Introduction
Gastric cancer (GC) is the fourth most common type
of cancer and ranks as the second most common cause of
cancer-associated mortalities, while the incidence rate is
gradually rising every year worldwide (1). Although certain advances, such as
combination chemotherapy, have been achieved in chemotherapy in
recent years, the therapeutic effect remains unsatisfactory
(2). Numerous molecular factors
associated with pathogenesis of GC have been reported, but the key
factors remain unclear. Therefore, finding out the controlling
factors of GC and their effects on its pathogenesis may increase
the current understanding of the mechanisms underlying gastric
carcinogenesis and facilitate the development gene-based
therapies.
Zinc finger proteins are highly abundant in human
eukaryotic cells and tissues and are important for the maintenance
of normal cellular functions (3). As
previously reported, numerous members of the zinc finger protein
family are associated with the initiation, development, invasion
and metastasis of tumors, including ZNF165 in urinary bladder
cancer and ZNF217 in colorectal adenoma and ovarian cancer
(4,5).
ZNF139, a member of the kruppel family of zinc-finger-containing
transcription factors, was found to be closely associated with GC
in a previous study (6). However, the
mechanism of ZNF139 in the growth and metastasis of GC cells was
not clarified. Therefore, the aim of the present study is to detect
the specific impact and the mechanism of ZNF139 on GC tumor cells
by using interference technology, orthotopic transplantation and
immunohistochemistry.
Materials and methods
Cell culture
The human GC SGC7901 cell line was purchased from
The Cell Bank of Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China) and were cultivated in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.), penicillin (100 unit/ml) and streptomycin (100 µg/ml). The
tumor cells were cultured in a humidified atmosphere of 5%
CO2 at 37°C.
Plasmid construction and
transfection
The vector RNAi-Ready pSIREN-RetroQ-ZsGreen plasmid
was obtained from Clontech Laboratories, Inc. (Mountainview, CA,
USA). The expression vectors containing either the
anti-Znf139 small interfering RNA (siRNA) sequence
ACCTCGGAAGATTCAGCAT for the negative control (scramble) sequence
GACGAGTTGACTGCGATTG were constructed, as previously described
(7), and termed Znf139-siRNA
or siRNA-scramble expression vector, respectively.
Znf139-siRNA expression vector (Znf139-siRNA group),
siRNA-scramble expression vector (siRNA-scramble group) or blank
expression vector (mock group) were transfected into SGC7901 cells
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. A fluorescence
microscope was used to observe the transfection efficiency at 24 h
after transfection and 80% transfection efficiency was considered
to be successful transfection. Transfection efficiency was
calculated as follows: Transfection efficiency (%) =(number of
fluorescent cells/total number of cells) × 100.
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) assay
SGC7901 cells (1×104 per well) were
planted into 96-well microtiter plates and incubated overnight
prior to being transfected with siRNA. At 24 h subsequent to
transfection, 20 µl MTT (Sigma-Aldrich, St. Louis, MO, USA) assay
solution (5 mg/ml) was added into the wells for 4 h at 37°C. After
discarding the medium, the formazan crystals were dissolved in
dimethyl sulfoxide (DMSO) and the absorbance was measured at 490 nm
on a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
5-ethynyl-2′-deoxyuridine (EdU)
assay
Briefly, cells (1×104 cells per well)
were seeded into 96-well plates with 100 µl EdU (50 µM;
Sigma-Aldrich) added, and cultured in an incubator for 2 h at room
temperature. The cells were fixed with 4% paraformaldehyde for 30
min, treated with glycine (2 mg/ml) for 5 min and washed with
phosphate-buffered saline (PBS) three times. Subsequently, 100 µl
1X Apollo® reaction cocktail (Sigma-Aldrich) was added
to the cells to react for 30 min in the dark, and 1X Hoechst 33342
(Sigma-Aldrich) was used to stain cell nuclei for 30 min at room
temperature. The nuclear morphology was then observed with an
immunofluorescence microscope and the percentage of EdU-labeled
(red) cells was noted.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from SGC7901 cells using
Trizol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. RNA (2 µg) was then reverse
transcribed into complementary DNA (cDNA) using the SuperScript
Reverse transcriptase kit (Invitrogen; Thermo Fisher Scientific,
Inc.). RT-qPCR was performed with the SYBR Premix Ex Taq II (Takara
Bio, Inc., Otsu, Japan) on an ABI-7500 Sequence Detection System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Housekeeping
gene β-actin was chosen as an internal control to detect the
relative expression of the target genes. The thermal cycling
conditions were 95°C for 30 sec and 58°C for 1 min. The sequences
of the PCR primers were as follows: Znf139 forward,
CTTCCTGAGTTCTTGGTTTCG and reverse, CCTTTGACCCACTGGTTTATG;
Bcl-2 forward, GGTGGGGTCATGTGTGTGG and reverse,
CGGTTCAGGTACTCAGTCATCC; survivin forward,
GGACCGCCTAAGAGGGCGTGC and reverse, AATGTAGAGATGCGGTGGTCCTT;
β-actin forward, CATGTACGTTGCTATCCAGGC and reverse,
CTCCTTAATGTCACGCACGAT. The comparative quantification (Cq) cycle
method (2−ΔΔCq) was used to calculate relative
expression levels of Znf139/β-actin,
Bcl-2/β-actin and survivin/β-actin
(8).
Western blot analysis
The cells were lysed using a ReadyPrep Protein
Extraction kit (Bio-Rad Laboratories, Inc.) for 5 min. After
removing cell debris by centrifugation at 12,000 × g for 15 min,
the supernatant containing the proteins was collected and boiled in
sample buffer for 5 min. The concentrated proteins were separated
by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis so
that the specific proteins could be transferred onto polyvinylidene
fluoride membranes (Roche Diagnostics, Basel, Switzerland). The
membranes were blocked using PBS containing 0.05% Tween 20 and 5%
skim milk for 1 h at room temperature. After being washed 3 times
in Tris-buffered saline containing Tween 20, the membranes were
incubated with rabbit anti-mouse ZNF139 polyclonal (1:800 dilution;
cat no. ab126124; Abcam, Cambridge, UK), Bcl-2 monoclonal (1:800
dilution; cat no. ab32124; Abcam), survivin polyclonal (1:800
dilution; cat no. ab469; Abcam) or β-actin polyclonal (1:5,000
dilution; cat no. ab8227; Abcam) primary antibodies overnight at
4°C, and then exposed to goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (1:5,000 dilution; cat no.
074-1506; KPL, Inc., Gaithersburg, MD, USA). Immunoreactive bands
were detected by the enhanced chemiluminescence system (GE
Healthcare Life Sciences, Little Chalfont, UK).
Mouse model establishment
Establishment of the GC tissue orthotopic
transplantation mouse model was separated into 2 steps. Firstly,
subcutaneous tumor-transplanted mouse model was established using
6–8 weeks old male BALB/C (nu/nu) nude mice. All mice were
acclimated to a natural day-night cycle at 21°C, and given free
access to food and water for 1 week prior to the trial. These nude
mice were divided into 2 groups: Group A was injected with
Znf139-knockdown SGC7901 cells and group B was injected with
SGC7901 cells transfected with siRNA-scramble. After the mice were
subcutaneously injected with 7×104 cells per mouse on
the backside, the mice were kept in specific pathogen free
conditions for 3 weeks until the implanted tumor grew to 1 cm
diameter. The mice were anesthetized with 6% pentobarbital sodium
and sacrificed by cervical dislocation. Then the tumor tissues were
removed, sliced into 1×1×1 mm pieces and implanted to the backside
of other nude mice. The source of the orthotopic transplantation
was coming from the sixth generation subcutaneously-transplanted
tumor slices. For the second step, the establishment of the
orthotopic transplanted mouse model, nude mice were anesthetized
with 6% pentobarbital sodium. A 5–10 mm abdominal incision was made
along the left paramedian line in order to expose the gastric wall.
The serosa of the greater curvature of stomach was cut so that the
tumor slice could be implanted. OB glue (1–2 drops; Guangzhou
Baiyun Medical Adhesive Co., Ltd., Baiyun, China) was used to seal
the rupture and the enterocoelia was closed using a suture. Mice
were weighed daily. After 90 days, all mice were sacrificed by
cervical dislocation due to the development of systemic failure,
and the tumors excised from stomach were measured using a vernier
caliper and analytical balance. The procedures for the care and use
of animals were approved by the Ethics Committee of the Fourth
Hospital of Hebei Medical University (Shijiazhuang, Hebei) and all
applicable institutional and governmental regulations concerning
the ethical use of animals were followed.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 17.0; SPSS Inc., Chicago, IL, USA). Data were
analyzed by one-way analysis of variance and are expressed as the
mean ± standard deviation. All experiments were repeated three
times. Multiple comparison between the groups was performed using
the Student-Newman-Keuls method. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of siRNA on Znf139 expression
in SGC7901 cells
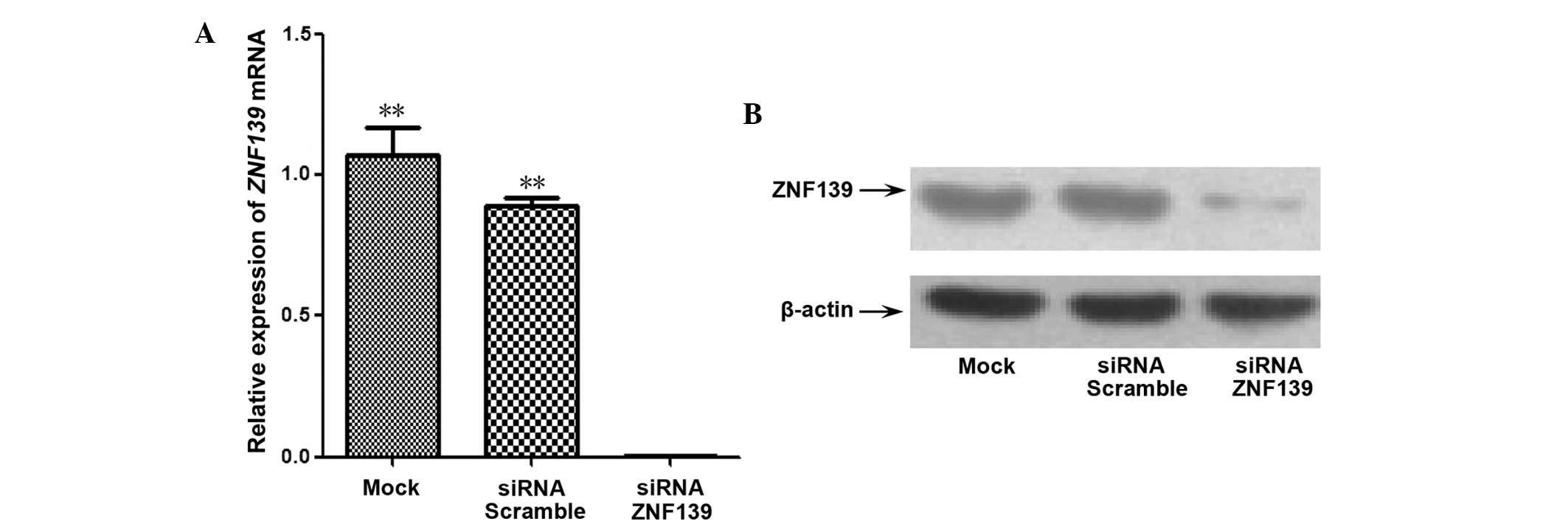
To demonstrate the role of ZNF139 on tumor growth
and apoptosis, a ZNF139 RNA interference vector was constructed
with green fluorescent protein transfected into the GC SGC7901 cell
line. In the cells transfected with Znf139-siRNA, the
expression of ZNF139 was evidently decreased in the messenger RNA
(mRNA) (Fig. 1A) and protein
(Fig. 1B) compared with the
siRNA-scramble and mock control groups (all P=0.001). There was no
significant difference between the siRNA-scramble and mock control
groups (Fig. 1). These results
indicated that the constructed Znf139-siRNA expression
vector was able to inhibit the transcription and translation of
endogenous Znf139 efficiently.
Effect of Znf139-siRNA on cell
growth
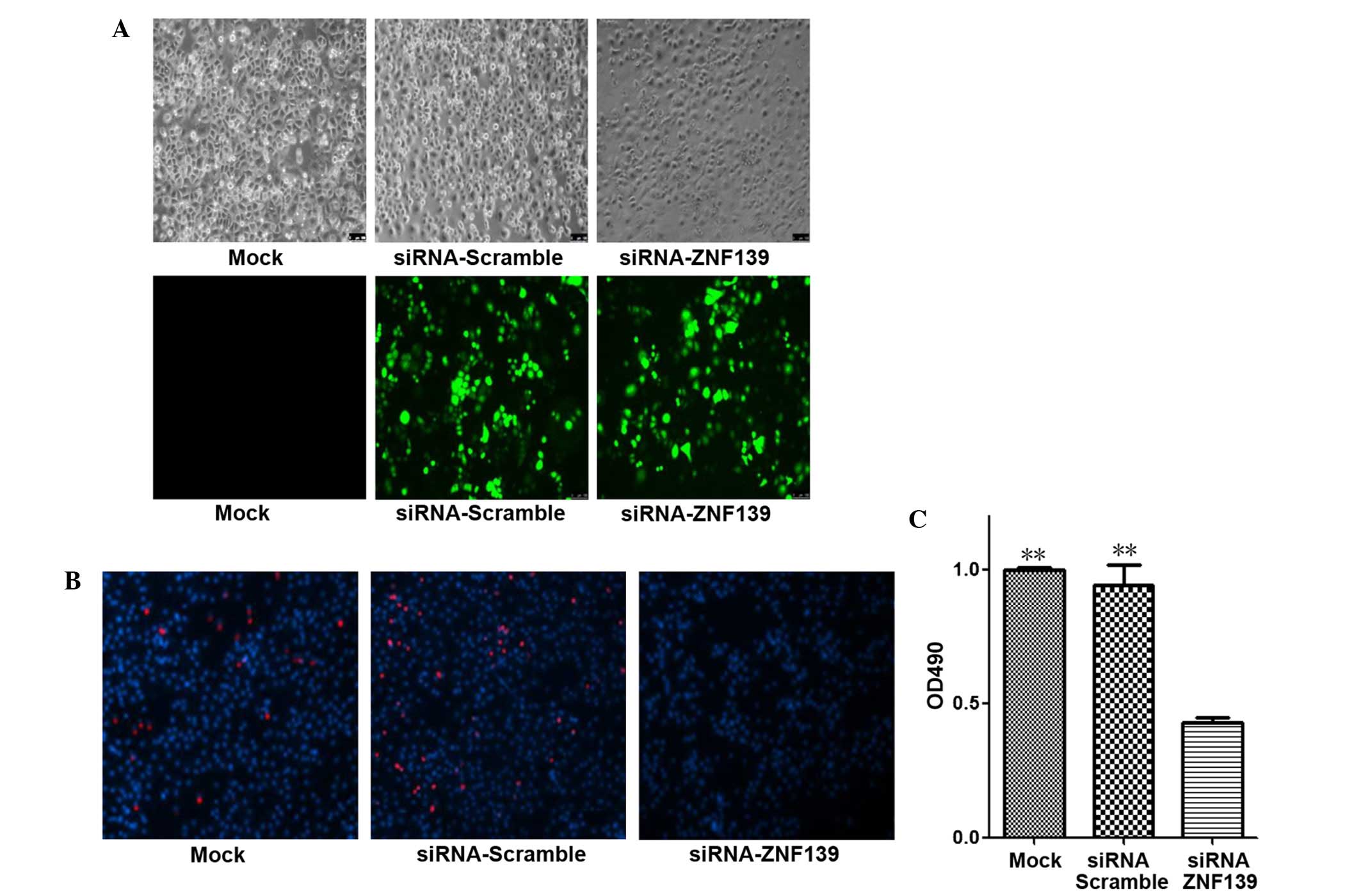
Further experiments were performed to detect the
effect of ZNF139 on cell growth. SGC7901 cells were transfected
with Znf139-siRNA and siRNA-scramble. As shown in Fig. 2, the growth of Znf139-knockdown
cells was inhibited significantly after 48 h transfection (Fig. 2A). Subsequently, EdU and MTT assays
were used to confirm whether cell viability and proliferation were
inhibited following the knockdown of Znf139. According to
the EdU analysis, the proliferation of the Znf139-knockdown
cells was evidently inhibited compared with the siRNA-scramble and
mock control groups (Fig. 2B). As
shown in the MTT assay, the viability of Znf139-silenced
cells was evidently inferior compared with the siRNA-scramble and
mock control groups (P=0.001 and P=0.003, respectively; Fig. 2C). These results suggest the
Znf139 gene is associated with the growth and proliferation
of gastric tumor cells.
Effect of Znf139-siRNA on cell
apoptosis
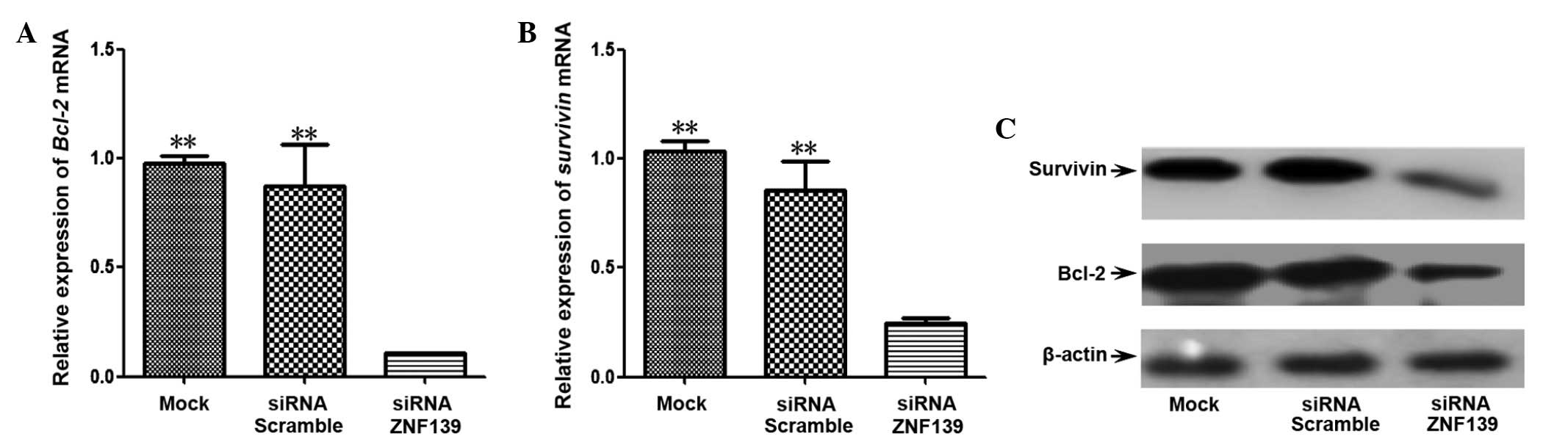
To further investigate the association between
ZNF139 and cell apoptosis, the Bcl-2 and survivin
genes were detected with qPCR and western blot analysis. The
results showed that the expression levels of Bcl-2 and survivin
mRNAs (P=0.001 and P=0.002, respectively) and proteins were
downregulated in Znf139-silenced cells compared with the
siRNA-scramble and mock groups (Fig.
3). These data indicated that Znf139 was able to induce
the growth of tumor cells by mediating the apoptosis pathway.
Znf139 knockdown downregulated the
expression of Bcl-2 and survivin in mice
Through weighing the mice daily, after the
establishment of the human GC nude mouse orthotopic transplantation
model, the mice appeared to experience systemic failure, showing
symptoms of emaciation, roachback, listlessness and hypoactivity by
90 days. The mice were then sacrificed by cervical dislocation and
the tumors excised from stomach were measured using a vernier
caliper and analytical balance. The tumors in mice orthotopically
transplanted with Znf139-knockdown SGC7901 cells were
significantly inhibited compared with those orthotopically
transplanted with siRNA-scramble SGC7901 cells, and there was no
metastasis of the tumor in peritoneum and lymph node in mice
orthotopically transplanted with Znf139-knockdown SGC7901
cells. The western blot analysis results revealed that the
expression levels of Bcl-2 and survivin were significantly
decreased in the tumors of orthotopic transplant mice that
interfered with Znf139 siRNA (Fig.
4), which was in agreement with the results of the in
vitro experiment.
Discussion
GC is one of the most common digestive system
malignant tumors worldwide, which is associated with a high
fatality rate, particularly in Asia. Therefore, researching the
mechanism of GC is of great importance (9). Various molecular factors have been be
shown to be associated with the pathogenesis of GC, but the major
genes affecting the growth and prognosis of GC tumors remains
unclear. The GC SGC7901 cell lines were moderately differentiated
adenocarcinoma cells, which are commonly found in clinical patients
(10). ZNF139, a member of the zinc
finger protein family, is a regulating factor at transcription
level (6). Dekken et al first
discovered the increased expression of ZNF139 in an adenocarcinoma
of the esophago-gastric junction (11). A previous study found that ZNF139
could affect gastric tumors by promoting the expression of fascin
and heterogeneous nuclear ribonucleoproteins A2/B1 (12). In addition, the expression of ZNF139
was found to be closely associated with GC, indicating that ZNF139
may be involved in gastric carcinogenesis and development (12). Another previous study showed that
ZNF139 was involved in GC multidrug resistance by simultaneously
promoting the expression of multidrug resistance protein 1 (MDR1),
multidrug resistance-associated protein 1 (MRP1) and Bcl-2, and
inhibiting Bcl-2-associated X protein (13). In order to explore the association
between ZNF139 and the growth of tumor cells, the expression of
ZNF139 was silenced in SGC7901 cells by first applying RNA
interference technology. As shown in the results, the viability and
proliferation were extremely suppressed in the SGC7901 cells with
Znf139 knockdown, indicating that the inhibition of ZNF139
was conducive to the growth of GC cells in vitro.
To investigate the influence of ZNF139 in
vivo, a human GC nude mouse orthotopic transplantation model
was created, which could simulate GC biological behavior, including
proliferation, invasion and metastasis, in the human body. The
orthotopic transplantation model of GC is stable, reliable and
considered to be the most optimal model of GC so far. In the
orthotopic transplantation mice with Znf139 knockdown, the
growth of the tumor was evidently suppressed and there were signs
of no lymphatic metastasis, suggesting that ZNF139 could affect the
development and metastasis of GC tumors in vivo. In order to
explore which genes are regulated by Znf139, changes of
apoptosis-associated genes were detected in the cells and mice of
each group. The Bcl-2 and survivin genes, the representative genes
of the apoptotic pathway, were significantly downregulated
following Znf139 knockdown, including their protein
products. Previous studies found that Bcl-2 enhances the
anti-apoptotic ability of the tumor cells by regulating the
mitochondrial redox state of thiol (14). Survivin inhibits caspase activation,
and therefore leads to the negative regulation of programmed cell
death (15). In summary, these
results show that the expression of anti-apoptotic associated
genes, Bcl-2 and survivin, was extremely reduced
following the silencing of Znf139. This indicates that
ZNF139 could facilitate the growth and metastasis of GC by
promoting Bcl-2 and survivin.
In the present study, ZNF139 was demonstrated to
affect the growth and metastasis of GC tumors by inhibiting cell
apoptosis. The establishment of a human GC nude mouse orthotopic
transplantation model partly enabled the simulation of GC
biological behavior the in human body. However, the anatomical
structure and microcirculatory system are varied in the human body
and nude mice, in addition to differences in the immunodeficiency
of nude mice. Recent studies have shown that ZNF139 may be
associated with multiple drug resistance by regulating the MDR1 and
MRP1 genes (13). Therefore, future
studies are recommended to focus on building animal models that are
closer to the internal environment of the human body, as well as
exploring the association between ZNF139, MDR1 and gene therapy in
GC.
Acknowledgements
The present study was supported by The National
Natural Science Foundation of China, Beijing, China (grant no.
81072033).
References
|
1
|
Ma Z, Wang W, Jin G, Chu P and Li H:
Effect of statins on gastric cancer incidence: A meta-Analysis of
case control studies. J Cancer Res Ther. 10:859–865. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Imamura T, Komatsu S, Ichikawa D, Kubota
T, Okamoto K, Konishi H, Shiozaki A, Fujiwara H, Morimura R,
Murayama Y, et al: Poor prognostic subgroup in T3N0 stage IIA
gastric cancer, suggesting an indication for adjuvant chemotherapy.
J Surg Oncol. 111:221–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei S, Zhang L, Zhou X, Du M, Jiang Z,
Hausman GJ, Bergen WG, Zan L and Dodson MV: Emerging roles of zinc
finger proteins in regulating adipogenesis. Cell Mol Life Sci.
70:4569–4584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh PK, Srivastava AK, Dalela D, Rath
SK, Goel MM and Bhatt ML: Frequent expression of zinc-finger
protein ZNF165 in human urinary bladder transitional cell
carcinoma. Immunobiology. 220:68–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li P, Maines-Bandiera S, Kuo WL, Guan Y,
Sun Y, Hills M, Huang G, Collins CC, Leung PC, Gray JW and
Auersperg N: Multiple roles of the candidate oncogene ZNF217 in
ovarian epithelial neoplastic progression. Int J Cancer.
120:1863–1873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Tan BB, Zhao Q, Fan LQ, Wang D and
Liu Y: ZNF139 promotes tumor metastasis by increasing migration and
invasion in human gastric cancer cells. Neoplasma. 61:291–298.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang S, Yang JH, Guo CK and Cai PC: Gene
silencing of TKTL1 by RNAi inhibits cell proliferation in human
hepatoma cells. Cancer Lett. 253:108–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livak and Schmittgen, . Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fock KM: Review article: The epidemiology
and prevention of gastric cancer. Aliment Pharmacol Ther.
40:250–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang S, Zuo L, Gui S, Zhou Q, Wei W and
Wang Y: Induction of cell differentiation and promotion of endocan
gene expression in stomach cancer by melatonin. Mol Biol Rep.
39:2843–2892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
vanDekken H, Tilanus HW, Hop WC, Dinjens
WN, Wink JC, Vissers KJ and van Marion R: Array comparative genomic
hybridization, expression array, and protein analysis of critical
regions on chromosome arms 1q, 7q, and 8p in adenocarcinomas of the
gastroesophageal junction. Cancer Genet Cytogenet. 189:37–42. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Tan BB, Fan LQ, Zhao Q, Song ZC and
Wang D: Proteomic identification and comparison of
differentiation-related proteins in gastric carcinoma cell lines.
Zhonghua Zhong Liu Za Zhi. 32:179–184. 2010.(In Chinese).
PubMed/NCBI
|
|
13
|
Li Y, Tan BB, Zhao Q, Fan LQ, Liu Y and
Wang D: Regulatory mechanism of ZNF139 in multi-drug resistance of
gastric cancer cells. Mol Biol Rep. 41:3603–3610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roy MJ, Vom A, Czabotar PE and Lessene G:
Cell death and the mitochondria: Therapeutic targeting of the BCL-2
family-driven pathway. Br J Pharmacol. 171:1973–1987. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rubio N, Garcia-Segura LM and Arevalo MA:
Survivin prevents apoptosis by binding to caspase-3 in astrocytes
infected with the BeAn strain of Theiler's murine encephalomyelitis
virus. J Neurovirol. 18:354–363. 2012. View Article : Google Scholar : PubMed/NCBI
|