Introduction
Pancreatic cancer (PC) is one of the most aggressive
malignancies, and is ranked as the fourth leading cause of
cancer-related mortality in developed countries (1,2). More than
85% of patients have already suffered local infiltration or distant
metastasis at the time of initial diagnosis due to the extremely
poor early diagnosis rate and highly aggressive behavior of PC
(3). The overall 5-year survival rate
is less than 5%. Thus, novel effective therapeutic methods or
agents for preventing and controlling this disease are urgently
required.
Deguelin is a natural compound of the flavonoid
family of products isolated from several plant species, including
Derris trifoliata Lour and Mundulea sericea
(Leguminosae) (4). Several studies
have already demonstrated its excellent potential as an
anticarcinogenic and antiproliferative agent against various
malignant tumors including gastric, lung and breast carcinoma, by
targeting apoptosis, cell cycle arrest and anti-angiogenesis
(5–7).
Deguelin also exhibits effects of anti-metastasis (7,8). It
inhibits cancers through a number of pathways, including the
PI3K-Akt, HIF-1-VEGF, IKK-IκBα-NF-κB, EMT and AMPK-mTOR-survivin
pathways (4–7). However, there are few studies on
deguelin regulating the hedgehog (Hh) signaling pathway, an
essential pathway in tumor growth and metastasis. In addition, the
correlation between deguelin and PC has rarely been researched to
date.
The Hh signaling pathway transmits information to
embryonic cells required for proper development. Mammals have three
Hh homologs: Desert (Dhh), Indian (Ihh) and Sonic (Shh), all of
which have distinct as well as overlapping roles (9–11). The
general organization of the Hh pathway consists of a series of
repressive interactions. In the absence of Hh proteins (off-state)
as the Hh receptors, Patched 1 (PTCH1), a 12-transmembrane protein,
suppresses the otherwise constitutively active signaling receptor
Smoothened (12–18). In the off-state, Suppressor of fused
(SUFU) suppresses Hh signaling by regulating the localization of
the Gli transcription factors (19,20). Gli1
is an effective activator of protein kinase A and itself.
Therefore, Gli1 localization within the nucleus is a biomarker of
Hh activity (21). Matrix
metalloproteinases (MMPs), a family of zinc-dependent
endopeptidases, play significant roles in tumor tissue remodeling,
and are associated with a number of physiological or pathological
processes. MMP-2 and MMP-9 are considered to be particularly
significant in metastasis (22,23). It is
well known that the activation of Hh has been implicated in the
development of cancers in various sites, including prostatic,
gastric, esophageal and ovarian organs (24–27).
In the present study, we further explored the
effects of deguelin as a chemotherapeutic agent against human PC
cells, and investigated its mechanism.
Materials and methods
Cell culture and reagents
Human PC cell lines Panc-1 and Bxpc-3 were purchased
from American Type Culture Collection (ATCC; Rockville, MD, USA).
The Panc-1 cells were cultured in Dulbecco's modified Eagle's
medium (Gibco, Rockville, MD, USA) supplemented with 10% fetal
bovine serum (FBS), 100 U/ml penicillin G and 100 U/ml streptomycin
in a humidified incubator containing 5% CO2 in air at 37°C. The
Bxpc-3 cells were cultured in RPMI-1640 medium (Gibco) containing
supplements as above. Deguelin was purchased from Sigma-Aldrich
(St. Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO) to
make 0.1 mmol/l stock solution and stored at −20°C. The final
concentration of DMSO was <0.1%.
Cell growth inhibition assay
Cell growth-inhibitory curves of 7 days were
observed by Cell Counting Kit-8 (CCK-8; Mai Bio, Ltd. Shanghai,
China) assay. Briefly, cells were plated at a density of 2×103
cells/well in 96-well microtiter plates. Following treatment, 20 µl
CCK-8 solution was added to each well and further incubated for 1–3
h. Then absorbance was measured with an absorbance reader (Bio-Tek
ELx800, Winooski, VT, USA) at a wavelength of 450 nm.
Flow cytometric assessment of
apoptosis
The Annexin V-FITC apoptosis detection kit (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China) was used for flow
cytometric analysis of apoptosis. Cells were treated with deguelin
for 48 h on a six-well plate, then 5×105 cells were harvested and
washed twice with phosphate-buffered saline, then resuspended in
500 µl binding buffer. Annexin V-FITC (5 µl) and propidium iodide
were added to each sample and incubated for 15 min in the dark. The
stained cells were analyzed directly by flow cytometry using the
CellQuest program (Becton Dickinson, San Jose, CA, USA).
Wound-healing assay
Cell migration in vitro was assessed by wound
healing assay. The culture insert (Ibidi, Martinsried, Germany) was
placed in a clean and dry six-well plate in advance. Suspended
cells (70 µl) were loaded at a density of 6×105/ml. When a
confluent layer was formed, medium with 0.5% FBS was added the well
after the culture insert was removed. Acquired images at 0, 12 and
24 h of follow-up incubation were analyzed online at wimasis.com.
Transwell migration assay
Transwell migration assays were performed using
polycarbonate membrane Transwell inserts (Corning Incorporated,
Corning, NY, USA) with an 8.0-µm pore size and 6.5-mm membrane
diameter on 24-well plates. The lower chambers were filled with 600
µl medium (10% FBS), and the upper chambers were loaded with cells
at a density of 1×105 cells/well) in 200 µl medium (2% FBS).
Following incubation for 24 h, the membranes were fixed in 4%
paraformaldehyde for 20 min and stained with hematoxylin and eosin
for 10 min, respectively. Cells on the upper surface of the
membranes were removed with cotton swabs. Cells adhered to the
lower surface were observed under the microscope.
Western blot analysis
Cells were lysed with RIPA buffer (Sigma-Aldrich)
supplemented with a complete protease inhibitor cocktail (Roche,
Basel, Switzerland). Following centrifugation at 13,000 × g for 30
min, the supernatant was collected and protein concentration was
determined using a bicinchoninic acid protein assay kit (Pierce
Biotechnology, Rockford, IL, USA). Protein (40 µg) was separated in
8–10% sodium dodecyl sulphate-polyacrylamide gel and transferred to
polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA).
The transblotted membranes were blocked in Tris-buffered saline
with Tween-20 (10 mM Tris-HCL pH 7.4, 150 mM NaCl and 0.1%
Tween-20) with 5% skimmed milk for 1.5 h at room temperature, then
incubated with primary antibodies overnight at 4°C, followed by
horseradish peroxidase-conjugated secondary antibodies. The
immunoreactive bands were visualized using enhanced
chemiluminescence (Biological Industries, Beit Haemek, Israel) and
exposed to X-ray films. All the antibodies were procured from Cell
Signaling Technology, Inc. (Danvers, MA, USA).
Statistical analysis
The results were expressed as the means ± standard
error. All experiments were performed in triplicate. The one-way
ANOVA or two-tailed Student's t-test were used to determine the
statistical significance between two unpaired groups. P<0.05 was
considered to indicate a statistically significant difference.
Analyses were performed using SPSS 13.0 statistical software
package (SPSS Inc., Chicago, IL, USA).
Results
Deguelin inhibits the growth of PC
cells
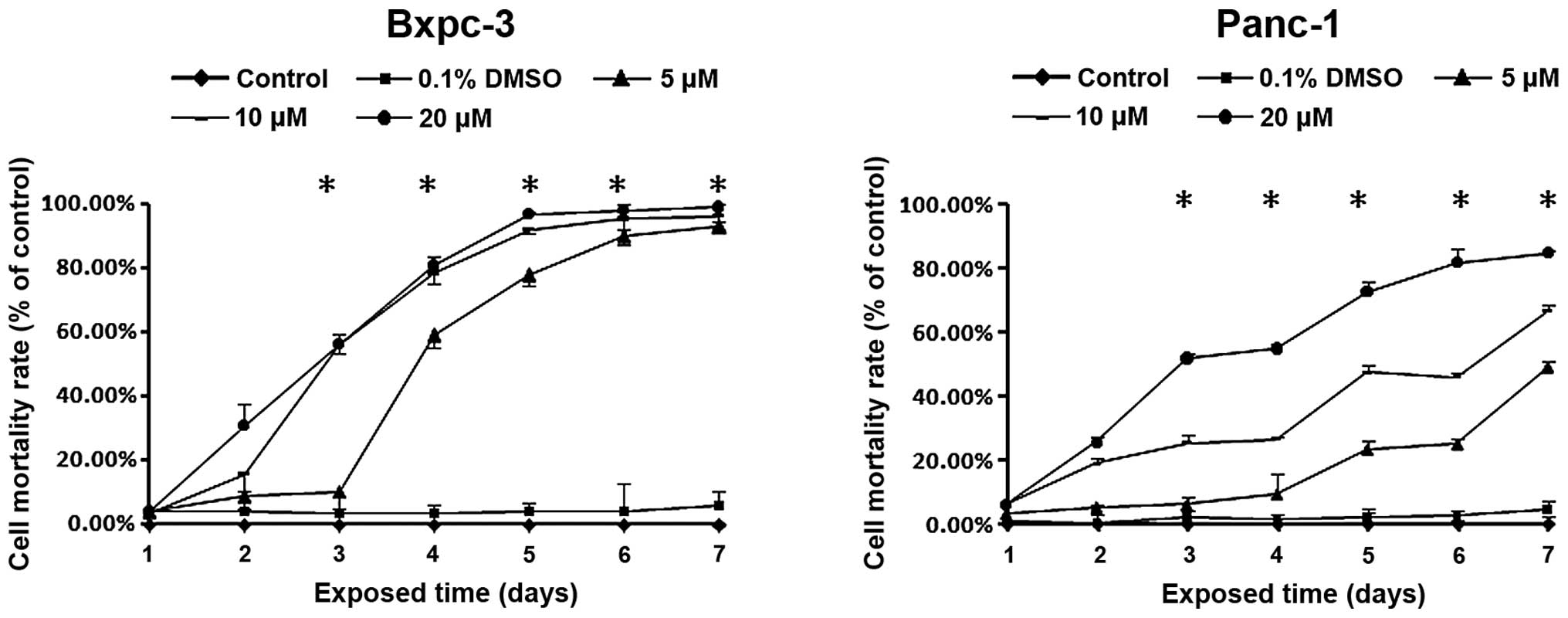
To assess the effects of deguelin on PC cell growth,
Bxpc-3 and Panc-1 cells were treated with deguelin at increasing
concentrations (0–20 µM) for a week. Deguelin inhibited the growth
of the two cell lines in a dose- and time-dependent manner under no
interference of the solvent (DMSO; Fig.
1A and B). These results indicate that deguelin was an
effective inhibitor of PC cell proliferation, and Bxpc-3 cells were
more sensitive to deguelin compared with Panc-1 cells.
Deguelin induces apoptosis of PC
cells
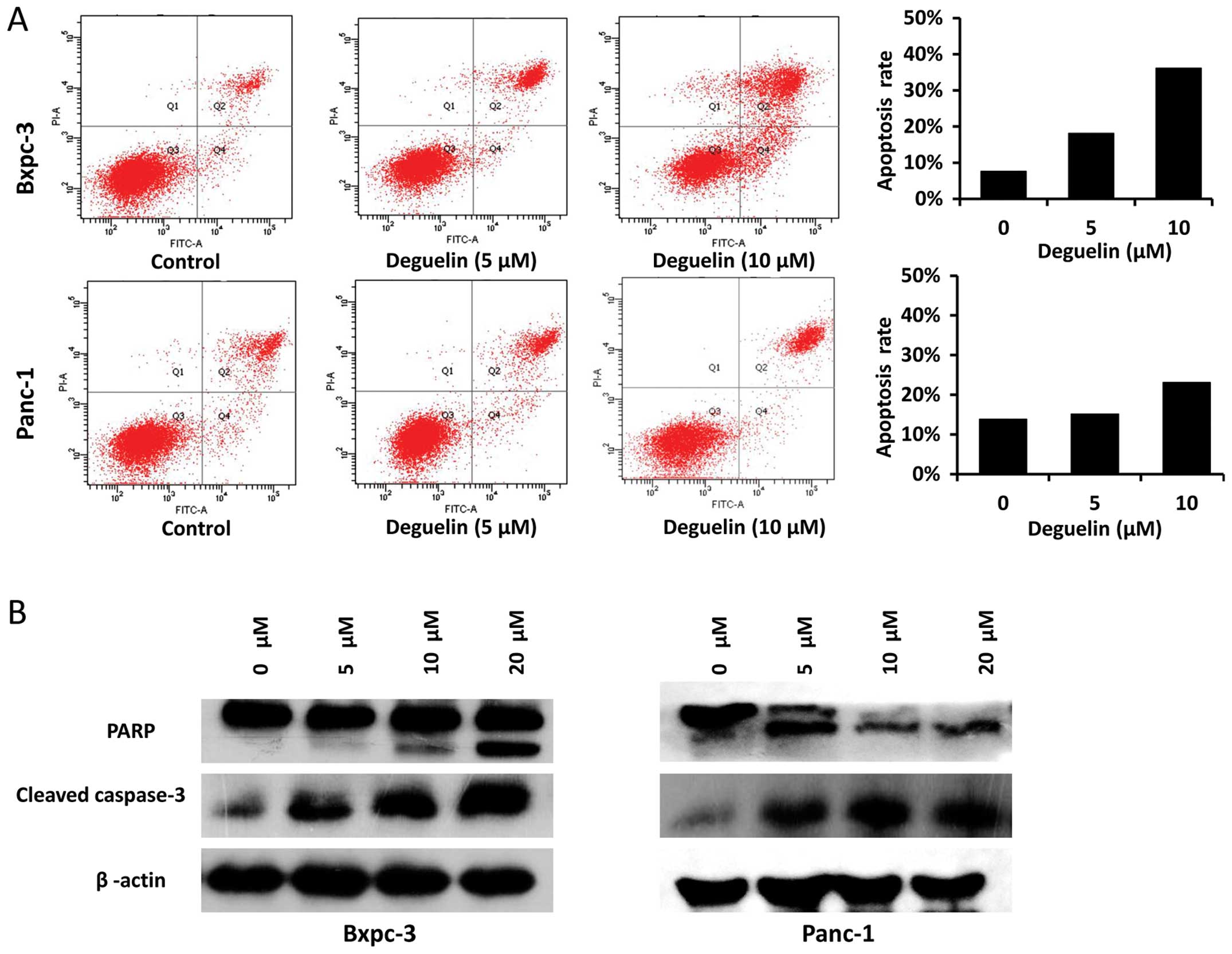
We subsequently investigated whether deguelin
induced apoptosis in PC cells. Flow cytometry revealed that
treatment with deguelin for 48 h induced apoptosis in the two cell
lines. Exposure to deguelin (5 µM) induced apoptosis by up to 18.1%
in Bxpc-3 and 15.1% in Panc-1 cells. The higher concentration (10
µM) induced more apoptosis (36.1% in Bxpc-3, 23.1% in Panc-1 cells)
(Fig. 2A). Western blot analysis
demonstrated that the expression of two apoptosis-related proteins,
cleaved caspase-3 and PARP, significantly increased with the
concentration of deguelin (Fig. 2B).
These data suggested that deguelin induces apoptosis through the
activation of caspase-3 and the cleavage of PARP.
Deguelin inhibits migration and
invasion of PC cells
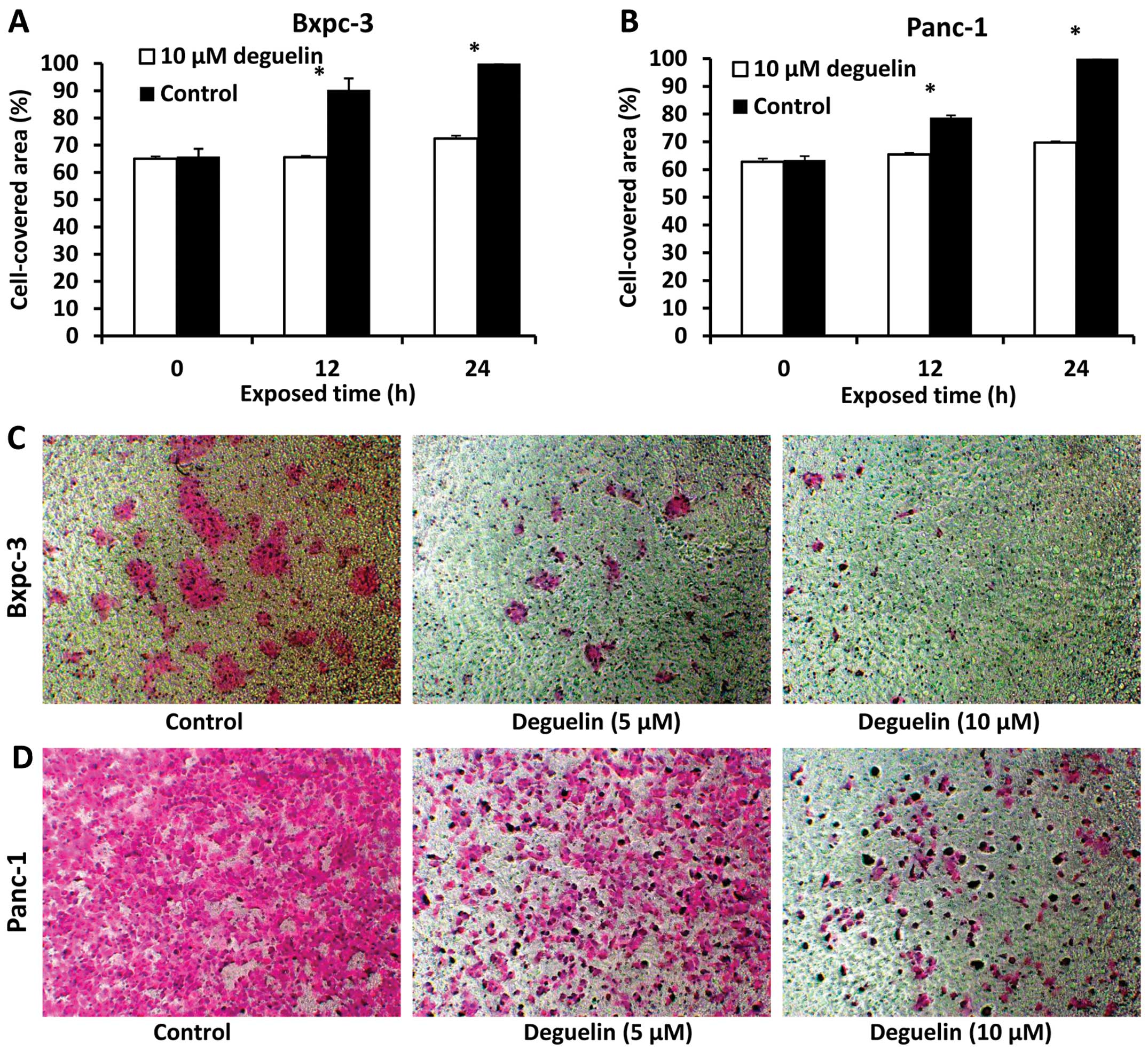
To verify whether deguelin inhibits the migration
and invasion ability of PC cells, wound healing assay and Transwell
cell invasion assay were performed. The data from wimasis.com demonstrated that the cell-covered area of
deguelin-treated cells was smaller than that of untreated cells
after 12 and 24 h (Fig. 3A and
B).
The results of the Transwell assay revealed that
cell invasion in the deguelin-treated group was notably decreased
compared with that in the control group (Fig. 3C and D). The results suggested that
deguelin inhibited the invasion and migration capability of the two
PC cell lines.
Deguelin downregulates MMP-2 and MMP-9
in Bxpc-3 and Panc-1 cells
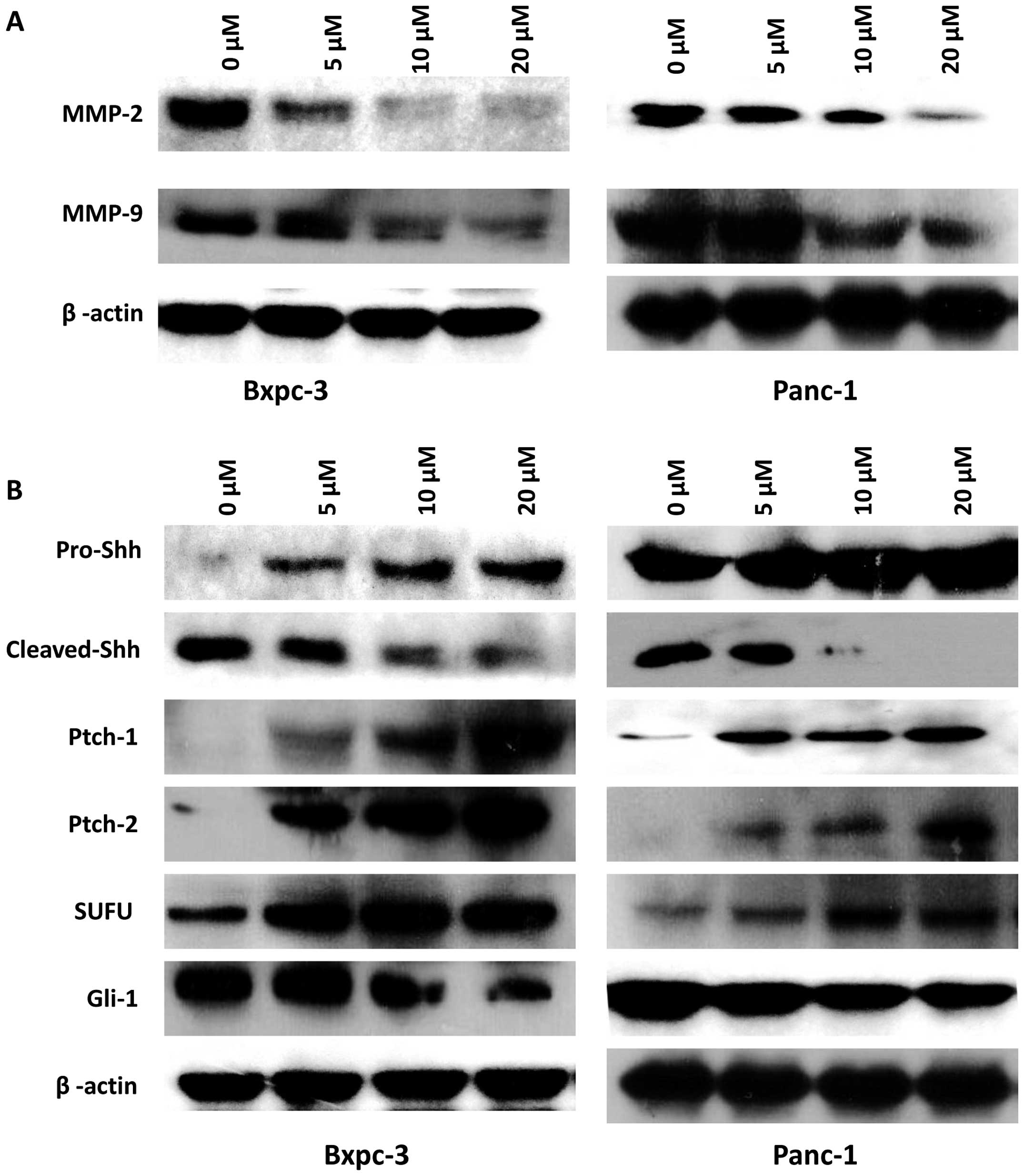
In order to verify our theory on the mechanism of
deguelin influencing PC, the expression levels of MMP-2 and MMP-9
were measured. Bxpc-3 and Panc-1 cells were treated with various
concentrations of deguelin for 48 h, and then total protein was
extracted for western blot analysis. We noted that the levels of
MMP-2 and MMP-9 protein were gradually decreased with the increase
in drug concentration (Fig. 4A). The
results revealed that deguelin inhibits metastasis in PC by
suppressing the activation of MMP-2 and MMP-9.
Deguelin inhibits Hh signaling pathway
in Bxpc-3 and Panc-1 cells
To further investigate whether deguelin regulates PC
through Hh signaling, we sought to examine the protein expression
levels of several key members of this pathway. The expression of
Gli1 was downregulated by the treatment of deguelin in Bxpc-3 and
Panc-1 cells. Conversely, the expression of SUFU was upregulated.
PTCH1 and PTCH2 were able to suppress Hh earlier than SUFU, and
their levels were increased in line with our expectations.
Furthermore, as the ligand located at the starting point of the Hh
signaling pathway, Shh is important for the regulation of Hh. The
spliceosome of Shh was increased following treatment with deguelin
(Fig. 4B). In sumary, the results
regarding the change of ligands in Hh indicated that deguelin
inhibited the Hh signaling pathway.
Discussion
PC patients are noted to have a rather poor survival
rate due to the high aggressiveness and early metastasis of the
disease. In recent years, various methods have been used to
restrain PC or increase the possibility of surgery. In our study,
deguelin demonstrated the potential to inhibit proliferation,
induce apoptosis and suppress migration in PC cells.
Deguelin is known to exhibit significant antitumor
properties in various types of cancer. However, its mechanisms have
not been fully elucidated.
The components of the Shh pathway are highly
expressed in PC cell lines (28,29). Our
results revealed that deguelin inhibits PC cells proliferation by
suppressing the Hh pathway.
MMPs, in particular MMP-2 and MMP-9, play a crucial
role in tumor metastasis and invasion (23,30,31). The
expression of MMP-2 and MMP-9 may be regulated by Hh pathways
(32–34). Our results demonstrated that deguelin
was capable of modulating migration and invasion in PC cells by
regulating the expression of MMP-2 and MMP-9. The interference of
the Hh pathway may induce the changes in MMP-2 and MMP-9.
In conclusion, the results from the present study
confirm that deguelin has the potential to inhibit the growth,
migration and invasion in PC by suppressing the Hh signaling
pathway. However, further research is necessary in order to obtain
further information before these results may be used in the
clinic.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81301889).
Glossary
Abbreviations
Abbreviations:
|
PC
|
pancreatic cancer
|
|
Hh
|
hedgehog
|
References
|
1
|
Maitra A and Hruban RH: Pancreatic cancer.
Annu Rev Pathol. 3:157–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pekala KR, Ma X, Kropp PA, Petersen CP,
Hudgens CW, Chung CH, Shi C, Merchant NB, Maitra A, Means AL and
Gannon MA: Loss of HNF6 expression correlates with human pancreatic
cancer progression. Lab Invest. 94:517–527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Murray T, Samuels A, Ghafoor A,
Ward E and Thun MJ: Cancer statistics, 2003. CA Cancer J Clin.
53:5–26. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Ma W and Zheng W: Deguelin, a
novel anti-tumorigenic agent targeting apoptosis, cell cycle arrest
and anti-angiogenesis for cancer chemoprevention. Mol Clin Oncol.
1:215–219. 2013.PubMed/NCBI
|
|
5
|
Mehta R, Katta H, Alimirah F, Patel R,
Murillo G, Peng X, Muzzio M and Mehta RG: Deguelin action involves
c-Met and EGFR signaling pathways in triple negative breast cancer
cells. PLoS One. 8:e651132013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee H, Lee JH, Jung KH and Hong SS:
Deguelin promotes apoptosis and inhibits angiogenesis of gastric
cancer. Oncol Rep. 24:957–963. 2010.PubMed/NCBI
|
|
7
|
Hu J, Ye H, Fu A, Chen X, Wang Y, Chen X,
Ye X, Xiao W, Duan X, Wei Y and Chen L: Deguelin - an inhibitor to
tumor lymphangiogenesis and lymphatic metastasis by downregulation
of vascular endothelial cell growth factor-D in lung tumor model.
Int J Cancer. 127:2455–2466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mehta RR, Katta H, Kalra A, Patel R, Gupta
A, Alimirah F, Murillo G, Peng X, Unni A, Muzzio M and Mehta RG:
Efficacy and mechanism of action of Deguelin in suppressing
metastasis of 4T1 cells. Clin Exp Metastasis. 30:855–866. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adolphe C, Narang M, Ellis T, Wicking C,
Kaur P and Wainwright B: An in vivo comparative study of sonic,
desert and Indian hedgehog reveals that hedgehog pathway activity
regulates epidermal stem cell homeostasis. Development.
131:5009–5019. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pathi S, PaganWestphal S, Baker DP, Garber
EA, Rayhorn P, Bumcrot D, Tabin CJ, Blake Pepinsky R and Williams
KP: Comparative biological responses to human Sonic, Indian and
Desert hedgehog. Mech Dev. 106:107–117. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang XM, RamalhoSantos M and McMahon AP:
Smoothened mutants reveal redundant roles for Shh and Ihh signaling
including regulation of L/R asymmetry by the mouse node. Cell.
105:781–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y and Struhl G: Dual roles for
patched in sequestering and transducing Hedgehog. Cell. 87:553–563.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ingham PW and McMahon AP: Hedgehog
signaling in animal development: paradigms and principles. Genes
Dev. 15:3059–3087. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McMahon AP, Ingham PW and Tabin CJ:
Developmental roles and clinical significance of hedgehog
signaling. Curr Top Dev Biol. 53:1–114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Motoyama J, Liu J, Mo R, Ding Q, Post M
and Hui CC: Essential function of Gli2 and Gli3 in the formation of
lung, trachea and oesophagus. Nat Genet. 20:54–57. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Motoyama J, Takabatake T, Takeshima K and
Hui C: Ptch2, a second mouse Patched gene is co-expressed with
Sonic hedgehog. Nat Genet. 18:104–106. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smyth I, Narang MA, Evans T, Heimann C,
Nakamura Y, ChenevixTrench G, Pietsch T, Wicking C and Wainwright
BJ: Isolation and characterization of human patched 2 (PTCH2), a
putative tumour suppressor gene in basal cell carcinoma and
medulloblastoma on chromosome 1p32. Hum Mol Genet. 8:291–297. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stone DM, Hynes M, Armanini M, Swanson TA,
Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, et
al: The tumour-suppressor gene patched encodes a candidate receptor
for Sonic hedgehog. Nature. 384:129–134. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barnfield PC, Zhang X, Thanabalasingham V,
Yoshida M and Hui CC: Negative regulation of Gli1 and Gli2
activator function by Suppressor of fused through multiple
mechanisms. Differentiation. 73:397–405. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Méthot N and Basler K: Suppressor of fused
opposes hedgehog signal transduction by impeding nuclear
accumulation of the activator form of Cubitus interruptus.
Development. 127:4001–4010. 2000.PubMed/NCBI
|
|
21
|
Matsushita S, Onishi H, Nakano K,
Nagamatsu I, Imaizumi A, Hattori M, Oda Y, Tanaka M and Katano M:
Hedgehog signaling pathway is a potential therapeutic target for
gallbladder cancer. Cancer Sci. 105:272–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Freije JM, Balbin M, Pendás AM, Sánchez
LM, Puente XS and López-Otín C: Matrix metalloproteinases and tumor
progression. Adv Exp Med Biol. 532:91–107. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vihinen P, AlaAho R and Kähäri VM: Matrix
metalloproteinases as therapeutic targets in cancer. Curr Cancer
Drug Targets. 5:203–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karhadkar SS, Bova GS, Abdallah N, Dhara
S, Gardner D, Maitra A, Isaacs JT, Berman DM and Beachy PA:
Hedgehog signalling in prostate regeneration, neoplasia and
metastasis. Nature. 431:707–712. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liao X, Siu MK, Au CW, Wong ES, Chan HY,
Ip PP, Ngan HY and Cheung AN: Aberrant activation of hedgehog
signaling pathway in ovarian cancers: effect on prognosis, cell
invasion and differentiation. Carcinogenesis. 30:131–140. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mori Y, Okumura T, Tsunoda S, Sakai Y and
Shimada Y: Gli-1 expression is associated with lymph node
metastasis and tumor progression in esophageal squamous cell
carcinoma. Oncology. 70:378–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoo YA, Kang MH, Kim JS and Oh SC: Sonic
hedgehog signaling promotes motility and invasiveness of gastric
cancer cells through TGF-beta-mediated activation of the ALK5-Smad
3 pathway. Carcinogenesis. 29:480–490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li SH, Fu J, Watkins DN, Srivastava RK and
Shankar S: Sulforaphane regulates self-renewal of pancreatic cancer
stem cells through the modulation of Sonic hedgehog-GLI pathway.
Mol Cell Biochem. 373:217–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rodova M, Fu J, Watkins DN, Srivastava RK
and Shankar S: Sonic hedgehog signaling inhibition provides
opportunities for targeted therapy by sulforaphane in regulating
pancreatic cancer stem cell self-renewal. PLoS One. 7:e460832012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
HadlerOlsen E, Winberg JO and Uhlin-Hansen
L: Matrix metalloproteinases in cancer: their value as diagnostic
and prognostic markers and therapeutic targets. Tumour Biol.
34:2041–2051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
StetlerStevenson WG: Type IV collagenases
in tumor invasion and metastasis. Cancer Metastasis Rev. 9:289–303.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen JS, Li HS, Huang JQ, Zhang LJ, Chen
XL, Wang Q, Lei J, Feng JT, Liu Q and Huang XH: Down-regulation of
Gli-1 inhibits hepatocellular carcinoma cell migration and
invasion. Mol Cell Biochem. 393:283–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen M, Qian Y, Dai J and Chu R: The sonic
hedgehog signaling pathway induces myopic development by activating
matrix metalloproteinase (MMP)-2 in Guinea pigs. PLoS One.
9:e969522014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fan HX, Wang S, Zhao H, Liu N, Chen D, Sun
M and Zheng JH: Sonic hedgehog signaling may promote invasion and
metastasis of oral squamous cell carcinoma by activating MMP-9 and
E-cadherin expression. Med Oncol. 31:412014. View Article : Google Scholar : PubMed/NCBI
|