Introduction
Epithelial ovarian cancer (EOC) affects almost
25,000 women annually and is the fifth most common malignancy in
women in North America, with a five-year mortality rate of >70%
(1). As the symptoms may not be
observed until the cancer has spread extensively, <25% of women
are diagnosed in the early stages of the disease. The combination
of surgery with cytotoxic chemotherapy produces favorable clinical
responses in 50–80% of patients (2,3).
The platinum-paclitaxel combination regimen is the
chemotherapy gold-standard for advanced ovarian cancer, with
response rates >80% and complete response rates of 40–60%
(4–8).
Carboplatin and cisplatin are two agents used in this combination
regimen. These compounds share the mechanism of the formation of
DNA adducts, and cross-resistance is frequently observed (9). As a result of the DNA adducts, signaling
pathways including cell cycle checkpoints, p53 signaling and
mitogen-activated protein kinases, are activated, ultimately
leading to cell death (10,11). However, the majority of patients
eventually experience a relapse even if they respond to
platinum-paclitaxel combination treatment in the beginning, with a
median progression-free survival time of 18 months (12). The mechanisms implicated in platinum
resistance include decreased platinum uptake, enhanced DNA damage
repair and increased resistance to apoptosis (9–11).
Patients that develop platinum-resistant or refractory disease are
treated with a range of other drugs, including paclitaxel,
bevacizumab and capecitabine (13).
However, improving the rate of curing EOC remains critical.
Therefore, it is necessary to search for novel, biologically
targeted treatment modalities. The understanding of the molecular
biology of cancer, including the mechanisms underlying cancer
processes and drug resistance, has facilitated the development of
targeted therapies, including small-molecule inhibitors. These
agents target proteins associated with malignant cell behavior,
including cell viability/death, metastasis and angiogenesis.
Far upstream element (FUSE) binding protein 1 (FBP1)
has been identified as an anti-apoptotic and pro-proliferative
oncoprotein that is overexpressed in hepatocellular carcinoma
(14,15). It has also been demonstrated that the
high expression of FBP1 is associated with carboplatin resistance
(16). FBP1 functions as a
transcriptional regulator by binding to the single-stranded DNA
element, FUSE, and interacting with the basal transcriptional
machinery (17). Target genes
regulated by FBP1 include the oncogene c-Myc (18), the cell cycle inhibitor p21 (15) and the deubiquitinating enzyme
ubiquitin-specific peptidase 29 (Usp29) (19). Recently, Rabenhorst et al
(20) demonstrated that FBP1
additionally serves a role in hematopoietic development and
homeostasis. In our previous studies, we identified that FBP1
physically interacts with p53 to suppress p53 transcriptional
activity during radiation-induced cellular stress, and that it
facilitates hepatitis C virus replication in hepatoma cells
(21,22). Therefore, the present study considered
the association of FBP1 expression with EOC progression and the
response to carboplatin treatment.
In the present study, it was identified that the
silencing of FBP1 enhanced the sensitivity of EOC cells to
carboplatin. Additionally, carboplatin treatment inhibited EOC cell
viability and migration by inhibiting the expression of FBP1.
Therefore, FBP1 may be a novel potential biological target for the
treatment of EOC.
Materials and methods
Clinical tissue samples and
immunohistochemical staining (IHS)
The study was conducted subsequent to obtaining
informed consent from all subjects, and the approval of the study
protocol by the Medical Ethics Committee of Guangzhou Red Cross
Hospital of Medical College, Jinan University (Guangzhou, China).
Samples were collected between January 2012 and June 2015; patients
had a median age of 45.5 years (range, 17–76 years). Samples were
assigned into three groups, including normal epithelial ovarian
tissue (4 samples), epithelial ovarian adenoma tissue (7 samples)
and epithelial ovarian cancer tissue (10 samples).
Paraffin sections (5-mm) were deparaffinized in 100%
xylene and rehydrated in a descending series of ethanol-water
solutions. The sections were boiled in 10 mmol/l citrate buffer (pH
6.0) for 20 min for antigen retrieval. Endogenous peroxidase
activity was blocked with 0.3% methanolic peroxide for 30 min at
room temperature. Anti-FBP1 antibody (cat. no. sc-136137, 1:100)
was obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Horseradish peroxidase (HRP)-conjugated secondary antibody was also
obtained from Santa Cruz Biotechnology (cat. no. sc-2004, 1:100).
The antigen-antibody reactions were visualized with the chromogen
diaminobenzidine (DAB).
Stained sections were observed under a microscope.
DAB density was quantified by a video camera mounted on an optical
microscope (Microphot; Nikon Corporation, Tokyo, Japan) connected
to a video capture card. Images were captured with a ×40 objective
and image processing and analyses were performed using Image-Pro
Plus 6.0 software (Media Cybernetics, Inc., Shanghai, China). The
intensity of the immunohistochemical reaction was expressed as the
integrated absorbance (IA) of the DAB reaction product. The results
of 5 separate measurements for each sample were expressed as the
mean ± standard deviation (SD).
Cell culture
SKOV3 ovarian cancer cells were obtained from the
American Type Culture Collection (Manassas, VA, USA). The cells
were grown in complete Dulbecco's modified Eagle's medium (DMEM)
containing 10% fetal bovine serum (FBS.), 100 U/ml penicillin, 100
µg/ml streptomycin (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and kept at 37°C in a humidified atmosphere with 5%
CO2.
Construction of FBP1 knockdown
lentivirus and generation of stable FBP1 knockdown cells
A pSi-LVRH1GP vector with a puromycin resistance
cassette (GeneCopoeia, Rockville, MD, USA) was used to express
short hairpin (sh)RNA to knock down FBP1 expression. The control
vector expressed a scrambled sequence (5′-GCTTCGCGCCGTAGTCTTA-3′)
and was designated pSi-LV-FBP1-C. To knock down FBP1, several shRNA
sequences were used, including the sequences from 1036-1056
(5′-GGACAACACCCGAAAGGATAG-3′), 1671-1671
(5′-GCAGGAACGGATCCAAATTCA-3′) and 1758–1778
(5′-GCAGGTGCACCAACTACAACT-3′) of FBP1; these vectors were
designated as pSi-LV-FBP1-KD. SKOV3 cells were transfected with
pSi-LV-FBP1-C or pSi-LV-FBP1-KD. First, 2×105 SKOV3
cells were seeded in 2 ml antibiotic-free DMEM medium supplemented
with FBS and cells were incubated until they reached 60–80%
confluence. After washing the cells once with 2 ml of antibiotic-
and FBS-free DMEM medium, 900 ul antibiotic- and FBS-free DMEM
medium was added to the cells, alongside 1 µg of pSi-LV-FBP1-C or
pSi-LV-FBP1-KD diluted in 100 ul antibiotic and FBS-free DMEM
medium. After 6 h, medium was changed for DMEM supplemented with
FBS and antibiotics. Puromycin was used as a selective marker.
After 48 h incubation, medium was changed to DMEM growth medium
containing 1.5 µg/ml of puromycin (Santa Cruz Biotechnology, Inc.).
Western blotting was used to confirm the knockdown. The
pSi-LV-FBP1-C-transfected cells were designated FBP1-C cells, and
the cells with the most complete FBP1 knockdown as FBP1-KD
cells.
Cell viability assay
SKOV3 FBP1-C or FBP1-KD cells were seeded at
2.0×104 cells/well in a 24-well plate overnight. They
were then treated with a range of concentrations of carboplatin
(including 0, 38, 77, 134 and 269 µmol/l) for 48 h. Cell viability
was determined by the inner salt (MTS) method, according to the
Cell Titer 96 Aqueous One Solution Viability assay manual (Promega
Corporation, Madison, WI, USA). The experiment was repeated at
least three times.
Western blot analysis
Whole-cell extracts were prepared with
radioimmunoprecipitation assay buffer [150 mM NaCl, 50 mM Tris (pH
7.4), 1% NP40, 0.1% SDS and 0.5% sodium deoxycholate] supplemented
with 10 mmol/l phenylmethanesulfonyl fluoride (Amresco, LLC, Solon,
OH, USA). The concentration of protein was determined with a
bicinchoninic protein assay kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). A total of 30 µg protein per lane were
separated by 10% SDS-PAGE gel and electro-blotted onto
nitrocellulose membranes (Bio-Rad Laboratories, Inc.). The
membranes were blocked with 5% (w/v) skimmed milk powder in
Tris-buffered saline with Tween-20 [TBST; 10 mM Tris (pH 7.5), 150
mM NaCl, 0.1% (v/v) Tween-20]. The membranes were then incubated at
4°C overnight with antibodies against FBP1 (Santa Cruz
Biotechnology, Inc.; cat. no. sc-136137; 1:200), β-catenin (Santa
Cruz Biotechnology, Inc.; cat. no. sc-7963; 1:200), cleaved
caspase-3 (Cell Signaling Technology, Inc., Danvers, MA, USA; cat.
no. 9664; 1:1,000) or matrix metalloproteinase (MMP) 9 (Santa Cruz
Biotechnology, sc-21733; 1:200) and GAPDH (Cell Signaling
Technology, Inc.; cat. no. 5174; 1:1,000) in 5% (w/v) skimmed milk
powder in TBST. Subsequent to washing three times in TBST,
membranes were incubated with HRP-conjugated secondary antibodies
(Cell Signaling Technology, Inc.; cat. nos. 7076 and 7074) diluted
in TBST (1:5,000) for 1 h at room temperature. Subsequent to
washing a further three times with TBST, the target proteins were
detected using an ECL Western Blotting Substrate kit (Thermo Fisher
Scientific, Inc.; cat. no. 32106) and quantified with a ChemiDoc
XRS+ Imaging system (Bio-Rad Laboratories, Hercules, CA,
USA).
Migration assay
SKOV3 FBP1-C or FBP1-KD cells were grown to
confluence in 6-well plates pre-coated with 0.1% gelatin, then
incubated with 10 µg/ml mitomycin C (both Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) for 2 h to inactivate cell viability. The
cell monolayer was wounded with a pipette tip and washed with PBS.
DMEM supplemented with 1% FBS was added into the wells with 5 ng/ml
vascular endothelial growth factor (VEGF; R&D Systems,
Minneapolis, MN, USA) or 5 ng/ml VEGF with 38 or 77 µmol/l
carboplatin. Images were captured at 48 h. The migration distance
of the cells was quantified as wound width at 0 h-wound width at 48
h. Three independent experiments were performed.
Statistical analysis
Data are presented as the mean ± SD. Statistical
analysis was performed using SPSS 18.0 software (SPSS, Inc.,
Chicago, IL, USA). A Student's t-test was performed to test
statistical significance between groups. A nonparametric Spearman's
rank correlation analysis was performed to evaluate the correlation
between FBP1 expression and increasing EOC grade. P<0.05 was
considered to indicate a statistically significant difference.
Results
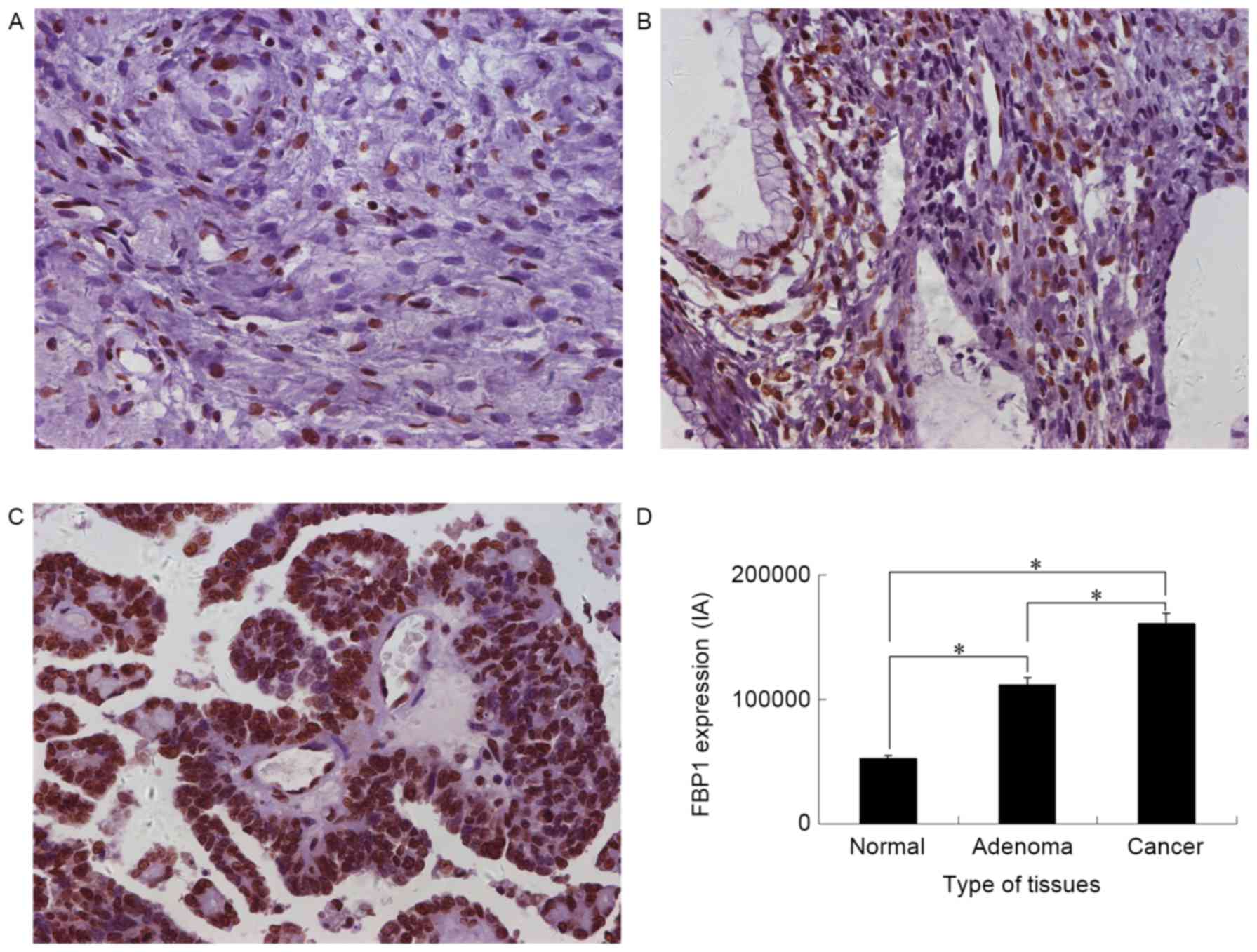
The expression of FBP1 in epithelial
ovarian tissue
To explore the potential role of FBP1 in EOC
development, the expression of FBP1 in normal epithelial ovarian,
epithelial ovarian adenoma and advanced EOC tissue was analyzed
with IHS. FBP1 expression was identified in all three groups
(Fig. 1). The FBP1 IA was
52,060±27,749 for normal epithelial ovarian, 111,770±44,299 for
epithelial ovarian adenoma and 161,067±58,531 for EOC tissue.
Significant differences in IA were identified between normal
epithelial ovarian tissue and epithelial ovarian adenoma or EOC
tissue, and between epithelial ovarian adenoma and EOC tissues
(P<0.05). The highest FBP1 expression was identified in EOC
tissue (Fig. 1D). FBP1 expression was
positively correlated with the epithelial ovarian grade, as
analyzed by a Spearman's rank correlation analysis; the correlation
coefficient was 0.637 (P<0.001).
The generation of ovarian cancer cells
with stable FBP1 knockdown
SKOV3 cells were transfected with pSi-LV-FBP1-C or
-KD vectors to generate FBP1-C and FBP1-KD SKOV3 cells. The
interference rate, as analyzed by RT-qPCR, was 57% for sequence
1,036–1,056, 76% for 1,671–1,691 and 56% for 1,758–1,778. The
result was confirmed by western blot analysis (Fig. 2A, lane 1 and 3); thus, cells knocked
down with sequence 1671–1691 were used for further experiments in
the study. The protein expression of FBP1 in FBP1-KD cells was ~15%
of FBP1-C cells.
 | Figure 2.Knockdown of FBP1 enhanced the
sensitivity of SKOV3 cells to carboplatin. (A) Protein expression
levels of FBP1, MMP-9 and β-catenin relative to GAPDH, as
determined by western blotting, compared between FBP1-C and FBP1-KD
cells with, or without, carboplatin treatment. (B) Relative
viability of SKOV3 cells treated with carboplatin for 48 h as
determined by an MTS assay. (C) Protein expression level of
c-caspase 3 relative to GAPDH, as determined by western blotting,
compared between FBP1-C and FBP1-KD cells with, or without,
carboplatin treatment. *P<0.05. FBP1, FUSE binding protein 1;
MMP-9, matrix metalloproteinase-9; FBP1-C, SKOV3 cells stably
transfected with control vector; FBP1-KD, SKOV3 cells stably
transfected with FBP1 knockdown vector; c-caspase 3, cleaved
caspase 3. |
FBP1 knockdown increases the
sensitivity of ovarian cancer cells to carboplatin
To assess the cytotoxicity of carboplatin, the
inhibitory effect on FBP1-C and FBP1-KD SKOV3 cell viability was
assessed with an MTS assay. Carboplatin treatment significantly
inhibited the viability of FBP1-C and FBP-KD SKOV3 cells from 38
µmol/l (Fig. 2B). The knockdown of
FBP1 increased the sensitivity of SKOV3 cells to carboplatin. The
decrease in cell viability following carboplatin treatment was
significantly enhanced by FBP1 knockdown in the range of 38–134
µmol/l carboplatin (P<0.05).
Carboplatin downregulates FBP1 and
β-catenin, and stimulates cleaved-caspase-3 expression
As the knockdown of FBP1 increased the sensitivity
of SKOV3 cells to carboplatin, the level of FBP1 expression in
SKOV3 cells treated with 38 µmol/l of carboplatin for 48 h was
determined. FBP1 expression was decreased by carboplatin treatment
in FBP1-C and in FBP1-KD cells, with a greater decrease in FBP1-KD
than in FBP1-C SKOV3 cells (Fig. 2A,
lanes 2 and 4; P<0.05).
It was previously reported that β-catenin expression
was elevated in EOC (23),
potentially contributing to the carboplatin resistance of ovarian
cancer cells (24). In the present
study, the expression of β-catenin in FBP1-KD SKOV3 cells was
decreased relative to FBP1-C (Fig.
2A, lanes 1 and 3). Carboplatin treatment inhibited β-catenin
expression by 4 and 35% in FBP1-C and FBP1-KD SKOV3 cells,
respectively (Fig. 2A, lanes 2 and 4,
P<0.05).
Cleaved caspase 3 is a marker for apoptosis.
Carboplatin treatment increased the expression of cleaved
caspase-3; the increase in cleaved caspase-3 expression was more
significant in FBP1-KD than in FBP1-C SKOV3 cells following
treatment with 38 µmol/l of carboplatin for 48 h (Fig. 2C, P<0.05). The result indicated
that carboplatin induced apoptosis.
Carboplatin inhibits the migration of
SKOV3 cells
Cell migration is critical for cancer invasion and
metastasis. The effect of carboplatin on the chemotactic motility
of FBP1-C and FBP1-KD SKOV3 cells was measured with a wound-healing
assay. Carboplatin inhibited VEGF-induced SKOV3 migration in FBP1-C
SKOV3 cells in a dose dependent manner (Fig. 3). The inhibition of cell migration by
carboplatin was more significant for the FBP1-KD SKOV3 cells,
compared with FBP1-C (P<0.05). The result indicated that FBP1
knockdown facilitated the inhibition of migration by
carboplatin.
MMPs promote extracellular matrix degradation and
cell migration, so the effects of carboplatin on MMP-9 expression
were investigated. FBP1-C and FBP1-KD SKOV3 cells were treated with
38 µmol/l carboplatin for 48 h. Carboplatin inhibited the
expression of MMP-9 (Fig. 2A). The
MMP-9 decrease in FBP1-C SKOV3 cells was 59% (Fig. 2A, lanes 1 and 2), whereas the MMP-9
decrease in FBP1-KD SKOV3 cells was 69% (Fig. 2A, lanes 3 and 4).
Discussion
In our previous study, it was demonstrated that FBP1
was abundantly expressed in hepatocellular carcinoma tumors with
chronic hepatitis C backgrounds (22). In the present study, the high
expression of FBP1 was identified in EOC and it was demonstrated
that FBP1 expression was associated with EOC progression.
Carboplatin is commonly used to treat EOC (4–9). However,
the majority of patients eventually experience a relapse, with a
median progression-free survival time of 18 months (12). A recent study in patients with
refractory breast cancer demonstrated that tumor profiling-based
therapy resulted in a survival benefit (23). Therefore, improving the understanding
of the mechanisms implicated in platinum resistance may improve the
management of EOC. In our previous study, it was identified that
FBP1 knockdown enhances the sensitivity of hepatoma cells to
γ-irradiation (21). In the present
study, it was demonstrated that the knockdown of FBP1 enhanced the
sensitivity of EOC cells to the growth- and migration-inhibiting
effect of carboplatin. This result confirmed the conclusion of a
previous study, in which FBP1 was identified as one of eight genes
associated with carboplatin resistance, based on the receiver
operating characteristics analysis of 1,452 patients (16).
The high expression of β-catenin was previously
identified in EOC (24) and elevated
β-catenin expression may contribute to the carboplatin resistance
of ovarian cancer cells (25). The
present study identified that the expression of β-catenin decreased
significantly in the cells with FBP1 knockdown and carboplatin
treatment inhibited the expression of β-catenin in FBP1-KN cells to
a greater extent than in FBP1-C cells. This result implied that low
β-catenin expression, resulting from FBP1 knockdown, increased the
sensitivity of epithelial ovarian cells to carboplatin. Carboplatin
treatment also upregulated the expression of cleaved-caspase 3, a
marker for apoptosis, in FBP1-KN to a greater extent than FBP1-C
cells. Therefore, carboplatin treatment induced a greater extent of
apoptosis in FBP1-KN cells than in FBP1-C cells.
MMPs belong to a family of Zn2+-binding,
Ca2+-dependent endopeptidases with the essential
function of proteolysis of the extracellular matrix, which is
associated with several cellular processes (26); MMPs are considered to regulate a
number of processes, including cell migration, viability,
apoptosis, angiogenesis, tumor expansion and metastasis (27–29).
Although they are generally expressed at low levels, MMPs are
upregulated during tissue remodeling, inflammation, wound healing
and cancer progression (30,31). In the present study, it was
demonstrated that carboplatin inhibited EOC cell migration, and
that the knockdown of FBP1 enhanced the inhibition of cell
migration by carboplatin. Additionally, carboplatin treatment
decreased the expression of MMP-9.
Understanding the mechanisms underlying cancer
processes, including drug resistance, may facilitate the
development of targeted therapies. FBP1 has been identified as an
anti-apoptotic and pro-proliferative oncoprotein that is
overexpressed in a number of cancer types, including human
hepatocellular carcinoma and non-small cell lung cancer (32). It has also been demonstrated that the
high expression of FBP1 is associated with carboplatin resistance
(16). In a recent study, it was
reported that FBP2, another member of the FBP family, regulates
doxorubicin resistance in human breast cancer cell lines (33). In addition to regulating the
expression of the oncogene c-Myc (18), FBP1 also regulates the expression of
p21 (15) and Usp29 (19) and suppresses p53 transcription
activity (21,22). In the present study, the expression of
β-catenin and MMP-9 was lower in FBP1-KD SKOV3 cells compared with
FBP1-C SKOV3 cells. This may imply that FBP1 serves a role in the
regulation of the expression of β-catenin and MMP-9. It is also
reasonable to hypothesize that the effect of carboplatin on the
expression of β-catenin and MMP-9 is mediated through the
inhibition on FBP1.
Based on the data in the present study, FBP1
knockdown enhanced the sensitivity of epithelial ovarian cancer
cells to carboplatin. FBP1 knockdown enhanced the inhibition of
cell viability and migration by carboplatin. Therefore, it is
concluded that the high expression of FBP1 is a potential mechanism
for carboplatin resistance, and inducing the downregulation of FBP1
is a potential strategy for a novel EOC treatment regimen.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81272222, 30973510,
81171104 and 81473211).
References
|
1
|
A JY, . Wang GJ, Sun JG, Gu YC, Wu MS and
Liu JH: Identification of phase I and phase II metabolites of
Guanfu base A hydrochloride in human urine. Eur J Drug Metab
Pharmacokinet. 28:265–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berkenblit A and Cannistra SA: Advances in
the management of epithelial ovarian cancer. J Reprod Med.
50:426–438. 2005.PubMed/NCBI
|
|
3
|
Salom E, Almeida Z and Mirhashemi R:
Management of recurrent ovarian cancer: Evidence-based decisions.
Curr Opin Oncol. 14:519–527. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agarwal R and Kaye SB: Ovarian cancer:
Strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R: Gynecologic Oncology Group: Phase III trial of
carboplatin and paclitaxel compared with cisplatin and paclitaxel
in patients with optimally resected stage III ovarian cancer: A
Gynecologic Oncology Group study. J Clin Oncol. 21:3194–3200. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
du Bois A, Neijt JP and Thigpen JT: First
line chemotherapy with carboplatin plus paclitaxel in advanced
ovarian cancer-a new standard of care? Ann Oncol. 10 Suppl
1:S35–S41. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Biagi JJ and Eisenhauer EA: Systemic
treatment policies in ovarian cancer: The next 10 years. Int J
Gynecol Cancer. 13 Suppl 2:S231–S240. 2003. View Article : Google Scholar
|
|
8
|
Neijt JP, Engelholm SA, Tuxen MK, Sorensen
PG, Hansen M, Sessa C, de Swart CA, Hirsch FR, Lund B and van
Houwelingen HC: Exploratory phase III study of paclitaxel and
cisplatin versus paclitaxel and carboplatin in advanced ovarian
cancer. J Clin Oncol. 18:3084–3092. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gore ME, Fryatt I, Wiltshaw E and Dawson
T: Treatment of relapsed carcinoma of the ovary with cisplatin or
carboplatin following initial treatment with these compounds.
Gynecol Oncol. 36:207–211. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malz M, Weber A, Singer S, Riehmer V,
Bissinger M, Riener MO, Longerich T, Soll C, Vogel A, Angel P, et
al: Overexpression of far upstream element binding proteins: A
mechanism regulating proliferation and migration in liver cancer
cells. Hepatology. 50:1130–1139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rabenhorst U, Beinoraviciute-Kellner R,
Brezniceanu ML, Joos S, Devens F, Lichter P, Rieker RJ, Trojan J,
Chung HJ, Levens DL and Zörnig M: Overexpression of the far
upstream element binding protein 1 in hepatocellular carcinoma is
required for tumor growth. Hepatology. 50:1121–1129. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pénzváltó Z, Lánczky A, Lénárt J,
Meggyesházi N, Krenács T, Szoboszlai N, Denkert C, Pete I and
Győrffy B: MEK1 is associated with carboplatin resistance and is a
prognostic biomarker in epithelial ovarian cancer. BMC Cancer.
14:8372014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chung HJ and Levens D: c-myc expression:
keep the noise down! Mol Cells. 20:157–166. 2005.PubMed/NCBI
|
|
18
|
Duncan R, Bazar L, Michelotti G, Tomonaga
T, Krutzsch H, Avigan M and Levens D: A sequence-specific,
single-strand binding protein activates the far upstream element of
c-myc and defines a new DNA-binding motif. Genes Dev. 8:465–480.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Chung HJ, Vogt M, Jin Y, Malide D,
He L, Dundr M and Levens D: JTV1 co-activates FBP to induce USP29
transcription and stabilize p53 in response to oxidative stress.
EMBO J. 30:846–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rabenhorst U, Thalheimer FB, Gerlach K,
Kijonka M, Böhm S, Krause DS, Vauti F, Arnold HH, Schroeder T,
Schnütgen F, et al: Single-Stranded DNA-Binding Transcriptional
Regulator FUBP1 Is Essential for Fetal and Adult Hematopoietic Stem
Cell Self-Renewal. Cell Rep. 11:1847–1855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dixit U, Liu Z, Pandey AK, Kothari R and
Pandey VN: Fuse binding protein antagonizes the transcription
activity of tumor suppressor protein p53. BMC Cancer. 14:9252014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dixit U, Pandey AK, Liu Z, Kumar S,
Neiditch MB, Klein KM and Pandey VN: FUSE Binding Protein 1
facilitates persistent hepatitis C virus replication in hepatoma
cells by regulating tumor suppressor p53. J Virol. 89:7905–7921.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jameson GS, Petricoin EF, Sachdev J,
Liotta LA, Loesch DM, Anthony SP, Chadha MK, Wulfkuhle JD,
Gallagher RI, Reeder KA, et al: A pilot study utilizing multi-omic
molecular profiling to find potential targets and select
individualized treatments for patients with previously treated
metastatic breast cancer. Breast Cancer Res Treat. 147:579–588.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rask K, Nilsson A, Brännström M, Carlsson
P, Hellberg P, Janson PO, Hedin L and Sundfeldt K: Wnt-signalling
pathway in ovarian epithelial tumours: Increased expression of
beta-catenin and GSK3beta. Br J Cancer. 89:1298–1304. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barghout SH, Zepeda N, Xu Z, Steed H, Lee
CH and Fu Y: Elevated β-catenin activity contributes to carboplatin
resistance in A2780cp ovarian cancer cells. Biochem Biophys Res
Commun. 468:173–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Murphy G and Nagase H: Localizing matrix
metalloproteinase activities in the pericellular environment. FEBS
J. 278:2–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zitka O, Kukacka J, Krizkova S, Huska D,
Adam V, Masarik M, Prusa R and Kizek R: Matrix metalloproteinases.
Curr Med Chem. 17:3751–3768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Butler GS and Overall CM: Updated
biological roles for matrix metalloproteinases and new
‘intracellular’ substrates revealed by degradomics. Biochemistry.
48:10830–10845. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rodríguez D, Morrison CJ and Overall CM:
Matrix metalloproteinases: What do they not do? New substrates and
biological roles identified by murine models and proteomics.
Biochim Biophys Acta. 1803:39–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hadler-Olsen E, Fadnes B, Sylte I,
Uhlin-Hansen L and Winberg JO: Regulation of matrix
metalloproteinase activity in health and disease. FEBS J.
278:28–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J and Chen QM: Far upstream element
binding protein 1: A commander of transcription, translation and
beyond. Oncogene. 32:2907–2916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang YY, Gu XL, Wang C, Wang H, Ni QC,
Zhang CH, Yu XF, Yang LY, He ZX, Mao GX and Yang SY: The
far-upstream element-binding protein 2 is correlated with
proliferation and doxorubicin resistance in human breast cancer
cell lines. Tumour Biol. 37:9755–9769. 2016. View Article : Google Scholar : PubMed/NCBI
|