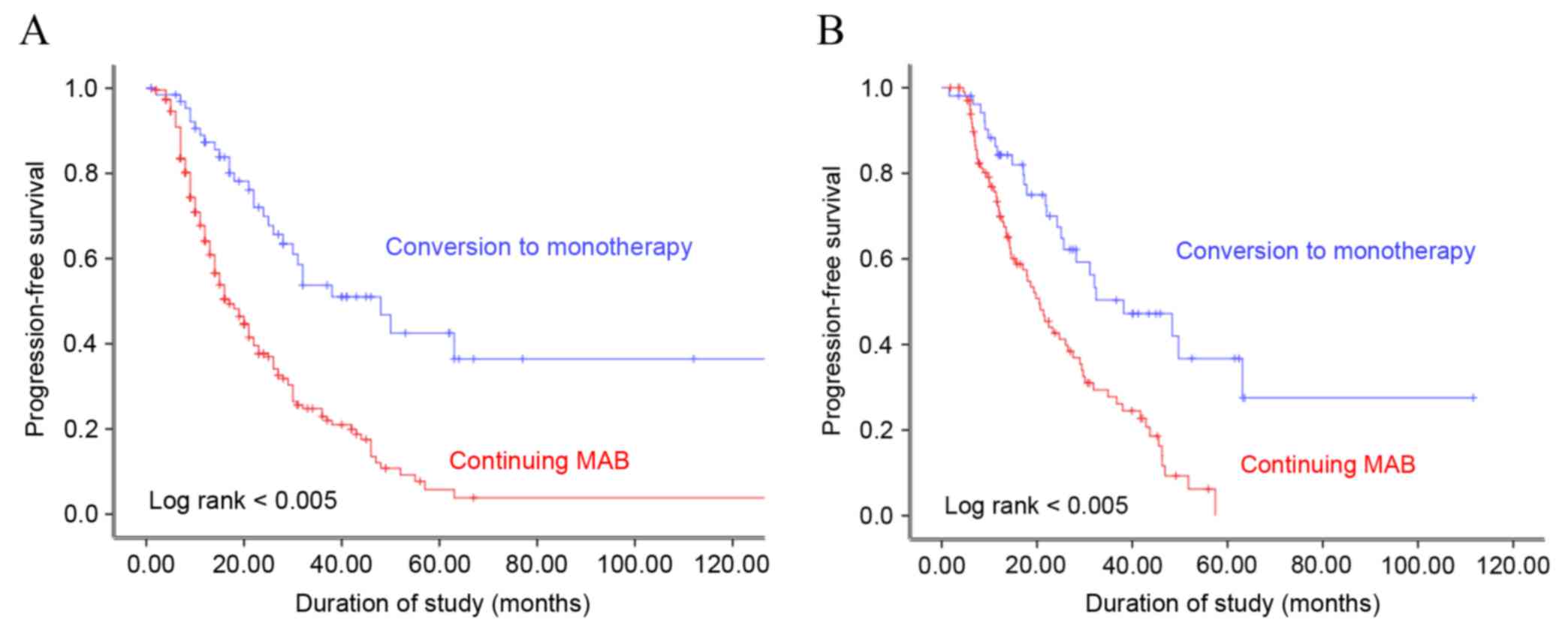
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jung KW, Won YJ, Kong HJ, Oh CM, Shin A
and Lee JS: Survival of Korean adult cancer patients by stage at
diagnosis, 2006–2010: National cancer registry study. Cancer Res
Treat. 45:162–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salonen AJ, Taari K, Ala-Opas M, Viitanen
J, Lundstedt S and Tammela TL; FinnProstate Group, : The
FinnProstate study VII: intermittent versus continuous androgen
deprivation in patients with advanced prostate cancer. J Urol.
187:2074–2081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taylor LG, Canfield SE and Du XL: Review
of major adverse effects of androgen-deprivation therapy in men
with prostate cancer. Cancer. 115:2388–2399. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwandt A and Garcia JA: Complications of
androgen deprivation therapy in prostate cancer. Curr Opin Urol.
19:322–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crawford ED, Eisenberger MA, McLeod DG,
Spaulding JT, Benson R, Dorr FA, Blumenstein BA, Davis MA and
Goodman PJ: A controlled trial of leuprolide with and without
flutamide in prostatic carcinoma. N Engl J Med. 321:419–424. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharifi N, Gulley JL and Dahut WL:
Androgen deprivation therapy for prostate cancer. JAMA.
294:238–244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mottet N, van Damme J, Loulidi S, Russel
C, Leitenberger A and Wolff JM; TAP22 Investigators Group, :
Intermittent hormonal therapy in the treatment of metastatic
prostate cancer: A randomized trial. BJU Int. 110:1262–1269. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seidenfeld J, Samson DJ, Hasselblad V,
Aronson N, Albertsen PC, Bennett CL and Wilt TJ: Single-therapy
androgen suppression in men with advanced prostate cancer: A
systematic review and meta-analysis. Ann Intern Med. 132:566–577.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alva AS and Hussain M: Initial management
of metastatic prostate cancer. In: Comprehensive Textbook of
Genitourinary OncologyScardino PT, Linehan WM, Zelefsky MJ and
Vogelzang NJ: Lippincott Williams & Wilkins; Philadelphia: pp.
251–261. 2011
|
|
12
|
da Silva F Calais, da Silva FM Calais,
Gonçalves F, Santos A, Kliment J, Whelan P, Oliver T, Antoniou N,
Pastidis S, Queimadelos A Marques and Robertson C: Locally advanced
and metastatic prostate cancer treated with intermittent androgen
monotherapy or maximal androgen blockade: Results from a randomised
phase 3 study by the South European Uroncological Group. Eur Urol.
66:232–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
da Silva FE Calais, Bono AV, Whelan P,
Brausi M, Queimadelos A Marques, Martin JA, Kirkali Z, da Silva FM
Calais and Robertson C: Intermittent androgen deprivation for
locally advanced and metastatic prostate cancer: Results from a
randomised phase 3 study of the South European Uroncological Group.
Eur Urol. 55:1269–1277. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsai HT, Penson DF, Makambi KH, Lynch JH,
Van Den Eeden SK and Potosky AL: Efficacy of intermittent androgen
deprivation therapy vs conventional continuous androgen deprivation
therapy for advanced prostate cancer: A meta-analysis. Urology.
82:327–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prapotnich D, Fizazi K, Escudier B, Mombet
A, Cathala N and Vallancien G: A 10-year clinical experience with
intermittent hormonal therapy for prostate cancer. Eur Urol.
43:233–240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salonen AJ, Viitanen J, Lundstedt S,
Ala-Opas M, Taari K and Tammela TL; FinnProstate Group, : Finnish
multicenter study comparing intermittent to continuous androgen
deprivation for advanced prostate cancer: Interim analysis of
prognostic markers affecting initial response to androgen
deprivation. J Urol. 180:915–920. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grossfeld GD, Small EJ and Carroll PR:
Intermittent androgen deprivation for clinically localized prostate
cancer: Initial experience. Urology. 51:137–144. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scher HI, Halabi S, Tannock I, Morris M,
Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ,
Dreicer R, et al: Design and end points of clinical trials for
patients with progressive prostate cancer and castrate levels of
testosterone: Recommendations of the Prostate Cancer Clinical
Trials Working Group. J Clin Oncol. 26:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
D'Agostino RB Jr: Propensity score methods
for bias reduction in the comparison of a treatment to a
non-randomized control group. Stat Med. 17:2265–2281. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ryan CJ, Elkin EP, Small EJ, Duchane J and
Carroll P: Reduced incidence of bony metastasis at initial prostate
cancer diagnosis: Data from CaPSURE. Urol Oncol. 24:396–402. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shiota M and Eto M: Current status of
primary pharmacotherapy and future perspectives toward upfront
therapy for metastatic hormone-sensitive prostate cancer. Int J
Urol. 23:360–369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hussain M, Tangen CM, Berry DL, Higano CS,
Crawford ED, Liu G, Wilding G, Prescott S, Sundaram S Kanaga, Small
EJ, et al: Intermittent versus continuous androgen deprivation in
prostate cancer. N Engl J Med. 368:1314–1325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maximum androgen blockade in advanced
prostate cancer: An overview of the randomised trials with 3283
deaths in 5710 patients. Prostate Cancer Trialists' Collaborative
Group. Lancet. 346:265–269. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akaza H, Hinotsu S, Usami M, Arai Y,
Kanetake H, Naito S and Hirao Y; Study Group for the Combined
Androgen Blockade Therapy of Prostate Cancer, : Combined androgen
blockade with bicalutamide for advanced prostate cancer: Long-term
follow-up of a phase 3, double-blind, randomized study for
survival. Cancer. 115:3437–3445. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmitt B, Bennett C, Seidenfeld J, Samson
D and Wilt T: Maximal androgen blockade for advanced prostate
cancer. Cochrane Database Syst Rev: CD001526. 2000.
|
|
26
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: EAU guidelines on prostate cancer. Part II:
Treatment of advanced, relapsing and castration-resistant prostate
cancer. Eur Urol. 65:467–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Horwich A, Parker C, de Reijke T and
Kataja V; ESMO Guidelines Working Group, : Prostate cancer: ESMO
clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 24 Suppl 6:vi106–vi114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Akaza H, Labrie F and Namiki M: A way of
thinking of a MAB therapy for local/locally advanced prostate
cancer: The theory and recent evaluation. Gan To Kagaku Ryoho.
34:657–669. 2007.(In Japanese). PubMed/NCBI
|
|
29
|
Sharifi N, Gulley JL and Dahut WL: An
update on androgen deprivation therapy for prostate cancer. Endocr
Relat Cancer. 17:R305–R315. 2010. View Article : Google Scholar : PubMed/NCBI
|