Introduction
Pancreatic neuroendocrine neoplasms (p-NENs)
originate from pancreatic neuroendocrine cells, and have increased
in incidence in American and Asian patients during the past 20
years (1,2). To improve prognosis, Capella et
al (3) developed a
clinicopathological classification in 1995 according to clinical,
radiographical and histopathological features. Based on this
stratification, the World Health Organization (WHO; Geneva,
Switzerland) published a classification system in 2000 that
distinguishes well-differentiated endocrine tumors from
well-differentiated and poorly differentiated endocrine carcinomas
(4). Subsequently, in 2010, the WHO
updated this classification system to reflect the proliferation
marker protein Ki-67 index and mitotic count (5). In this revised classification system,
p-NENs are classified as neuroendocrine tumor (NET) grade (G)1
(Ki-67 ≤2%), NET G2 (Ki-67 >2–20%), neuroendocrine carcinoma
(NEC) G3 (Ki-67 >20%) and mixed adenoneuroendocrine carcinoma
(5).
In 2006 the European Neuroendocrine Tumor Society
(ENETS; Berlin, Germany), and in 2010 the American Joint Cancer
Committee (Chicago, IL, USA), advocated tumor-node-metastasis (TNM)
staging systems for the prognosis of p-NENs (6,7), which
referenced previous results from retrospective studies highlighting
potential prognostic factors (5,8).
Conversely, according to hormone secretion status and clinical
presentation, p-NENs are divided into functioning and
non-functioning tumors (9).
Non-functioning p-NENs may also secrete elevated amounts of
hormones while remaining asymptomatic (10). Therefore, non-functioning p-NENs
frequently present later in the course of the disease with symptoms
resulting from local expansion or distant metastasis (11).
Surgical resection is the only potentially curative
therapy for p-NENs, and palliative surgery is also an accepted
course of action in cases of liver metastatic disease (12–15). With
the development of surgical technology, improved long-term survival
of patients with liver-metastatic p-NENs following cytoreductive
surgery has also been recently reported (16). However, certain patients exhibit a
short survival period following curative surgery and the
significance of prognostic factors following surgical resection
remains unclear (17). A unified
standard to identify critical prognostic factors in p-NENs remains
to be performed. Therefore, in the present study the clinical
characteristics and prognostic factors of Chinese patients with
p-NENs following surgical treatment were analyzed, in order to
identify potential risk factors and to detail the outcomes of p-NEN
treatment.
Materials and methods
Patient selection
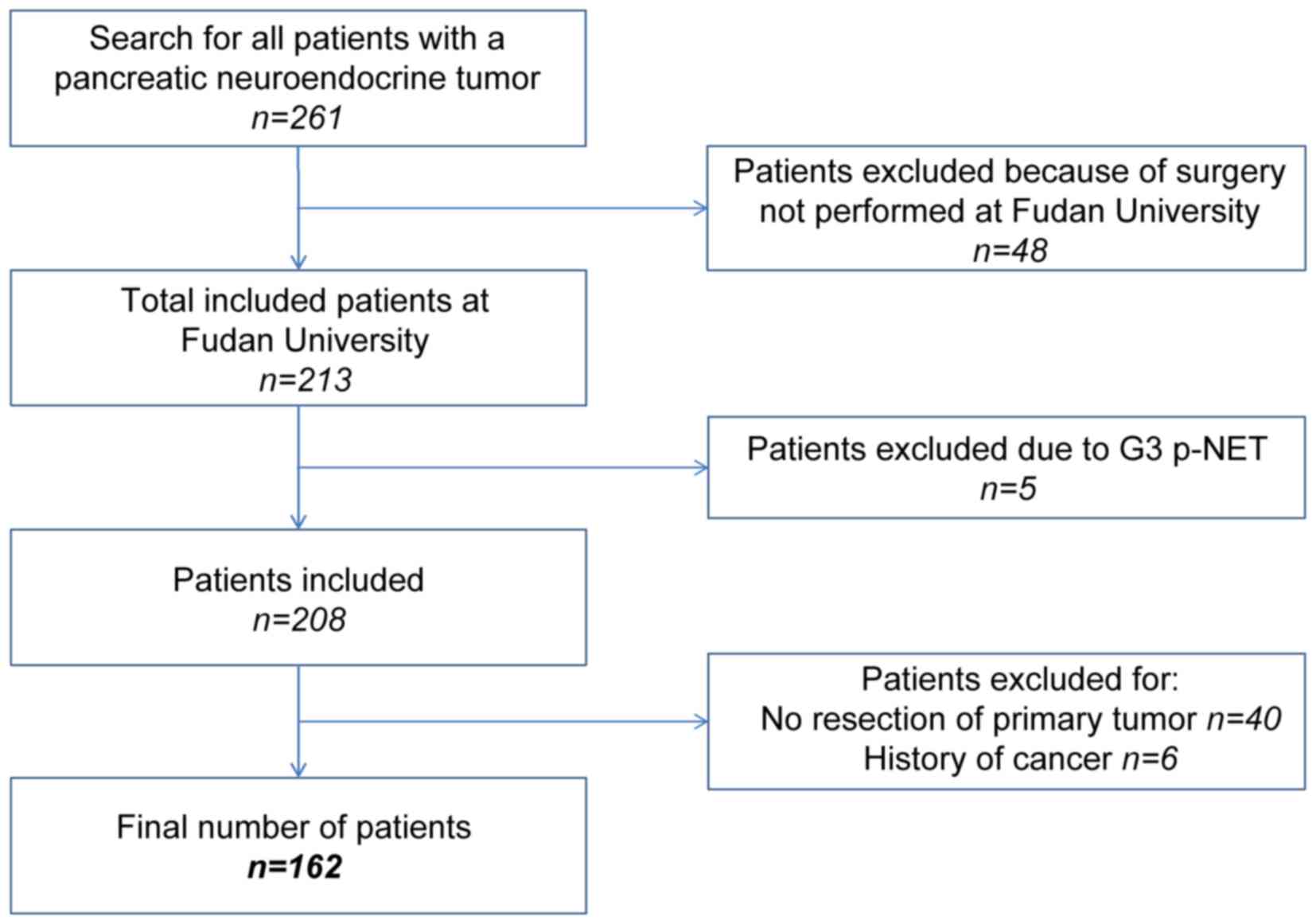
The present study retrospectively analyzed the
medical records of a prospectively maintained database. Between
January 2003 and July 2015, 162 patients were pathologically
diagnosed with p-NEN and surgically treated at the Department of
Pancreatic Oncology of the Fudan University Shanghai Cancer Center
(Shanghai, China). The following eligibility criteria were applied
(Fig. 1): i) Patients exhibited
histologically confirmed p-NENs; ii) patients underwent surgery
exclusively at the Fudan University Shanghai Cancer Center; iii)
patients did not exhibit p-NET G3, which was defined as NET with
high proliferative activity; iv) patients did not present with an
unresectable primary tumor or have a history of other types of
cancer.
Tumor characteristics
Patient demographics (age and gender), hormone
secretion status (functioning or non-functioning) and tumor
characteristics (size, location and presence of lymph node/distant
metastasis) are presented in Table I.
The WHO 2010 grading classifications and ENETS 2006 TNM staging
system were used to assess the clinical outcomes of patients with
p-NEN.
 | Table I.Clinical, surgical and pathological
characteristics of the study population (n=162). |
Table I.
Clinical, surgical and pathological
characteristics of the study population (n=162).
| Characteristic | Total, n (%) |
|---|
| Mean age ± SD,
years | 51.2±12.6 |
| Gender |
|
| Male | 69 (42.6) |
|
Female | 93 (57.4) |
| Hormone secretion
status |
|
|
Functioning | 21 (13.0) |
|
Non-functioning | 141 (87.0) |
| Mean tumor size ± SD,
cm | 4.1±2.8 |
| Location |
|
| Head | 64 (39.5) |
| Body and
tail | 95 (58.6) |
|
Multicentricity | 3 (1.9) |
| Lymph node
metastasis | 40 (24.7) |
| Distant
metastasis | 13 (8.0) |
| Surgical
approach |
|
|
PD/PPPD | 42 (26.0) |
| DP | 88 (54.3) |
| LP | 24 (14.8) |
| TP | 8 (4.9) |
| R0 resection | 147 (90.7) |
| ENETS stage |
|
| I | 40 (24.7) |
| II | 77 (47.5) |
| III | 32 (19.8) |
| IV | 13 (8.0) |
| 2010 WHO grading
classification |
|
| NET
G1 | 79 (48.8) |
| NET
G2 | 67 (41.3) |
| NEC
G3 | 16 (9.9) |
Follow-up and survival
Follow-up was performed via telephone, clinic visit,
or outpatient visit between January 2015 and September 2015.
Medical records of the included patients were reviewed to collect
the following information: Age, gender, hormone secretion status,
tumor size, tumor location, tumor invasion, lymphatic metastasis,
distant metastasis, surgical approach, surgical margin status,
ENETS 2006 TNM staging and WHO 2010 grading. A complete dataset was
obtained following the exclusion of patients who succumbed to other
factors during follow-up. The data were collected in a prospective
manner.
Statistical analysis
Survival estimates were constructed using the
Kaplan-Meier estimator method and survival curves were compared
using the log-rank test. Differences between NET G1/G2 and NEC G3
were compared by the χ2 test. Univariate and
multivariate Cox proportional hazards models were used to
investigate the effects of several prognostic factors.
Statistically significant factors following the univariate analysis
were included in the multivariate analysis. Statistical analyses
were performed using SPSS software (version 22.0; IBM SPSS, Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
The patient clinicopathological data following
diagnosis are summarized in Table I.
The mean age (± standard deviation) of the patients was 51.2±12.6
years and 42.6% of the patients were male. A total of 141 patients
(87.0%) presented with a non-functioning tumor, whereas 21 patients
(13.0%) presented with a functioning tumor. The mean tumor diameter
was 4.1±2.8 cm. In 64 (39.5%) patients, the primary disease site
was the pancreatic head. A total of 40 (24.7%) patients were
pathologically confirmed to exhibit lymph node invasion, whereas 13
(8.0%) patients exhibited distant metastases.
All patients with locoregional or metastatic disease
received surgical treatment. Pancreatoduodenectomy, distal
pancreatectomy and local resection of the pancreatic tumor were the
most frequently performed surgical procedures. R0 resection was
performed in 147 (90.7%) patients, whereas the surgery was
palliative in 15 (9.3%) cases. There were 40 (24.7%), 77 (47.5%),
32 (19.8%) and 13 (8.0%) cases, classified as stage I, II, III and
IV, respectively, according to the 2006 ENETS staging system. All
13 patients with stage IV p-NEN presented with a liver metastasis
at the time of diagnosis.
The WHO 2010 grading classification was performed
for all patients, yielding a distribution of 79 (48.8%), 67 (41.3%)
and 16 (9.9%), G1, G2 and G3 cases, respectively. Details of the
surgical procedures and features are presented in Table II. Patients with p-NEC exhibited a
significantly increased lymph node metastasis rate, compared with
patients with G1/G2 p-NETs (62.5 vs. 20.5%, respectively,
P=0.003).
 | Table II.Surgical procedures and features of
the patients with pancreatic neuroendocrine neoplasms (n=162). |
Table II.
Surgical procedures and features of
the patients with pancreatic neuroendocrine neoplasms (n=162).
| Characteristic | NET G1/G2
(n=146) | NEC G3 (n=16) |
|---|
| Tumor size, mean ±
SD, cm | 4.05±2.75 | 4.84±3.21 |
| Location, n (%) |
|
|
| Head | 59 (40.4) | 5 (31.2) |
| Body
and tail | 84 (57.5) | 11 (68.8) |
|
Multicentricity | 3 (2.1) | 0 (0.0) |
| Lymph node
metastasis, n (%) | 30 (20.5) | 10 (62.5) |
| Distant metastasis,
n (%) | 10 (6.9) | 3 (18.8) |
| Surgical approach,
n (%) |
|
|
|
PD/PPPD | 38 (36.0) | 4 (25.0) |
| DP | 78 (53.4) | 10 (62.5) |
| LP | 24 (13.7) | 0 (0.0) |
| TP | 6 (4.1) | 2 (12.5) |
| R0 resection, n
(%) | 132 (90.4) | 15 (93.8) |
| ENETS stage, n
(%) |
|
|
| I | 39 (26.7) | 1 (6.3) |
| II | 72 (49.3) | 5 (31.2) |
|
III | 25 (17.1) | 7 (43.8) |
| IV | 10 (6.9) | 3 (18.8) |
Survival analyses
The presence of lymphatic metastasis [hazard ratio
(HR)=4.802; 95% confidence interval (CI), 1.824–12.645; P=0.001],
distant metastasis (HR=3.267; 95% CI, 1.038–10.284; P=0.043), and
R1/R2 resection (HR=3.277; 95% CI, 1.119–9.592; P=0.030) led to a
decrease in overall survival (OS) compared with their absence
(Table III). By contrast, gender,
age, surgical approach, primary tumor size, hormone status, vessel
invasion and perineural invasion had no significant effect on
OS.
 | Table III.Univariate analysis of the clinical
factors influencing the prognosis of patients with pancreatic
neuroendocrine neoplasms. |
Table III.
Univariate analysis of the clinical
factors influencing the prognosis of patients with pancreatic
neuroendocrine neoplasms.
|
|
| Univariate
analysis |
|---|
|
|
|
|
|---|
| Variable prognostic
factor | Mean survival time,
months | Hazard ratio | 95% CI | P-value |
|---|
| Gender |
|
|
|
|
|
Male | 84 | – | – | – |
|
Female | 126 | 0.476 | 0.174–1.174 | 0.147 |
| Age, years |
|
|
|
|
|
≤51 | 109 | – | – | – |
|
>51 | 91 | 0.996 | 0.384–2.384 | 0.994 |
| Surgical
approaches |
|
|
|
|
|
PD/PPPD | 87 | – | – | – |
| DP | 116 | 0.789 | 0.263–2.263 | 0.672 |
| LP | 96 | 0.611 | 0.118–3.118 | 0.558 |
| TP | 44 | 1.723 | 0.196–15.196 | 0.624 |
| Primary tumor size,
cm |
|
|
|
|
| ≤4 | 118 | – | – | – |
|
>4 | 88 | 1.516 | 0.570–4.570 | 0.404 |
| Hormone status |
|
|
|
|
|
Functioning | 56 | – | – | – |
|
Non-functioning | 111 | 1.792 | 0.231–13.231 | 0.576 |
| Lymph node
metastasis |
|
|
|
|
| No | 120 | – | – | – |
|
Yes | 71 | 4.802 | 1.824–12.824 | 0.001a |
| Vessel
invasion |
|
|
|
|
| No | 113 | – | – | – |
|
Yes | 81 | 2.380 | 0.911–6.911 | 0.077 |
| Perineural
invasion |
|
|
|
|
| No | 113 | – | – | – |
|
Yes | 84 | 1.445 | 0.531–3.531 | 0.471 |
| Distant
metastasis |
|
|
|
|
| No | 115 | – | – | – |
|
Yes | 56 | 3.267 | 1.038–10.038 | 0.043a |
| Resection |
|
|
|
|
| R0 | 115 | – | – | – |
|
R1/R2 | 62 | 3.277 | 1.119–9.119 | 0.030a |
| Ki-67 (ENETS/WHO
2010) |
|
|
|
|
| ≤2 | 126 | – | – | – |
|
>2–20 | 88 | 2.605 | 0.688–9.688 | 0.159 |
|
>20 | 21 | 28.134 | 6.219–127.219 |
<0.001a |
| Ki-67 modified |
|
|
|
|
| ≤5 | 128 | – | – | – |
|
>5–20 | 80 | 4.470 | 1.273–15.273 | 0.019a |
|
>20 | 21 | 27.857 | 7.058–109.058 |
<0.001a |
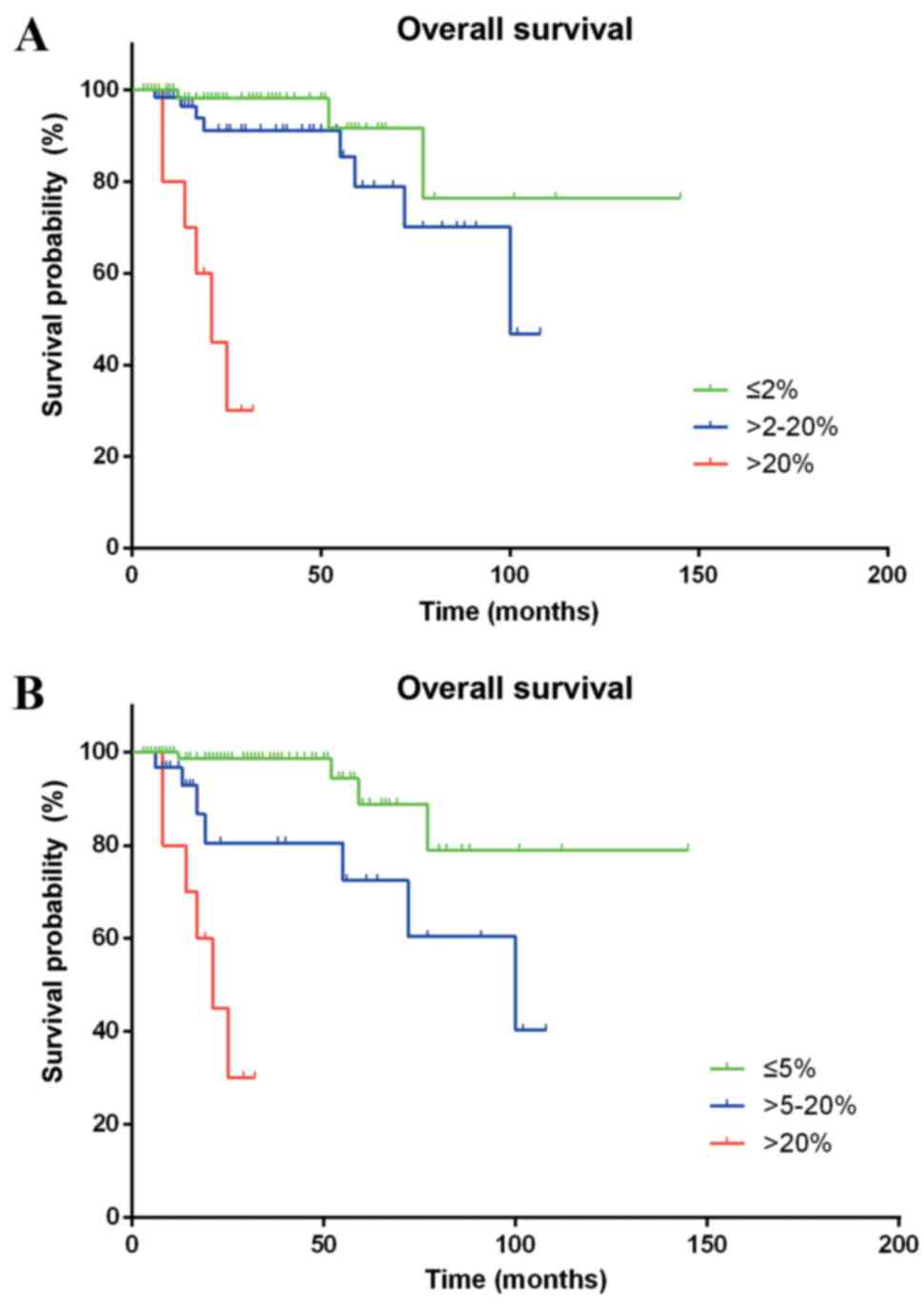
According to the WHO 2010 grading system, the
differences in the survival time of patients classified as G1 and
G3 (HR=28.134; 95% CI, 6.219–127.272; P<0.001) were
statistically significant. However, a statistically significant
difference between G1 and G2 was not observed (HR=2.605; 95% CI,
0.688–9.866; P=0.159). Furthermore, Ki-67 staining analysis defined
a proliferative index of ≤5% as G1, between 5 and 20% as G2, and
>20% as G3, providing a more efficient stratification of Chinese
patients with p-NENs (G1 vs. G2, HR=4.470; 95% CI, 1.273–15.699;
P=0.019; G1 vs. G3, HR=27.857; 95% CI, 7.058–109.944; P<0.001),
as compared with the ENETS/WHO classification systems (Fig. 2).
In Table IV, when the
Cox proportional hazards model was adjusted for grade, residual
tumor classification, lymphatic metastasis and distant metastases,
NEC G3 was a significant factor for poor prognosis on multivariate
analysis (HR=12.593; 95% CI, 3.476–45.622; P<0.001, vs. NET
G1/G2).
 | Table IV.Multivariate analysis of the clinical
factors influencing the prognosis of patients with pancreatic
neuroendocrine neoplasms. |
Table IV.
Multivariate analysis of the clinical
factors influencing the prognosis of patients with pancreatic
neuroendocrine neoplasms.
|
| Multivariate
analysis |
|---|
|
|
|
|---|
| Variable prognostic
factor | Hazard ratio | 95% CI | P-value |
|---|
| Lymph invasion |
|
|
|
| No | – | – | – |
|
Yes | 2.904 | 0.970–8.970 | 0.057 |
| Distant
metastasis |
|
|
|
| No | – | – | – |
|
Yes | 2.460 | 0.391–15.391 | 0.338 |
| Resection |
|
|
|
| R0 | – | – | – |
|
R1/R2 | 3.695 | 0.696–19.696 | 0.125 |
| Tumor grade |
|
|
|
| NET
G1/G2 | – | – | – |
| NEC
G3 | 12.593 | 3.476–45.476 |
<0.001a |
Discussion
Based on the results of the present study, four
prognostic factors, including lymphatic metastasis, distant
metastasis, R1/R2 resection and p-NEC, predicted a poor prognosis
following univariate analysis, and were subsequently used in
multivariate analysis. p-NEC was an independent predictor of poor
prognosis in patients with p-NEN following multivariate analysis.
In addition, p-NEC exhibited increased development of lymph node
metastasis compared with G1/G2 p-NET.
Conversely, the present single-center study was not
able to distinguish a difference in OS between G1 and G2 tumors,
using 2% as the threshold value of the Ki-67 index. The following
grading index thresholds classified Chinese patients with p-NENs
into three distinct survival groups more efficiently than the WHO
2010 grading classification: G1 ≤5%; G2 >5–20%; and G3
>20%.
The WHO 2010 grade classification system (5) was determined to be an independent
predictor of clinical outcomes, thereby corroborating previously
published studies (18,19). However, Bettini et al (20) published conflicting results, in which
the Ki-67 index was not demonstrated to have predictive value
between G1 and G2 tumors. Furthermore, certain studies have
demonstrated that a Ki-67 index >5% is the most efficient
predictor of recurrence following resection for p-NENs (21,22). In a
multicenter study of 202 p-NEN cases, it was revealed that patients
with a Ki-67 index of >5% had a notably unfavorable prognosis
compared with patients with a Ki-67 index of >2% (8). Rindi et al (23) also demonstrated that a Ki-67 index of
5% is more efficient, compared with 2%, for distinguishing between
G1 and G2. In the receiver operating characteristic analysis of
that study, the optimal threshold value for the prediction of
tumor-associated mortality at five years was identified to be a
Ki-67 index of ≥4.85 (23). These
findings suggest that a Ki-67 index of 5% is a more efficient
threshold value to distinguish between G1 and G2 in patients with
p-NENs. Therefore, the revision of the Ki-67 index threshold value
for classifying G1/G2 tumors from 2 to 5% is advised.
p-NEC exhibits a poor prognosis (24) and previous evidence has demonstrated
the decreased survival rate of patients with p-NEC (25,26).
Furthermore, increased lymph node metastasis in p-NEC was observed
in the present study. This difference suggested increased malignant
biological behavior in p-NEC, which was consistent with other
studies (19,27). Therefore, radical surgery with
lymphadenectomy is typically recommended for the treatment of
localized p-NEC (28).
There were several limitations to the present study.
The mean duration of follow-up was 30.3 months, which was shorter
compared with other p-NEN studies. The use of an increased sample
size is necessary to confirm potential prognostic factors
associated with an obvious decrease in survival. Furthermore,
relapse-free survival was not analyzed due to the limitation of
data integrity, and more effective models are essential in order to
reduce loss to follow-up.
In conclusion, the WHO grade classification is a key
prognostic factor, while p-NEC is a crucial predictor of poorer OS
in Chinese patients with p-NENs. A Ki-67 staining index of 5% is a
more efficient threshold value for the identification of G1 and G2.
Therefore, the results of the present study suggest that the
threshold for classifying G1/G2 tumors be revised from 2 to 5% in
patients with p-NENs.
Acknowledgements
The present study was supported in part by the
Sino-German Center (grant no. GZ857), the Science Foundation of
Shanghai (grant no. 13ZR1407500) and the National Science
Foundation of China (grant no. 81101807).
References
|
1
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: Epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsai HJ, Wu CC, Tsai CR, Lin SF, Chen LT
and Chang JS: The epidemiology of neuroendocrine tumors in Taiwan:
A nation-wide cancer registry-based study. PLoS One. 8:e624872013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Capella C, Heitz PU, Höfler H, Solcia E
and Klöppel G: Revised classification of neuroendocrine tumours of
the lung, pancreas and gut. Virchows Arch. 425:547–560. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Solcia E, Klöppel G and Sobin LH:
Histological Typing of Endocrine TumorsWHO International
Histological Classification of Tumors. Springer; Berlin, Germany:
2000
|
|
5
|
Rindi GAR and Bosman FT: ea: Nomenclature
and classification of neuroendocrine neoplasms of the digestive
systemBosman T, Carneiro F, Hruban R, et al: In: Bosman T, Carneiro
F, Hruban R, et al (eds). WHO Classification of Tumours of
the Digestive System. 4th. Lyon, France: International Agency for
Research on Cancer (IARC); 3. pp. 4172010
|
|
6
|
Rindi G, Klöppel G, Alhman H, Caplin M,
Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M,
Komminoth P, et al: TNM staging of foregut (neuro)endocrine tumors:
A consensus proposal including a grading system. Virchows Arch.
449:395–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Edge SBBDR and Compton CC: ea. AJCC Cancer
Staging Manual. New York, NY: Springer; 2010
|
|
8
|
Panzuto F, Boninsegna L, Fazio N, Campana
D, Brizzi Pia M, Capurso G, Scarpa A, De Braud F, Dogliotti L,
Tomassetti P, et al: Metastatic and locally advanced pancreatic
endocrine carcinomas: Analysis of factors associated with disease
progression. J Clin Oncol. 29:2372–2377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kulke MH, Bendell J, Kvols L, Picus J,
Pommier R and Yao J: Evolving diagnostic and treatment strategies
for pancreatic neuroendocrine tumors. J Hematol Oncol. 4:292011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dixon E and Pasieka JL: Functioning and
nonfunctioning neuroendocrine tumors of the pancreas. Curr Opin
Oncol. 19:30–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheema A, Weber J and Strosberg JR:
Incidental detection of pancreatic neuroendocrine tumors: An
analysis of incidence and outcomes. Ann Surg Oncol. 19:2932–2936.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haugvik SP, Janson ET, österlund P, Langer
SW, Falk RS, Labori KJ, Vestermark LW, Grønbæk H, Gladhaug IP and
Sorbye H: Surgical treatment as a principle for patients with
high-grade pancreatic neuroendocrine carcinoma: A nordic
multicenter comparative study. Ann Surg Oncol. 23:1721–1728. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim MJ, Choi DW, Choi SH, Heo JS, Park HJ,
Choi KK, Jang KT and Sung JY: Surgical strategies for
non-functioning pancreatic neuroendocrine tumours. Br J Surg.
99:1562–1568. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hill JS, McPhee JT, McDade TP, Zhou Z,
Sullivan ME, Whalen GF and Tseng JF: Pancreatic neuroendocrine
tumors: The impact of surgical resection on survival. Cancer.
115:741–751. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Franko J, Feng W, Yip L, Genovese E and
Moser AJ: Non-functional neuroendocrine carcinoma of the pancreas:
Incidence, tumor biology, and outcomes in 2,158 patients. J
Gastrointest Surg. 14:541–548. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cusati D, Zhang L, Harmsen WS, Hu A,
Farnell MB, Nagorney DM, Donohue JH, Que FG, Reid-Lombardo KM and
Kendrick ML: Metastatic nonfunctioning pancreatic neuroendocrine
carcinoma to liver: Surgical treatment and outcomes. J Am Coll
Surg. 215:117–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ekeblad S, Skogseid B, Dunder K, Oberg K
and Eriksson B: Prognostic factors and survival in 324 patients
with pancreatic endocrine tumor treated at a single institution.
Clin Cancer Res. 14:7798–7803. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ballian N, Loeffler AG, Rajamanickam V,
Norstedt PA, Weber SM and Cho CS: A simplified prognostic system
for resected pancreatic neuroendocrine neoplasms. HPB (Oxford).
11:422–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fischer L, Bergmann F, Schimmack S, Hinz
U, Prieß S, Müller-Stich BP, Werner J, Hackert T and Büchler MW:
Outcome of surgery for pancreatic neuroendocrine neoplasms. Br J
Surg. 101:1405–1412. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bettini R, Boninsegna L, Mantovani W,
Capelli P, Bassi C, Pederzoli P, Fave GF Delle, Panzuto F, Scarpa A
and Falconi M: Prognostic factors at diagnosis and value of WHO
classification in a mono-institutional series of 180
non-functioning pancreatic endocrine tumours. Ann Oncol.
19:903–908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boninsegna L, Panzuto F, Partelli S,
Capelli P, Fave G Delle, Bettini R, Pederzoli P, Scarpa A and
Falconi M: Malignant pancreatic neuroendocrine tumour: Lymph node
ratio and Ki67 are predictors of recurrence after curative
resections. Eur J Cancer. 48:1608–1615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khan MS, Luong TV, Watkins J, Toumpanakis
C, Caplin ME and Meyer T: A comparison of Ki-67 and mitotic count
as prognostic markers for metastatic pancreatic and midgut
neuroendocrine neoplasms. Br J Cancer. 108:1838–1845. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rindi G, Falconi M, Klersy C, Albarello L,
Boninsegna L, Buchler MW, Capella C, Caplin M, Couvelard A,
Doglioni C, et al: TNM staging of neoplasms of the endocrine
pancreas: Results from a large international cohort study. J Natl
Cancer Inst. 104:764–777. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vélayoudom-Céphise FL, Duvillard P, Foucan
L, Hadoux J, Chougnet CN, Leboulleux S, Malka D, Guigay J, Goere D,
Debaere T, et al: Are G3 ENETS neuroendocrine neoplasms
heterogeneous. Endocr Relat Cancer. 20:649–657. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hashim YM, Trinkaus KM, Linehan DC,
Strasberg SS, Fields RC, Cao D and Hawkins WG: Regional
lymphadenectomy is indicated in the surgical treatment of
pancreatic neuroendocrine tumors (PNETs). Ann Surg. 259:197–203.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Strosberg JR, Cheema A, Weber J, Han G,
Coppola D and Kvols LK: Prognostic validity of a novel american
joint committee on cancer staging classification for pancreatic
neuroendocrine tumors. J Clin Oncol. 29:3044–3049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wong J, Fulp WJ, Strosberg JR, Kvols LK,
Centeno BA and Hodul PJ: Predictors of lymph node metastases and
impact on survival in resected pancreatic neuroendocrine tumors: A
single-center experience. Am J Surg. 208:775–780. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Garcia-Carbonero R, Sorbye H, Baudin E,
Raymond E, Wiedenmann B, Niederle B, Sedlackova E, Toumpanakis C,
Anlauf M, Cwikla JB, et al: ENETS consensus guidelines for
high-grade gastroenteropancreatic neuroendocrine tumors and
neuroendocrine carcinomas. Neuroendocrinology. 103:186–194. 2016.
View Article : Google Scholar : PubMed/NCBI
|