination leading to hepatic metastasis.
In the present study, we developed a hepatic
metastasis mouse model by injecting human SW480 colon cancer cells
into the spleen of BALB/c mice. The animals were also treated with
GHR siRNA-interfering plasmid and 5-FU was added. The formation of
hepatic metastatic tumor in BALB/c mice was investigated. The
experimental results showed that GHR siRNA is capable of inhibiting
the hepatic metastasis of human SW480 colon cancer cells. In the
joint group of FU+G4+GH, a comparison of the inhibition ratio of
hepatic metastasis using 5-FU alone for the group FU+G4 yielded no
statistically significant difference. However, in order to improve
food intake in the body mass of mice, the latter two groups have an
advantage over the group of FU+G4+GH. When GH binds with GHR-siRNA
and 5-FU, the hepatic metastatic ratio of SW480 cells is not
increased. Additionally, GHR-siRNA is capable of selectively
inhibiting the metastasis of human SW480 colon cancer cells,
confirming the significant role GHR plays in tumor metastasis
(26–28). Therefore, results of the present study
further enhance understanding for treating colon cancer through the
combination therapy of 5-FU and siGHR.
References
|
1
|
Nordlinger B, Sorbye H, Glimelius B,
Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole
ET, Finch-Jones M, et al: EORTC Gastro-Intestinal Tract Cancer
Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen
und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie
(ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG);
Fédération Francophone de Cancérologie Digestive (FFCD):
Perioperative chemotherapy with FOLFOX4 and surgery versus surgery
alone for resectable liver metastases from colorectal cancer (EORTC
Intergroup trial 40983): A randomised controlled trial. Lancet.
371:1007–1016. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tropea A, Biondi A, Corsaro A, Donati M,
Basile F and Gruttadauria S: Combined microwave thermal ablation
and liver resection for single step treatment of otherwise
unresectable colorectal liver metastases; a monoistitutional
experiences. Eur Rev Med Pharmacol Sci. 18(Suppl 2): 6–10.
2014.PubMed/NCBI
|
|
3
|
Giovinale M, Fonnesu C, Soriano A,
Cerquaglia C, Curigliano V, Verrecchia E, De Socio G, Gasbarrini G
and Manna R: Atypical sarcoidosis: Case reports and review of the
literature. Eur Rev Med Pharmacol Sci. 13(Suppl 1): 37–44.
2009.PubMed/NCBI
|
|
4
|
Otchy D, Hyman NH, Simmang C, et al:
Standards Practice Task Force; American Society of Colon and Rectal
Surgeons: Practice parameters for colon cancer. Dis Colon Rectum.
47:1269–1284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou D, Liang D and Zhang Y: The
expression of human growth hormone receptor in colon cancer tissues
and its clinical significance. China J Curr Adv Gen Surg.
10:490–492. 2007.(In Chinese).
|
|
6
|
Liang DM, Chen JY and Zhang Y: The
expression of human growth hormone receptor in rectal cancer
tissues. China J Gen Surg. 18:414–416. 2009.(In Chinese).
|
|
7
|
Shen XY, Holt RI, Miell JP, Justice S,
Portmann B, Postel-Vinay MC and Ross RJ: Cirrhotic liver expresses
low levels of the full-length and truncated growth hormone
receptors. J Clin Endocrinol Metab. 83:2532–2538. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Waters MJ, Hoang HN, Fairlie DP, Pelekanos
RA and Brown RJ: New insights into growth hormone action. J Mol
Endocrinol. 36:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei C, Tan J, Xu L, Juan L, Zhang SW, Wang
L and Wang Q: Differential diagnosis between hepatic metastases and
benign focal lesions using DWI with parallel acquisition technique:
A meta-analysis. Tumour Biol. 36:983–990. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Waisberg J and Ivankovics IG: Liver-first
approach of colorectal cancer with synchronous hepatic metastases:
A reverse strategy. World J Hepatol. 7:1444–1449. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Watzka FM, Fottner C, Miederer M, Schad A,
Weber MM, Otto G, Lang H and Musholt TJ: Surgical therapy of
neuroendocrine neoplasm with hepatic metastasis: Patient selection
and prognosis. Langenbecks Arch Surg. 400:349–358, erratum 359.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kemeny NE, Chou JF, Capanu M, Gewirtz AN,
Cercek A, Kingham TP, Jarnagin WR, Fong YC, DeMatteo RP, Allen PJ,
et al: KRAS mutation influences recurrence patterns in patients
undergoing hepatic resection of colorectal metastases. Cancer.
120:3965–3971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang XY, Zhang XP, Huang JH, Luo RG, Miao
BJ and Wang Y: Effects of intra-arterial infusion of
3-bromopyruvate on metastases and survival benefit of hepatic VX2
tumor in rabbits. Zhonghua Yi Xue Za Zhi. 93:3139–3142. 2013.(In
Chinese). PubMed/NCBI
|
|
14
|
Cai C: Reflections on clinical problems of
hepatic metastasis from gastric and colorectal cancer. China J Gen
Surg. 14:721–722. 2005.(In Chinese).
|
|
15
|
Choti MA, Sitzmann JV, Tiburi MF, et al:
Trends in long-term survival following liver resection for hepatic
colorectal metastases. Ann Surg. 235:759–766. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tschoep K, Kohlmann A, Schlemmer M,
Haferlach T and Issels RD: Gene expression profiling in sarcomas.
Crit Rev Oncol Hematol. 63:111–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Zhang B and Wang L: The targeting
effect of pro-drug thermochemotherapy on mice hepatic metastasis of
gene transfection of colon cancer. China J Gen Surg. 18:348–352.
2009.(In Chinese).
|
|
18
|
Wang X and Zhao J: KLF8 transcription
factor participates in oncogenic transformation. Oncogene.
26:456–461. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Campbell GS: Growth-hormone signal
transduction. J Pediatr. 131:S42–S44. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yi HK, Hwang PH, Yang DH, Kang CW and Lee
DY: Expression of the insulin-like growth factors (IGFs) and the
IGF-binding proteins (IGFBPs) in human gastric cancer cells. Eur J
Cancer. 37:2257–2263. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu H and Yang D: The research development
on the relation between GH-IGFs and tumor. China Cancer.
11:720–722. 2002.
|
|
22
|
Yang XD, Liu FK, Xu Z and Li JS: Growth
hormone receptor expression in human colorectal cancer and its
implication. Zhonghua Wei Chang Wai Ke Za Zhi. 8:252–254. 2005.(In
Chinese). PubMed/NCBI
|
|
23
|
Lim C, Broqueres-You D, Brouland JP,
Merkulova-Rainon T, Faussat AM, Hilal R, Rouquie D, Eveno C,
Audollent R, Levy BI, et al: Hepatic ischemia-reperfusion increases
circulating bone marrow-derived progenitor cells and tumor growth
in a mouse model of colorectal liver metastases. J Surg Res.
184:888–897. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paschos KA, Majeed AW and Bird NC: Natural
history of hepatic metastases from colorectal cancer -
pathobiological pathways with clinical significance. World J
Gastroenterol. 20:3719–3737. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamao T, Hayashi H, Higashi T, Takeyama H,
Kaida T, Nitta H, Hashimoto D, Chikamoto A, Beppu T and Baba H:
Colon cancer metastasis mimicking intraductal papillary neoplasm of
the extra-hepatic bile duct. Int J Surg Case Rep. 10:91–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Y, Ning SL, Chen YX, Xu KS and Shou
NH: Role of vascular cell adhesion molecule-1 in the mouse model of
hepatic ischemia/reperfusion and the hematogenic metastasis.
Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 36:426–431. 2014.(In Chinese).
PubMed/NCBI
|
|
27
|
Barber EL, Schink JC and Lurain JR:
Hepatic metastasis in gestational trophoblastic neoplasia: Patient
characteristics, prognostic factors, and outcomes. J Reprod Med.
59:199–203. 2014.PubMed/NCBI
|
|
28
|
Padda RS, Gkouvatsos K, Guido M, Mui J,
Vali H and Pantopoulos K: A high-fat diet modulates iron metabolism
but does not promote liver fibrosis in hemochromatotic Hjv-/- mice.
Am J Physiol Gastrointest Liver Physiol. 308:G251–G261. 2015.
View Article : Google Scholar : PubMed/NCBI
|