Introduction
Renal cell carcinoma (RCC) accounts for 3% of all
adult malignancies and is the third most common urological cancer
worldwide, with the highest mortality rate of RCC at >40%
(1). Approximately 65,150 novel cases
and 13,680 mortalities were estimated for 2013 in the USA (1). Clear cell RCC (ccRCC) is the largest
subtype of RCC and accounts for 70% of RCCs (2). Despite the increasingly early detection
of RCC and more frequent use of surgery, the mortality rate has not
changed significantly since 1990 and ~30% patients with localized
RCC develop post-operative metastatic recurrence (3). The current adjuvant therapy for
metastatic RCC remains extremely limited, as RCC is relatively
unaffected by chemotherapy or radiotherapy (4). Therefore, a better understanding of the
mechanisms involved in the pathogenesis of RCC and more effective
therapeutic approaches are urgently required.
MicroRNAs (miRNAs) are a small class of non-coding
RNAs of 19–25 nucleotides in length. miRNAs are known to be
important in the pathogenesis of cancer, from initiation to
metastasis, primarily through interaction with the 3′ untranslated
region (3′UTR) of various target genes, which results in target
gene translational silence or cleavage (5). miRNAs are known to be involved in
multiple tumorigenic steps in human cancers, including in cell
growth, apoptosis, migration and invasion (6–9). Of these
miRNAs, miR-137 is an notable member that is located on chromosome
1p22 and has been indicated to be underexpressed in non-small lung
(10), gastric (11), colorectal (12) and breast cancer (13). The restoration of miR-137 expression
has been shown to inhibit cancer cell growth, migration and
invasion, and to induce cancer cell apoptosis (10–13). These
previous studies suggest that miR-137 could act as a diagnostic and
prognostic marker in human cancers. However, the clinical
significance and role of miR-137 in RCC remain unknown at
present.
Therefore, the aim of the present study was to
analyze the expression pattern of miR-137 in clinical RCC samples
and examine the effects of miR-137 on the proliferation, migration,
invasion, apoptosis and cell cycle of RCC cell lines, and to
evaluate the effects of miR-137 on tumor growth in 786-O xenograft
nude mice models.
Materials and methods
RCC clinical specimens
Subsequent to obtaining informed consent from all
patients, surgical specimens (paired normal and cancerous tissue)
were collected from 50 patients with RCC in the Department of
Urology, China-Japan Union Hospital of Jilin University (Changchun,
China) between August 2012 and August 2014. Samples were flash
frozen in liquid nitrogen and stored at −80°C until required. The
present study was approved by the ethics committee of Jilin
University. Clinical and pathological information from patient
records was also gathered and is listed in Table I.
 | Table I.Association between the relative level
of miR-137 in the tumor tissues of patients with RCC and the
clinicopathological features of RCC. |
Table I.
Association between the relative level
of miR-137 in the tumor tissues of patients with RCC and the
clinicopathological features of RCC.
| Feature | Patients, n | miR-137 expression
levela | P-value |
|---|
| Age, years |
|
|
0.797 |
|
<60 | 22 | 0.54±0.06 |
|
| ≥60 | 28 | 0.56±0.07 |
|
| Gender |
|
|
0.914 |
| Male | 26 | 0.55±0.08 |
|
|
Female | 24 | 0.56±0.07 |
|
| TNM stage |
|
| <0.001 |
| I–II | 34 | 0.68±0.12 |
|
|
III–IV | 16 | 0.35±0.05 |
|
| Tumor size |
|
|
0.006 |
| <5
cm | 30 | 0.62±0.11 |
|
| ≥5
cm | 20 | 0.47±0.07 |
|
| Metastasis |
|
| <0.001 |
| No | 35 | 0.70±0.14 |
|
| Yes | 15 | 0.23±0.04 |
|
Cell culture
The human RCC A498 and 786-O cell lines and normal
renal HK-2 cell line were purchased from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
All cells were cultured in Dulbecco's modified Eagle medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) with 1% penicillin/streptomycin (Sigma-Aldrich,
St. Louis, MO, USA) at 37°C in 5% CO2.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA of tissues and cells was extracted with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The concentration and
quality of RNA was determined using the Nanodrop 2000c
spectrophotometer (Thermo Fisher Scientific, Inc.). Total RNA (2
µg) was reverse transcribed into cDNA using First-Strand cDNA
Synthesis kit (Invitrogen; Thermo Fisher Scientific, Inc.) with
specific primers, qualified with a Taqman probe, as per the
manufacturer's protocol. The expression of miR-137 was detected
using the SYBR® Premix Ex Taq™ II kit (Takara
Biotechnology Co., Ltd., Dalian, China) under an ABI PRISM 7900
Sequence Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). In brief, the reaction volume was 20 µl, and the
mixture contained 10 µl SYBR® Premix Ex Taq, 2 µl cDNA,
0.5 µl (10 µM) miR-137 forward primer and miR-137 reverse primer or
0.5 µl (10 µM) U6 forward primer and U6 reverse primer, and 7 µl
dH2O. The primers were as follows: miR-137 forward,
5′-GCGCTTATTGCTTAAGAATAC-3′ and reverse, 5′-CAGTGCAGGGTCCGAGGT-3′;
U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The PCR amplification conditions
were as follows: 95°C for 30 sec and 40 cycles of 95°C for 5 sec,
62°C for 30 sec, and final extension at 72°C for 5 min. The
relative expression levels were evaluated using the
2−∆∆Cq method (14). Three
replicates were performed for each reaction.
Cell transfection
miR-137 mimic and corresponding negative control
miRNA (miR-NC) were purchased from Shanghai GenePharma (Shanghai,
China), and were transiently transfected into 786-O cells in 6-well
plates at a concentration of 100 nM using Oligofectamine™
Transfection Reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions.
Cell viability
Cell viability was assessed at 24, 48 and 72 h after
transfection with miR-137 or miR-NC using the
3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide
(MTT) method (Sigma-Aldrich). In brief, transfected cells
(5×103) were seeded in 96-well plates in triplicate,
with MTT working solution (Sigma-Aldrich) and cultured for 4 h at
37°C. The medium was then removed and 200 µl dimethyl sulphoxide
(DMSO; Sigma-Aldrich) was added to dissolve the formazan crystals.
Cell viability was assessed at an absorbance of 570 nm using an
iMark microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Cells transfected with PBS were used as a control. All
experiments were repeated three times.
Cell cycle and cell apoptosis
assay
Cell cycle and cell apoptosis assays were performed
by flow cytometry. First, cells were transfected with either
miR-137 or miR-NC and cultured at 37°C in 5% CO2 for 48
h. For cell cycle analysis, the cells were harvested by
trypsinisation, and the fixed cells were incubated with DNA binding
dye propidium iodide (PI; 20 µg/ml; Sigma-Aldrich) and RNase (1.0
mg/ml; Sigma-Aldrich) for 30 min at 37°C in the dark, and then
analyzed by flow cytometry (BD Biosciences, Franklin Lakes, NJ,
USA). For the cell apoptosis assay, apoptosis was determined by PE
Annexin V/Apoptosis Detection Kit I (BD Pharmingen, San Diego, CA,
USA) according to the manufacturer's protocol, and analyzed by
fluorescence activated cell sorting in flow cytometry (FACSCalibur
4 Colour; BD Biosciences). Flow cytometry data were analyzed using
FlowJo software (version 7.65; FlowJo, LLC, Ashland, OR, USA).
Experiments were performed in triplicate.
Cell migration and invasion assay
A 24-well Transwell plate with 8-µm pore
polycarbonate membrane inserts (BD Biosciences) was used to analyze
the migration and invasive potential of cells transfected with
miR-137 mimic or miR-NC. For the invasion assay, the membrane was
coated with the Matrigel (200 µg/ml; BD Biosciences). Transfected
cells (2.5×104) suspended in serum free DMEM were added
to the upper chamber. In the bottom chamber, medium containing 10%
FBS was added as a chemoattractant. Subsequent to 24 h for the
migration assay or 48 h for the invasion assay, migrated or invaded
cells were fixed with 70% ethanol (Sigma-Aldrich) for 30 min and
stained with 0.2% crystal violet (Sigma-Aldrich) for 10 min.
Migrating or invading cells were counted by taking photomicrographs
in randomly five fields under a light microscope (ECLIPSE TS100;
Nikon Corporation, Tokyo, Japan).
Western blot analysis
Protein was extracted from tissues and cells using
radioimmunoprecipitation assay lysis buffer containing proteinase
inhibitor (Sigma-Aldrich) supplemented with a protease inhibitor
mixture stock solution (Roche Molecular Biochemicals, Mannheim,
Germany) and phenylmethanesulfonyl fluoride (Sigma-Aldrich).
Concentrations of total cellular protein were determined using a
bicinchoninic acid assay kit (Pierce; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Protein (20 µg)
was separated by a 10% sodium dodecylsulfate-polyacrylamide gel,
and then transferred onto a nitrocellulose membrane (Bio-Rad
Laboratories GmbH, Munich, Germany). The membrane was incubated
with 5% nonfat skim milk to block nonspecific binding. The
membranes were incubated overnight at 4°C with the following
antibodies: Mouse anti-human monoclonal glyceraldehyde 3-phosphate
dehydrogenase (GAPDH; dilution, 1:5,000; catalog no. sc-365062;
Cell Signaling Technology, Inc., Danvers, MA, USA); mouse
anti-human monoclonal phosphoinositide 3-kinase (PI3K; dilution,
1:2,000; catalog no. sc-23962; Cell Signaling Technology, Inc.);
rabbit anti-mouse monoclonal phosphorylated-PI3K (p-PI3K) (Tyr458;
dilution, 1:500; catalog no. 4228; Cell Signaling Technology,
Inc.); mouse anti-human monoclonal protein kinase B (AKT; dilution,
1:2,000; catalog no. sc-5298; Cell Signaling Technology, Inc.); and
mouse anti-human monoclonal phosphorylated-AKT (p-AKT; Ser473;
dilution, 1:1,500; catalog no. sc-293125; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). The membranes were washed and incubated
with horseradish peroxidase-conjugated goat anti-mouse IgG
(dilution, 1:5,000; catalog no. sc-2004; Santa Cruz Biotechnology,
Inc.) or goat anti-rabbit IgG (dilution, 1:5,000; catalog no.
sc-2005; Santa Cruz Biotechnology, Inc) for 2 h at room
temperature. The protein bands were visualized by enhanced
chemiluminescence (SuperSignal West Femto Maximum Sensitivity
Substrate; catalog no. 32209; Thermo Fisher Scientific, Inc.).
Blots were stripped and reprobed with anti-GAPDH to control for
loading variations.
Tumor growth in vivo
A total of 30 male BALB/c mice, 5–6 weeks old, were
purchased from the Experiments Animal Center of Changchun
Biological Institute (Changchun, China), maintained under specific
pathogen-free conditions and provided with food and water ad
libitum. The present study was approved by the Animal Ethics
Committee of Jilin University (Changchun, China).
For animal xengraft model assays, 2×106
cells stably expressing miR-137 or miR-NC were injected into the
flanks of mice (n=10 per group). The control group was injected
with 2×106 786-O cells into the flanks of mice (n=10).
The tumors were measured using vernier calipers every week, and
tumor volume was calculated according to the formula: Volume
(mm3) = width2 × length / 2. At 30 days
subsequent to injection, the mice were sacrificed and the tumors
were resected and weighed. Total RNAs of tumor tissues were
extracted to measure the miR-137 level uing the aformentioned
RT-qPCR method.
Statistical analysis
Data from at least three independent experiments
were expressed as mean ± standard deviation. Statistical analysis
between two samples was performed using two-sided Student's
t-test, and more than two groups was performed using one-way
analysis of variance followed a Tukey's post-hoc test. All data
were analyzed using the GraphPad Prism version 5.01 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-137 is frequently reduced in RCC
tissues and cell lines
To determine whether miR-137 expression is
associated with RCC pathogenesis, the expression levels of miR-137
were determined using RT-qPCR analysis in 50 pairs of RCC and
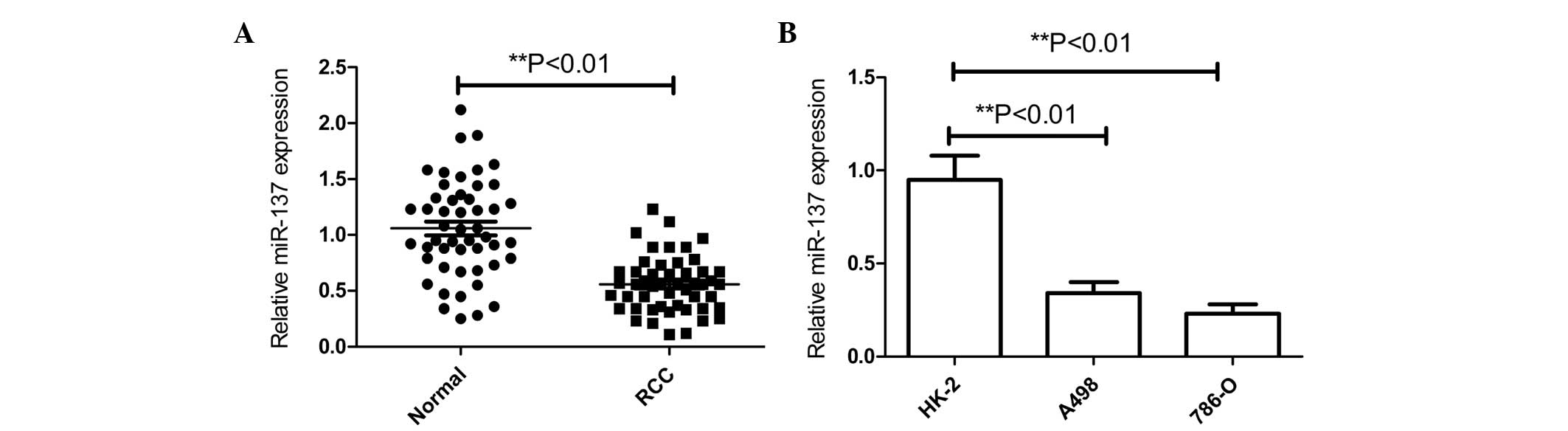
adjacent normal tissues. Notably, there was a significant decrease
in miR-137 expression in ~78% (39/50) of tumor tissues compared
with normal tissues (Fig. 1A). In
addition, the levels of miR-137 expression in the human RCC A498
and 786-O cell lines and normal renal HK-2 cell line were examined
by qRT-PCR (Fig. 1B). The expression
level of miR-137 in the RCC cell lines was found to be decreased
compared with the expression in the normal renal HK-2 cell line
(Fig. 1B). The 786-O cell line, which
possessed the lower level of miR-137 expression of the two RCC cell
lines (Fig. 1B), was selected for
further studies.
The association between miR-137 expression and the
clinicopathological parameters of the patients, including age,
gender, TNM stage, tumor diameter and metastasis was assessed
(Table I). The level of miR-137
expression in tissues was found to be negatively associated with a
larger tumor diameter, advanced TNM stage and metastasis
(P<0.01), which are all indicators of poor prognosis. There was
no correction between miR-137 expression and age and sex. These
data suggested that miR-137 might play a key role in RCC
development.
Overexpression of miR-137 attenuates
RCC cell proliferation and motility and induces cell apoptosis
To explore the biologic significance of miR-137, we
stably overexpressed miR-137 in 786-O cell by transfection with
miR-137 mimic or miR-NC. The efficacy of transfection was tested by
qRT-PCR (Fig. 2A). It was found that
miR-137 expression in miR-137 group was elevated in 786-O cells
compared to miR-NC group (Fig. 2A).
Then, the effect of miR-137 on cell proliferation was assessed with
the MTT assay. MTT assay showed that that overexpression of miR-137
suppressed RCC cell proliferation (P<0.05; Fig. 2B). As proliferation directly linked to
cell cycle distribution, we investigate the effect of miR-137 on
RCC cell cycle progression, and found that overexpression of
miR-137 caused cell-cycle arrest at G0-G1 phase and decreased the
S-phase population (Fig. 2C).
Furthermore, to reveal the biological role of miR-137 on migration
and invasion, transwell assay were performed. It was found that
overexpression of miR-137 markedly impaired RCC cell migration
(Fig. 2D) and invasiveness (Fig. 2E) compared with miR-NC (P<0.01). In
addition, PE Annexin-V staining was used to reveal miR-137 on
apoptosis in RCC cells, and found that overexpression of miR-137
markedly induced cell apoptosis compared with the miR-NC group
(Fig. 2F). These findings suggest
that miR-137 acts as a tumor suppressor in RCC by inhibiting cell
proliferation and motility and inducing cell apoptosis.
Effect of miR-137 on PI3 K/AKT
signaling pathway
It has been showed that the PI3K/AKT pathway play
crucial role in cell growth and survival for RCC (15,16). In
addition, recently a study showed that miR-137 functions as a tumor
suppressor in gastric cancer cell through targeting
Cyclooxygenase-2 (Cox-2) through inhibiting the activation of
PI3K/AKT signaling pathway (11).
Thus, in this study, we wonder whether miR-137 affects activation
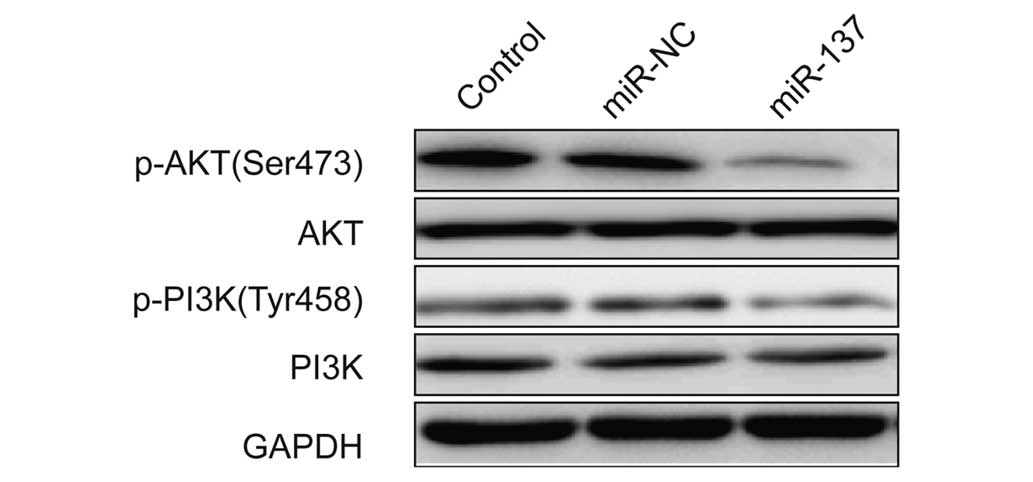
PI3K/AKT signaling pathway. Measurements of the
phosphorylation/activation pattern of PI3K and AKT was performed by
western blot. Western blot assay showed that overexpression of
miR-137 markedly inhibited the phosphorylation of PI3K, p-AKT
expression compared to untreated group and miR-NC group, without
altering the total protein levels of PI3K or AKT in 786-O cells
(Fig. 3), suggesting that miR-137
suppressed RCC growth in vitro and in vivo, at least
in part, via inhibiting activation of PI3K/AKT signaling
pathway.
Overexpression of miR-137 suppresses
tumor growth in vivo
As we showed that miR-137 acts as a tumor suppressor
in RCC by inhibiting cell proliferation and motility and inducing
cell apoptosis, we further investigated whether miR-137 would have
similar antitumor effect in vivo. 786-O cells stable
expression miR-NC or miR-137 were subcutaneously inoculated in nude
mice (n=10 for each group). The volumes of tumors established in
mice were measured with caliper every weeks. It was found that
overexpression of miR-137 significantly inhibited tumor volumes
than controls group and miR-NC group (Fig. 4A). The tumors were extracted 28 days
after implantation, tumor weight were measured. The result showed
that tumors tissue weight was significantly lower in miR-137 mimic
group relative to control group and miR-NC group (Fig. 4B and C). In addition, miR-137
expression level in xenograft tumors was determined by qRT-PCR. The
results of qRT-PCR showed that miR-137 expression was higher in the
xenograft tumors of miR-137 mimic group than that of the xenograft
tumors of miR-NC group and control group (P<0.05; Fig. 4D). These results might imply that
overexpression of miR-137 could inhibit RCC growth in
vivo.
Discussion
Recently, miRNAs have been reported to play key
roles in the initiation and maintenance of human various cancers
(7). It has drawn more attention in
the recent years because of it role in the gene transcriptional and
posttranscriptional regulation. Growing evidence showed that miRNAs
could act as novel biomarkers or therapy agent for various cancers,
inculding RCC (17). For instance,
the expression of was miR-129-3p significantly decreased in RCC, as
a molecular diagnostic marker to distinguish RCC from benign tumors
and normal tissues and a potential prognostic marker for RCC
(18). Chen et al showed that
miR-141 serves as a potential biomarker for discriminating RCC from
normal tissues and a crucial suppressor of RCC cell proliferation
and metastasis by modulating the EphA2/p-FAK/p-AKT/MMPs signaling
cascade (19). Lu et al
demonstrated that the expression of miR-145 was downregulated in
RCC compared to their normal adjacent tissues, and that restoring
miR-145 expression in RCC cell lines dramatically suppressed cell
proliferation, migration and invasion, and induced cell apoptosis
and G2-phase arrest (20). In this
study, we found that the expression of miR-137 was downregulated in
RCC compared to their normal adjacent tissues, and that
overexpression of miR-137 in RCC cells dramatically suppressed cell
proliferation, migration and invasion, and induced cell apoptosis
in vitro and suppressed tumor growth in vivo. These
studies and together with our study provide a solid foundation for
RCC utilization of miRNAs in diagnostic and anticancer therapy in
future.
Downregulation of miR-137 has been frequently
observed in several cancer cells including non-small lung cancer
(10), gastric cancer (11), and oral cancer cells (21) colorectal cancer (12) and breast cancer (13), glioblastoma cells (22). However, recently a study showed that
miR-137 expression was upregulated in squamous cells carcinoma of
the tongue (23), which suggests that
miR-137 may play different roles depending on different tumor
microenvironments. In this study, our results demonstrate that
miR-137 expression was frequently decreased in clinical specimens
of RCC and RCC cell lines, and its expression level was
significantly associated with poor prognostic clinicopathological
parameters, such as larger tumor diameter, advanced TNM stage, and
metastasis. We also showed that restored expression of miR-137 in
RCC cell line 786-O resulted in the inhibition of cell
proliferation, migration and invasion, and induction cell apoptosis
in vitro. In addition, in vivo assay demonstrated
that enforced miR-137 suppressed tumor growth and decreased tumor
volume and weight in xenograft nude mice models. These results
suggest that miR-137 functions as a tumor suppressor in RCC.
PI3K/AKT signaling is dysregulated in numerous
cancers, including RCC (24), and the
activation of this pathway has been suggested to correlate with
aggressive behavior and a poor prognosis in RCC tumors (25). Inhibition of the PI3K/AKT signaling
pathway using small molecule compounds acts as an attractive
potential therapeutic approach for RCC (15,16). A
recent study showed that the overexpression of miR-137 in gastric
cancer may inhibit the activation of PI3K/AKT signaling pathway
(11). Consistent with this result,
the results of the present study demonstrated that the
overexpression of miR-137 significantly inhibited the
phosphorylation of PI3K and p-AKT expression without altering the
total protein levels of PI3K or AKT in 786-O cells; which indicates
that miR-137 suppressed the growth of RCC in vitro and in
vivo, at least in part, by inhibiting the activation of the
PI3K/AKT signaling pathway.
In summary, the results of the present study
demonstrate that miR-137 was frequently reduced in clinical
specimens of RCC and in RCC cell lines. In addition, the expression
level of miR-137 was significantly associated with poor prognostic
clinicopathological parameters, including larger tumor diameter,
advanced TNM stage and metastasis. miR-137 showed a significant
suppressive effect on RCC proliferation, migration and invasion
in vitro and on the growth of tumors in xenograft nude mice
models, suggesting that miR-137 functions as a tumor suppressor in
RCC. In addition, miR-137 also inhibited the activation of the
PI3K/AKT signaling pathway, which may contribute to the inhibition
of RCC cell growth. These findings indicate that miR-137 may
present a useful biomarker for poor prognosis and a therapeutic
target for patients with RCC.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pantuck AJ, Zisman A and Belldegrun AS:
The changing natural history of renal cell carcinoma. J Urol.
166:1611–1623. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DiBiase SJ, Valicenti RK, Schultz D, Xie
Y, Gomella LG and Corn BW: Palliative irradiation for focally
symptomatic metastatic renal cell carcinoma: Support for dose
escalation based on a biological model. J Urol. 158:746–749. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gurtan AM and Sharp PA: The role of miRNAs
in regulating gene expression networks. J Mol Biol. 425:3582–3600.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ambros V and Lee RC: Identification of
microRNAs and other tiny noncoding RNAs by cDNA cloning. Methods
Mol Biol. 265:131–158. 2004.PubMed/NCBI
|
|
7
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death and tumorigenesis. Br J Cancer.
94:776–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu X, Li Y, Shen H, Li H, Long L, Hui L
and Xu W: miR-137 inhibits the proliferation of lung cancer cells
by targeting Cdc42 and Cdk6. FEBS Lett. 587:73–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng Y, Li Y, Liu D, Zhang R and Zhang J:
miR-137 effects on gastric carcinogenesis are mediated by targeting
Cox-2-activated PI3K/AKT signaling pathway. FEBS Lett.
588:3274–3281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu M, Lang N, Qiu M, Xu F, Li Q, Tang Q,
Chen J, Chen X, Zhang S, Liu Z, et al: miR-137 targets Cdc42
expression, induces cell cycle G1 arrest and inhibits invasion in
colorectal cancer cells. Int J Cancer. 128:1269–1279. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao Y, Li Y, Lou G, Zhao L, Xu Z, Zhang Y
and He F: MiR-137 targets estrogen-related receptor alpha and
impairs the proliferative and migratory capacity of breast cancer
cells. PLoS One. 7:e391022012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Porta C and Figlin RA:
Phosphatidylinositol-3-kinase/AKT signaling pathway and kidney
cancer, and the therapeutic potential of
phosphatidylinositol-3-kinase/AKT inhibitors. J Urol.
182:2569–2577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pal SK and Quinn DI: Differentiating mTOR
inhibitors in renal cell carcinoma. Cancer Treat Rev. 39:709–719.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li M, Wang Y, Song Y, Bu R, Yin B, Fei X,
Guo Q and Wu B: MicroRNAs in renal cell carcinoma: A systematic
review of clinical implications (Review). Oncol Rep. 33:1571–1578.
2015.PubMed/NCBI
|
|
18
|
Chen X, Ruan A, Wang X, Han W, Wang R, Lou
N, Ruan H, Qiu B, Yang H and Zhang X: miR-129-3p, as a diagnostic
and prognostic biomarker for renal cell carcinoma, attenuates cell
migration and invasion via downregulating multiple
metastasis-related genes. J Cancer Res Clin Oncol. 140:1295–1304.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X, Wang X, Ruan A, Han W, Zhao Y, Lu
X, Xiao P, Shi H, Wang R, Chen L, et al: miR-141 is a key regulator
of renal cell carcinoma proliferation and metastasis by controlling
EphA2 expression. Clin Cancer Res. 20:2617–2630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu R, Ji Z, Li X, Zhai Q, Zhao C, Jiang Z,
Zhang S, Nie L and Yu Z: miR-145 functions as tumor suppressor and
targets two oncogenes, ANGPT2 and NEDD9, in renal cell carcinoma. J
Cancer Res Clin Oncol. 140:387–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kozaki K, Imoto I, Mogi S, Omura K and
Inazawa J: Exploration of tumor-suppressive microRNAs silenced by
DNA hypermethylation in oral cancer. Cancer Res. 68:2094–2105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen L, Wang X, Wang H, Li Y, Yan W, Han
L, Zhang K, Zhang J, Wang Y, Feng Y, et al: miR-137 is frequently
down-regulated in glioblastoma and is a negative regulator of
Cox-2. Eur J Cancer. 48:3104–3111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wiklund ED, Gao S, Hulf T, Sibbritt T,
Nair S, Costea DE, Villadsen SB, Bakholdt V, Bramsen JB, Sørensen
JA, et al: MicroRNA alterations and associated aberrant DNA
methylation patterns across multiple sample types in oral squamous
cell carcinoma. PLoS One. 6:e278402011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Husseinzadeh HD and Garcia JA: Therapeutic
rationale for mTOR inhibition in advanced renal cell carcinoma.
Curr Clin Pharmacol. 6:214–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pantuck AJ, Seligson DB, Klatte T, Yu H,
Leppert JT, Moore L, O'Toole T, Gibbons J, Belldegrun AS and Figlin
RA: Prognostic relevance of the mTOR pathway in renal cell
carcinoma: Implications for molecular patient selection for
targeted therapy. Cancer. 109:2257–2267. 2007. View Article : Google Scholar : PubMed/NCBI
|