Introduction
Lung cancer is the leading cause of cancer
mortality, worldwide (1). Although
cigarette smoking is the primary risk factor for lung cancer, the
epidemiology of lung cancer remains partially unresolved, as the
majority of cigarette smokers do not develop lung tumors.
Furthermore, lung cancer is currently the seventh leading cause of
cancer-related mortality in non-smokers (2). Human papillomavirus (HPV) infection has
been postulated as a possible risk factor for the disease (3).
Recent studies have identified differences in the
prevalence of HPV infection in lung cancer between different
ethnicities and geographical variations (4,5). A lack of
consensus with regard to detection methods, as well as
methodological flaws may have been responsible for the large
discrepancies reported, particularly between studies reporting
conflicting results with regard to the prevalence of HPV in areas
within the same geographical locations (6). Due to the variability in HPV prevalence
reported, the involvement of HPV in lung cancer, specifically in
non-small cell lung cancer (NSCLC), which accounts for 80% of all
lung cancer cases, remains to be elucidated (7,8).
Previous studies in Taiwanese and Japanese
populations have reported that female non-smokers with lung
adenocarcinoma exhibit an elevated incidence of HPV infections
(9) and observed that lung cancer
patients with HPV infections were responsive to gefitinib treatment
(10). Recently, two studies
demonstrated that mutations in the epidermal growth factor receptor
(EGFR) gene were associated with the presence of HPV
infection in Taiwanese and Japanese patients (11,12).
However, these studies did not evaluate the response rate to
gefitinib as all subjects were diagnosed with primary lung cancer
and subsequently underwent surgery rather than gefitinib
administration.
In the present study, 95 patients with advanced lung
adenocarcinoma were enrolled to retrospectively investigate the
association between HPV status, EGFR mutations and clinical
characteristics, as well as their impact on progression-free
survival (PFS).
Materials and methods
Clinical specimens
A total of 95 paraffin-embedded tissue samples were
obtained from advanced lung adenocarcinoma patients (stage III–IV)
(13) prior to adjuvant therapy at
the Department of Respiratory Oncology, Anhui Provincial Hospital
(Hefei, China) between August 2011 and December 2013. Patients with
complete clinicopathological and follow-up data were included. The
tissues included 20 surgical specimens, 18 lung biopsy specimens,
18 bronchoscopic biopsy specimens, 23 pleural effusion specimens,
13 lymph node biopsy specimens and 3 bone biopsy specimens. All
patients provided written informed consent was provided and the
study was approved by the Institutional Review Board of Anhui
Provincial Hospital.
Clinical characteristics including patient age,
gender, smoking history, histological differentiation, lymph node
metastasis, distant metastasis and treatment type [tyrosine kinase
inhibitors (TKIs) (gefitinib or erlotinib) and platinum-based
chemotherapy (cisplatin or carboplatin)] were collected for
subsequent analyses. PFS was defined as the time from treatment to
recurrence or the date of censorship (the last date of
follow-up).
DNA extraction
DNA was deparaffinized and extracted from tissue
samples using the QIAamp DNA FFPE Tissue kit (Qiagen, Hilden,
Germany) according to the manufacturer's instructions. Briefly, 5–6
formalin-fixed, paraffin-embedded 8-µm tissue sections were soaked
in xylene and vortexed vigorously. A tissue pellet was obtained and
purified according to the manufacturer's instructions. DNA samples
were quantified spectrophotometrically and normalized aliquots were
obtained for each sample.
HPV DNA detection
HPV testing of lung cancer samples was performed
using polymerase chain reaction (PCR) amplification of a fragment
of the HPV L1 gene. The presence of HPV infection was
determined using a Tellgenplex™ HPV DNA Test kit (Tellgen Life
Science Co., Ltd., Shanghai, China) using the Luminex®
technique, which enables the detection of 26 HPV genotypes,
including those of 19 high-risk HPV types (16, 18, 26, 31, 33, 35,
39, 45, 51, 52, 53, 55, 56, 58, 59, 66, 68, 82 and 83) and 7
low-risk HPV types (6, 11, 40, 42, 44, 61 and 73). The experiments
were performed in a Bio-Plex® 200 system (Bio-Rad
Laboratories, Hercules, CA, USA) according to the manufacturer's
instructions. Briefly, 2 µg extracted DNA was amplified by PCR
under the following conditions: 5 cycles of 95°C for 30 sec, 58°C
for 30 sec and 72°C for 30 sec followed by 35 cycles of 95°C for 30
sec, 55°C for 30 sec and 72°C for 30 sec. PCR products were
subjected to rapid hybridization and the data were analyzed using
the software provided by the manufacturer (Tellgen Software,
version IS2.3; Tellgen Life Science Co., Ltd.) (14).
Mutations in EGFR exons 18–21 were detected
using previously described methods (15).
Statistical analysis
Statistical analyses were performed using SPSS 16.0
statistical software (SPSS, Inc., Chicago, IL, USA). χ2
tests were used to compare the associations between HPV status,
EGFR mutation status and clinical characteristics. To
investigate the combined effect of HPV infection and EGFR
mutation on PFS, four subgroups were determined
(HPV+/mutant+,
HPV+/mutant−,
HPV−/mutant+, and
HPV−/mutant−). The survival curves were
estimated by Kaplan-Meier methods and the differences in survival
distributions were compared using the log-rank test. To evaluate
the mortality risk, the hazard ratio (HR) and corresponding 95%
confidence interval (CI) were estimated using Cox
proportional hazards models to identify potential prognostic
factors. All statistical tests were two-sided and P<0.05 was
considered to indicate statistically significant differences.
Results
High-risk HPV infection in advanced
lung adenocarcinoma patients
Of the 95 DNA samples tested, 27 (28.4%) were
positive for high-risk HPV. The most common high-risk HPV types
identified in lung adenocarcinoma patients were HPV16 and HPV18;
however, other genotypes including HPV33 and 58 were also
detected.
Association between EGFR mutation and
HPV infection
HPV infection was associated with lymph node
metastasis (P=0.016) (Table I). A
total of 44/95 cases (46.3%) exhibited EGFR mutations (in
exons 19/21). EGFR mutations were more common in females
(P<0.006) and never-smokers (P<0.019) when compared with
males and ever-smokers, respectively. The presence of HPV DNA was
significantly associated with EGFR mutations (P=0.012)
(Table I). Multivariate analysis also
revealed that HPV DNA was significantly associated with EGFR
mutations (odds ratio=3.971) in advanced lung adenocarcinoma
(Table II).
 | Table I.Clinicopathological characteristics,
HPV infection status and EGFR mutation status of advanced lung
adenocarcinoma patients. |
Table I.
Clinicopathological characteristics,
HPV infection status and EGFR mutation status of advanced lung
adenocarcinoma patients.
|
| HPV |
| EGFR |
|
|---|
|
|
|
|
|
|
|---|
| Characteristics | Positive, n | Negative, n | P-value | Mutated, n | Wild-type, n | P-value |
|---|
| Age, years |
|
| 0.415 |
|
| 0.448 |
|
<64 | 16 | 34 |
| 25 | 25 |
|
|
≥64 | 11 | 34 |
| 19 | 26 |
|
| Gender |
|
| 0.255 |
|
| 0.006 |
|
Male | 12 | 39 |
| 17 | 34 |
|
|
Female | 15 | 29 |
| 27 | 17 |
|
| Smoking status |
|
| 0.078 |
|
| 0.019 |
|
Never | 20 | 37 |
| 32 | 25 |
|
|
Ever | 7 | 31 |
| 12 | 26 |
|
| Histological
differentiation |
|
| 0.109 |
|
| 0.110 |
|
Well | 4 | 21 |
| 10 | 15 |
|
|
Poor | 23 | 47 |
| 29 | 41 |
|
| Lymph node
metastasis |
|
| 0.016 |
|
| 0.801 |
|
Yes | 11 | 46 |
| 27 | 30 |
|
| No | 16 | 22 |
| 17 | 21 |
|
| Distant
metastasis |
|
| 0.255 |
|
| 0.326 |
|
Yes | 12 | 39 |
| 26 | 25 |
|
| No | 15 | 29 |
| 18 | 26 |
|
| EGFR mutations |
|
| 0.012 |
|
|
|
|
Mutated | 18 | 26 |
|
|
|
|
|
Wild-type | 9 | 42 |
|
|
|
|
 | Table II.Multivariate analysis of the
association between human papillomavirus infection status and the
clinicopathological characteristics of patients with advanced lung
adenocarcinoma. |
Table II.
Multivariate analysis of the
association between human papillomavirus infection status and the
clinicopathological characteristics of patients with advanced lung
adenocarcinoma.
|
| Multivariate
logistic regression |
|---|
|
|
|
|---|
| Variables | Odds ratio | 95% confidence
interval | P-value |
|---|
| Gender (male vs.
female) | 0.920 |
0.252–3.358 | 0.899 |
| Age, years (<64
vs. ≥64) | 0.779 |
0.279–2.179 | 0.635 |
| Smoking status
(never vs. ever) | 0.652 |
0.165–2.579 | 0.542 |
| Histological
differentiation (well vs. poor) | 0.321 |
0.087–1.192 | 0.090 |
| Lymph node
metastasis (yes vs. no) | 0.361 |
0.125–1.039 | 0.059 |
| Distant metastasis
(yes vs. no) | 0.645 |
0.220–1.892 | 0.424 |
| EGFR (mutated vs.
wild-type) | 3.971 | 1.364–11.562 | 0.011 |
Impact of HPV infection and EGFR
mutations on PFS
To determine the clinical significance of HPV
infection status and EGFR mutations in advanced lung
adenocarcinoma, the association between HPV infection, EGFR
mutations and PFS was investigated in the present study. The
Kaplan-Meier survival curve estimates are shown in Fig. 1. Patients with HPV infections
exhibited a longer median PFS time than those without (13 vs. 9
months; P=0.013).
To evaluate the clinicopathological characteristics
of patients and the impact of such on PFS in advanced lung
adenocarcinoma, the HR and corresponding 95% CI were estimated
using the Cox proportional hazards analysis, which was adjusted for
smoking (never vs. ever), lymph node metastasis (yes vs. no),
EGFR (mutated vs. wild-type) and HPV infection (positive vs.
negative). The presence of EGFR mutations and lymph node
metastasis were independent prognostic factors for survival,
however, this trend was not observed in patients with HPV infection
(Table III).
 | Table III.Univariate and multivariate analyses
of progression-free survival. |
Table III.
Univariate and multivariate analyses
of progression-free survival.
|
| Univariate |
| Multivariate |
|
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender (male vs.
female) | 0.888 | 0.569–1.387 | 0.602 |
|
|
|
| Age, years (<64
vs. ≥64) | 1.034 | 0.665–1.608 | 0.881 |
|
|
|
| Smoking status
(never vs. ever) | 1.709 | 1.086–2.690 | 0.020 | 1.174 | 0.738–1.866 | 0.498 |
| Histological
differentiation (well vs. poor) | 0.829 | 0.494–1.391 | 0.478 |
|
|
|
| Lymph node
metastasis (yes vs. no) | 2.756 | 1.618–4.696 | <0.001 | 2.750 | 1.566–4.829 | <0.001 |
| Distant metastasis
(yes vs. no) | 1.441 | 0.922–2.254 | 0.109 |
|
|
|
| EGFR status
(mutated vs. wild-type) | 0.229 | 0.134–0.392 | <0.001 | 0.229 | 0.132–0.397 | <0.001 |
| HPV infection
(positive vs. negative) | 0.556 | 0.337–0.915 | 0.021 | 0.893 | 0.518–1.539 | 0.683 |
For advanced lung adenocarcinoma, treatment modality
is one of the most important determinants of clinical outcome,
thus, the PFS of patients treated with TKIs and platinum-based
chemotherapy was analyzed using the Cox proportional hazards model.
No significant difference in survival was identified between
patients with HPV infections and those without in the subgroups
receiving TKI and platinum-based chemotherapy (Table IV). The results indicated that the
longest PFS times, which were observed in patients with positive
HPV infection status, may have been due to a good response to TKI-
or platinum-based-adjuvant therapy.
 | Table IV.Subgroup analyses for PFS stratified
by treatments. |
Table IV.
Subgroup analyses for PFS stratified
by treatments.
| Treatment | HPV status | Median PFS time,
months | HR (95% CI) | P-value |
|---|
| TKI | Positive | 16 | 0.689 | 0.304 |
|
| Negative | 14 | (0.338–1.402) |
|
| Platinum | Positive | 10 | 0.600 | 0.170 |
|
| Negative | 7 | (0.290–1.245) |
|
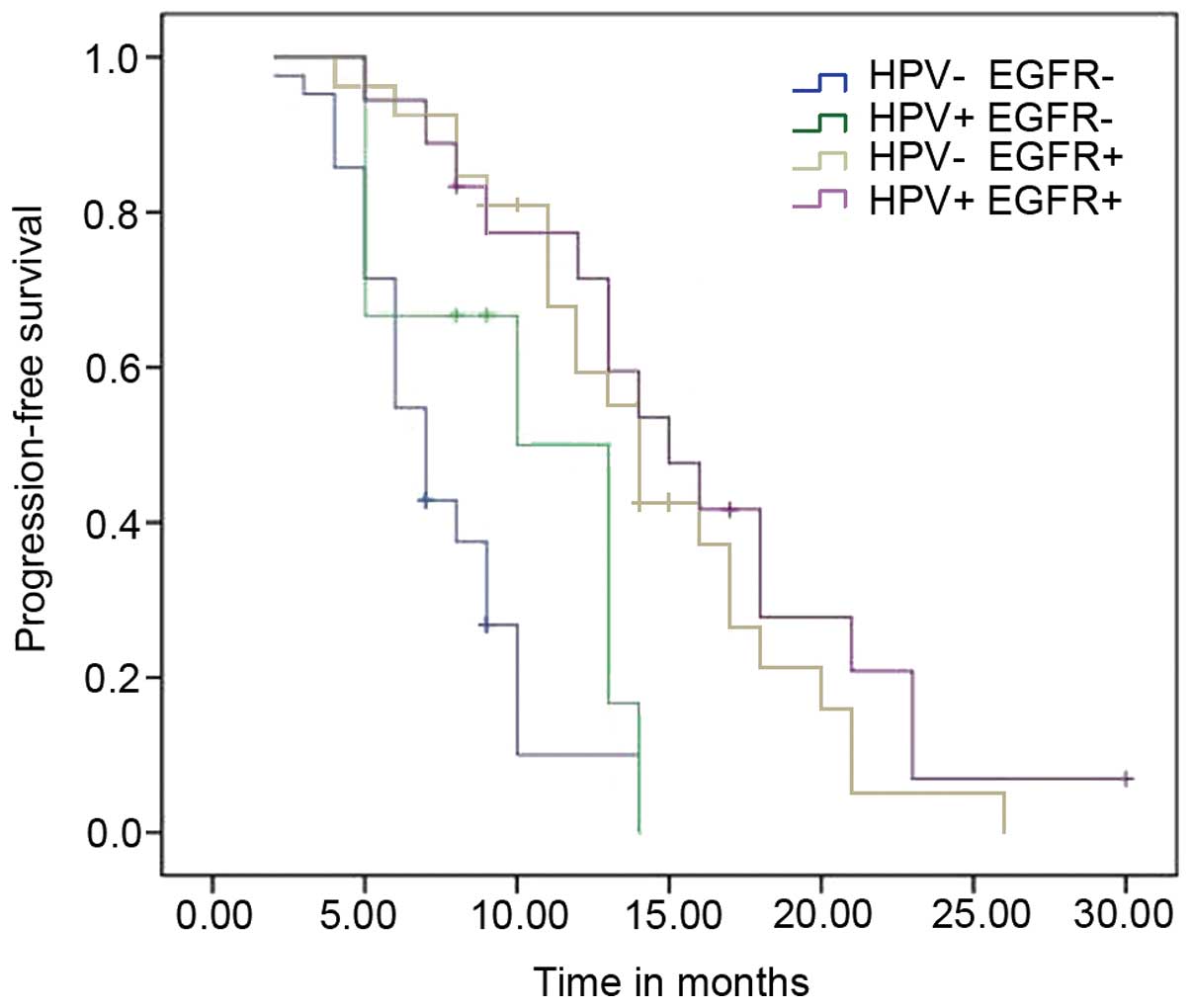
HPV infection and EGFR mutation
exhibit a synergistic effect on PFS time
Multivariate analysis demonstrated that HPV DNA was
significantly associated with EGFR mutations in lung adenocarcinoma
and thus, the combined effects of these two biomarkers on PFS was
evaluated, which revealed that they exhibited a synergistic benefit
on PFS time. Patients with both HPV infection and EGFR
mutations exhibited a significantly reduced risk when compared with
those exhibiting neither biomarker (adjusted HR=0.640; 95% CI:
0.488–0.840; P=0.001; Table V). The
PFS curves are shown in Fig. 2. In
addition, the results demonstrated that patients with EGFR
mutations exhibited a better prognosis compared with those
exhibiting wild-type EGFR, regardless of HPV status.
 | Table V.HRs for PFS according to
subgroups. |
Table V.
HRs for PFS according to
subgroups.
| HPV/EGFR
mutant | Patients, n | Median PFS time,
months | Unadjusted HR (95%
CI) | P-value | Adjusted
HRa (95% CI) | P-value |
|---|
| HPV+
mutant+ | 18 | 15 | 0.581
(0.445–0.758) | <0.001 | 0.640
(0.488–0.840) |
0.001 |
| HPV+
mutant− | 9 | 10 | 0.763
(0.507–1.148) | 0.194 | 0.829
(0.538–1.275) |
0.393 |
| HPV−
mutant+ | 26 | 14 | 0.216
(0.113–0.416) | <0.001 | 0.224
(0.117–0.428) | <0.001 |
| HPV−
mutant− | 42 | 7 | 1.0 |
| 1.0 |
|
Discussion
In the present study, 27/95 (28.4%) advanced lung
adenocarcinoma patients were positive for high-risk HPV infection.
Multivariate analysis revealed a significant association between
HPV infection and EGFR mutations. Subgroup analyses for PFS
stratified by treatments indicated that patients with HPV
infections exhibited the longest PFS times in both the TKI and
platinum-based-adjuvant treatment groups, which may be due to
patients exhibiting a good response to TKI- or
platinum-based-adjuvant therapy. Patients with both EGFR
mutations and HPV infections (HPV+/mutant+)
exhibited the longest survival time when compared with the other
subgroups (HPV+/mutant−,
HPV−/mutant+ and
HPV−/mutant−). However, patients with
EGFR mutations exhibit a better prognosis compared with
those exhibiting wild-type EGFR, regardless of HPV
status.
The reported frequency of HPV infection in lung
cancer patients varies worldwide. A comprehensive literature review
revealed mean frequencies of 17 and 15% in Europe and the United
States, respectively, with a higher frequency in Asia (35.7%)
(16). The most common high-risk HPV
types identified in lung cancer patients are HPV16 and HPV18,
however, other HPV types such as HPV6, 11, 31, 33, 39, 52, 58 and
82 have also been reported (17). In
accordance with these reports, in the present study HPV16 was
identified as the most common genotype and positivity for HPV18,
33, 58, 6 and 11 was also observed (data not shown). The overall
frequency of HPV DNA in advanced NSCLC patients was 25.2% (data not
shown). A previous study by Wang et al (18) demonstrated that HPV infections were
present in localized and earlier stages of lung cancer, however, in
the present study HPV infections were more commonly identified in
patients with lymph node metastasis. The reason for these
discrepancies remains unclear. We hypothesize that HPV infection
may be associated with certain disease stages and metastasis in
lung cancer.
Recently, NSCLC in never-smokers has emerged as a
global public health issue. The cause remains unclear and few
studies have focused on the prevalence of HPV in this population
(19). A previous study revealed that
Taiwanese never-smokers exhibited a high prevalence of HPV16/18
infection, indicating that this is a potential etiological agent of
lung cancer (20). In accordance with
these results, in the present study, HPV infections were observed
in never-smokers (data not shown).
EGFR mutations have been demonstrated to be
more common in never-smokers, women, individuals of Asian ethnicity
and adenocarcinoma patients (21,22).
Furthermore, lung adenocarcinoma patients harboring EGFR
mutations are considered to exhibit an increased sensitivity to
EGFR TKIs, such as gefitinib and erlotinib (23). Notably, these clinicopathological
features of EGFR mutations were evident in the present study
and a significant association was identified between HPV infections
and EGFR mutations in advanced lung adenocarcinoma patients.
However, the present study also indicated that patients with
EGFR mutations exhibited a better prognosis compared with
those exhibiting wild-type EGFR, regardless of HPV status,
and those patients exhibited the longest PFS times, which may be
due to good response to TKI- or platinum-based-adjuvant therapy.
However, these findings contradict previous studies. For example,
Hsu et al (24) reported
survival benefits for stage I NSCLC patients that expressed the
HPV16/18 E6 oncoprotein. Furthermore, Wang et al (18) demonstrated that patients with HPV
infections exhibited a better survival than those without in a
subgroup receiving platinum-based chemotherapy; however, this trend
was not observed in subgroups receiving TKI and radiation. We
hypothesize that this difference may be related to the survival
index using PFS but not overall survival, and the fact that the
majority of patients with EGFR mutations received TKI therapy but
not platinum-based chemotherapy.
Multivariate analysis in the present study revealed
that HPV DNA was significantly associated with EGFR
mutations in advanced lung adenocarcinoma. Furthermore, patients
with both EGFR mutations and HPV infections exhibited the
longest PFS times. Márquez-Medina et al (25) previously reported that HPV infections
were more common in patients harboring EGFR mutations or
those sensitive to erlotinib. In addition, Wu et al
(26), Zhang et al (27) and Sung et al (28) demonstrated that HPV16 E6 upregulates
cellular inhibitor of apoptosis 2 (cIAP2), which is located
downstream of EGFR signaling, via the
EGFR/phosphatidylinositide 3-kinase (PI3K)/protein kinase B (AKT)
cascade, and that cIAP2 expression was positively correlated with
EGFR mutations. Therefore, the EGFR/PI3K/AKT cascade may
exhibit a pivotal function in HPV-associated lung carcinogenesis in
individuals with EGFR mutations.
In conclusion, the present study indicates a
significant association between HPV infections and EGFR
mutations in advanced lung adenocarcinoma patients. However,
further large-scale prospective studies are required to confirm
this hypothesis. Currently, the authors of the present study are
planning a basic study to elucidate the association between HPV
infections and EGFR mutations using patient-derived tumor
xenograft models, which may increase current understanding with
regard to the pathogenesis of HPV infections in lung cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81172172). The authors would
like to thank Mr. Guo Zhen-Li (Department of Pathology, Affiliated
Provincial Hospital of Anhui Medical University, Hefei, China), for
collecting tissue samples and Mr. Wang Xu (Science and Education
Division, Anhui Provincial Children's Hospital, Hefei, China) for
statistical analysis.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thun MJ, Hannan LM, Adams-Campbell LL,
Boffetta P, Buring JE, Feskanich D, Flanders WD, Jee SH, Katanoda
K, Kolonel LN, et al: Lung cancer occurrence in never-smokers: An
analysis of 13 cohorts and 22 cancer registry studies. PLoS Med.
5:e1852008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klein F, Amin Kotb WF and Petersen I:
Incidence of human papilloma virus in lung cancer. Lung Cancer.
65:13–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yanagawa N, Wang A, Kohler D, Santos Gda
C, Sykes J, Xu J, Pintilie M and Tsao MS: Human papilloma virus
genome is rare in North American non-small cell lung carcinoma
patients. Lung Cancer. 79:215–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sagerup CM, Nymoen DA, Halvorsen AR,
Lund-Iversen M, Helland A and Brustugun OT: Human papilloma virus
detection and typing in 334 lung cancer patients. Acta Oncol.
53:952–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sarchianaki E, Derdas SP, Ntaoukakis M,
Vakonaki E, Lagoudaki ED, Lasithiotaki I, Sarchianaki A,
Koutsopoulos A, Symvoulakis EK, Spandidos DA, et al: Detection and
genotype analysis of human papillomavirus in non-small cell lung
cancer patients. Tumour Biol. 35:3203–3209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anantharaman D, Gheit T, Waterboer T,
Halec G, Carreira C, Abedi-Ardekani B, McKay-Chopin S, Zaridze D,
Mukeria A, Szeszenia-Dabrowska N, et al: No causal association
identified for human papillomavirus infections in lung cancer.
Cancer Res. 74:3525–3534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simen-Kapeu A, Surcel HM, Koskela P,
Pukkala E and Lehtinen M: Lack of association between human
papillomavirus type 16 and 18 infections and female lung cancer.
Cancer Epidemiol Biomarkers Prev. 19:1879–1881. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hasegawa Y, Ando M, Kubo A, Isa S,
Yamamoto S, Tsujino K, Kurata T, Ou SH, Takada M and Kawaguchi T:
Human papilloma virus in non-small cell lung cancer in never
smokers: A systematic review of the literature. Lung Cancer.
83:8–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baba M, Castillo A, Koriyama C, Yanagi M,
Matsumoto H, Natsugoe S, Shuyama KY, Khan N, Higashi M, Itoh T, et
al: Human papillomavirus is frequently detected in
gefitinib-responsive lung adenocarcinomas. Oncol Rep. 23:1085–1092.
2010.PubMed/NCBI
|
|
11
|
Tung MC, Wu HH, Cheng YW, Wang L, Chen CY,
Yeh SD, Wu TC and Lee H: Association of epidermal growth factor
receptor mutations with human papillomavirus 16/18 E6 oncoprotein
expression in non-small cell lung cancer. Cancer. 119:3367–3376.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kato T, Koriyama C, Khan N, Samukawa T,
Yanagi M, Hamada T, Yokomakura N, Otsuka T, Inoue H, Sato M, et al:
EGFR mutations and human papillomavirus in lung cancer. Lung
Cancer. 78:144–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wood DE, Kazerooni E, Baum SL, Dransfield
MT, Eapen GA, Ettinger DS, Hou L, Jackman DM, Klippenstein D, Kumar
R, et al: Lung cancer screening, version 1.2015: featured updates
to the NCCN guidelines. J Natl Compr Canc Netw. 13:23–34.
2015.PubMed/NCBI
|
|
14
|
Wei W, Shi Q, Guo F, Zhang BY, Chen C,
Zhang NS and Dong XP: The distribution of human papillomavirus in
tissues from patients with head and neck squamous cell carcinoma.
Oncol Rep. 28:1750–1756. 2012.PubMed/NCBI
|
|
15
|
Zhou Q, Zhang XC, Chen ZH, Yin XL, Yang
JJ, Xu CR, Yan HH, Chen HJ, Su J, Zhong WZ, et al: Relative
abundance of EGFR mutations predicts benefit from gefitinib
treatment for advanced non-small-cell lung cancer. J Clin Oncol.
29:3316–3321. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Syrjänen K: Detection of human
papillomavirus in lung cancer: Systematic review and meta-analysis.
Anticancer Res. 32:3235–3250. 2012.PubMed/NCBI
|
|
17
|
Ragin C, Obikoya-Malomo M, Kim S, Chen Z,
Flores-Obando R, Gibbs D, Koriyama C, Aguayo F, Koshiol J, Caporaso
NE, et al: HPV-associated lung cancers: An international pooled
analysis. Carcinogenesis. 35:1267–1275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang JL, Fang CL, Wang M, Yu MC, Bai KJ,
Lu PC and Liu HE: Human papillomavirus infections as a marker to
predict overall survival in lung adenocarcinoma. Int J Cancer.
134:65–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng YW, Chiou HL, Sheu GT, Hsieh LL,
Chen JT, Chen CY, Su JM and Lee H: The association of human
papillomavirus 16/18 infection with lung cancer among nonsmoking
Taiwanese women. Cancer Res. 61:2799–2803. 2001.PubMed/NCBI
|
|
20
|
Pallis AG and Syrigos KN: Lung cancer in
never smokers: Disease characteristics and risk factors. Crit Rev
Oncol Hematol. 88:494–503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mitsudomi T, Kosaka T and Yatabe Y:
Biological and clinical implications of EGFR mutations in lung
cancer. Int J Clin Oncol. 11:190–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shigematsu H, Lin L, Takahashi T, Nomura
M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, et
al: Clinical and biological features associated with epidermal
growth factor receptor gene mutations in lung cancers. J Natl
Cancer Inst. 97:339–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paez JG, Janne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsu NY, Cheng YW, Chan IP, Ho HC, Chen CY,
Hsu CP, Lin MH and Chou MC: Association between expression of human
papillomavirus 16/18 E6 oncoprotein and survival in patients with
stage I non-small cell lung cancer. Oncol Rep. 21:81–87.
2009.PubMed/NCBI
|
|
25
|
Márquez-Medina D, Gasol-Cudós A,
Taberner-Bonastre MT, Samamé Pérez-Vargas JC, Salud-Salvia A and
Llombart-Cussac A: Human papillomavirus in non-small-cell lung
cancer: The impact of EGFR mutations and the response to erlotinib.
Arch Bronconeumol. 49:79–81. 2013.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu HH, Wu JY, Cheng YW, Chen CY, Lee MC,
Goan YG and Lee H: cIAP2 upregulated by E6 oncoprotein via
epidermal growth factor receptor/phosphatidylinositol 3-kinase/AKT
pathway confers resistance to cisplatin in human papillomavirus
16/18-infected lung cancer. Clin Cancer Res. 16:5200–5210. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang E, Feng X, Liu F, Zhang P, Liang J
and Tang X: Roles of PI3K/Akt and c-Jun signaling pathways in human
papillomavirus type 16 oncoprotein-induced HIF-1α, VEGF, and IL-8
expression and in vitro angiogenesis in non-small cell lung cancer
cells. PLoS One. 9:e1034402014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sung WW and Lee H: The role of
interleukin-10 in the progression of human
papillomavirus-associated lung carcinoma. Oncoimmunology.
2:e258542013. View Article : Google Scholar : PubMed/NCBI
|