Introduction
Cervical carcinoma, also known as invasive carcinoma
of cervix uteri, is a cancer arising from the cervix. It is ranked
as the second most common type of cancer among women worldwide
(1,2).
Furthermore, cervical cancer was associated with 275,000
mortalities in 2008, ~88% of which occurred in developing countries
(3). Therefore, the identification of
molecular genes involved in the regulation of cervical carcinoma
progression is warranted in order to develop novel therapeutic
approaches for this cancer.
Several molecular genes have been reported to be
associated with tumor progression, including deleted in pancreatic
carcinoma locus 4 (DPC4/Smad4), vascular endothelial growth factor
(VEGF) and thrombospondin-1 (TSP-1) (4). Loss of DPC4 contributes to the switch of
transforming growth factor (TGF)-β from a tumor-suppressive to a
tumor-promoting pathway in cancer (5). Previous research demonstrated that a
decrease in DPC4 mRNA expression is associated with lack of growth
inhibition in human cervical carcinoma (6). VEGF and TSP-1 are regulatory molecules
of angiogenesis (7), which is
essential for the progression of cervical carcinoma (8). High expression of VEGF is associated
with the degree of vascularization (9). In addition, the positive expression rate
of VEGF in cervical carcinoma is higher compared with that in
normal, inflammatory and cervical intraepithelial neoplasia (CIN)
cervix (10). It has also been
revealed that decreased TSP-1 expression correlates inversely with
microvessel count in cervical carcinoma (11) and alters tumor growth by modulating
angiogenesis (12). Furthermore,
recent research suggests that VEGF and TSP-1 are key target genes
for DPC4 (4), and the stable
expression of DPC4 downregulates the expression of VEGF in cervical
carcinoma (13). Although DPC4 and
VEGF have been identified to be associated with cervical carcinoma,
the involvement of the two with tumorigenesis, and the correlation
between DPC4 and VEGF/TSP-1 in cervical carcinoma remain
unclear.
In the present study, the expression levels of DPC4
and VEGF in cervical carcinoma were assessed in order to
investigate the association between DPC4, VEGF, and
clinicopathological characteristics of patients with cervical
carcinoma. Furthermore, the microvessel density (MVD) of each
cervical squamous-cell carcinoma (CSCC) sample was detected to
investigate the effect of DPC4, VEGF and TSP-1 on angiogenesis. In
addition, the correlation between DPC4, VEGF and TSP-1 was
investigated in order to elucidate the roles of DPC4 and VEGF in
the progression of tumorigenesis, and their possible associations
in carcinogenesis.
Materials and methods
Patients
The field study took place at the Second Clinical
Hospital of Jilin University (Changchun, China) between August 2012
and August 2013. A total of 115 patients with invasive cervical
carcinoma aged between 25 and 60 years (mean age, 46 years) were
surgically treated to remove a tumor. All the patients were treated
without radiotherapy, chemotherapy or other adjuvant therapy prior
to surgery, and did not recently use drugs that affected the
metabolism of prostaglandin and thromboxane. The 115 samples were
identified by three pathologists independently, and the final
diagnosis was confirmed when ≥2 of the same results were obtained
from the three pathologists. Lymph node metastasis and
classification of tumors were regarded as the criteria. The samples
included 19 cervical inflammation, 10 CIN I, 11 CIN II, 14 CIN III
and 61 CSCC. Local pathologists were asked to provide complete
clinical data of the patients with CSCC. The 61 CSCC samples, which
were staged according to the International Federation of Gynecology
and Obstetrics (14), were divided
into stage I (n=20), stage II (n=25) and stage III (n=16).
Pathological grade assessment was also performed, and the CSCC
samples were graded into G1 (n=18), G2 (n=26) and G3 (n=17)
according to the proportion of differentiated cells (15). A total of 21 lymph node metastasis
samples were involved in these CSCC samples. Finally, 12 fresh
normal cervix tissue samples, which were acquired from individuals
confirmed via necropsy to have succumbed to natural causes (and
therefore unassociated with cervical complications) at the Second
Clinical Hospital of Jilin University between August 2012 and
August 2013, were used as normal controls.
Written informed consent was obtained from all the
subjects or their parents. The present study was approved by the
Ethics Committee of the Second Clinical Hospital of Jilin
University and all study procedures were performed according to
ethical standards.
Immunohistochemical analysis
Excess tissue and moisture were removed from the
freshly obtained tissue samples. The samples were then dehydrated
routinely and embedded in paraffin. Embedded tissues were sliced
into 5-µm thick sections, deparaffinized in xylene followed by
treatment with 95, 70 and 50% ethanol, and rehydration with PBS (pH
7.4). Paraffin-embedded tissues were used for hematoxylin and eosin
staining, and immunohistochemical analysis. Pathological diagnosis
and pathological grade were established by two pathologists,
respectively. Sections analyzed for protein expression (DPC4, VEGF
and TSP-1) were microwaved for 5 min at 98°C in citrate buffer (pH
6.0) for antigen retrieval. Following these pretreatment
procedures, all samples were incubated with a solution of 1%
hydrogen peroxide in methanol for 15 min at room temperature to
block endogenous peroxidase. Sections were washed three times with
PBS (pH 7.5) and then incubated for 10 min at room temperature in
50 µl normal non-immune goat serum (YeSen Biotechnology Co., Ltd.,
Shanghai, China). The rabbit anti-human primary monoclonal
antibodies for DPC4, TSP-1 or VEGF (all at a dilution of 1:100;
Shanghai Westang Bio-Tech Co., Ltd., Shanghai, China) were applied
to the sections overnight at 4°C. The next day the sections were
rinsed three times in PBS and incubated for 10 min with the
addition of goat anti-rabbit peroxidase-conjugated secondary
antibody (1:50; Wuhan Boster Biological Technology, Ltd., Wuhan,
China) for 1 h at room temperature. Following rinsing three times
in PBS, the samples were incubated with 50 µl
streptavidin-peroxidase for another 10 min at room temperature.
Then, the samples were washed with PBS and visualized with 100 µl
stable 3,3′-diaminobenzidine (Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA) for 10 min at room temperature. The reaction
was stopped by washing with water and the sections were
counterstained with Gill No. 3 hematoxylin solution (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). Following rinsing in PBS, the
slides were dehydrated with 50, 70, 95% and absolute ethanol, and
mounted with neutral balsam. The slides were observed using an
optical microscope (CH20BIMF2000; Olympus Corporation, Tokyo,
Japan). Negative controls involved the same procedure; however, the
primary antibody was replaced with PBS. As a positive control, a
specimen of CSCC with strong expression of DPC4, VEGF or TSP-1 was
used.
Protein expression was determined by two independent
observers who semi-quantitatively assessed the staining intensity
and the percentage of stained tumor cells. The staining intensity
was rated as follows: 0 points, negative; 1 point, weak intensity;
2 points, moderate intensity; and 3 points, strong intensity. The
percentage of positive cells was rated as follows: 0 points, 0%
positive tumor cells; 1 point, <30% positive cells; 2 points,
30–60% positive cells; and 3 points, >60% positive cells. Points
of staining intensity and percentage of positive cells were added,
positive expression was regarded as ≥3 points.
ELISA
The protein expression levels of DPC4 and VEGF in
different pathological periods of CCSC were detected using an ELISA
kit (Wuhan Boster Biological Technology, Ltd.) according to the
manufacturer's protocol. The serum samples (100 µl) were obtained
by drawing peripheral blood and centrifuging for 10 min (1,760 × g
at 4°C). The substrate used as the enzyme conjugate was
tetramethylbenzidine. Optical density values were read at 450 nm.
Normal cervix samples were used as controls.
Quantification of MVD
The MVD of CSCC samples were determined according to
the Winder method (16). Microvessels
were highlighted by staining endothelial cells for Factor VIII
(FVIII) with immunohistochemistry. The most vascularized areas (hot
spots) with a high density of FVIII-positive cells were picked up
using a low power field magnification (×40). The MVD was quantified
using a ×200 magnification high-power field (×10 ocular and ×20
objective; field area; 0.25 mm2). All positively stained
discrete cells or cell clusters with, or without visible lumina
were counted as a microvessel. The branch structure of discrete
cells, which did not connect with the primary structure, was also
counted as a microvessel. However, vessels with a diameter greater
than the sum of eight red blood cells, which had an evident
muscular layer, were not counted. A total of three fields of view
in each sample were counted and the average was represented as the
MVD.
Statistical analysis
Statistical analysis was performed using SPSS 11.0
software (SPSS, Inc., Chicago, IL, USA). All data are expressed as
the mean ± standard deviation. Statistical significance of protein
expression (DPC4, TSP-1 or VEGF) in the different groups was
conducted using the χ2 test. In addition, paired t-tests
were performed to estimate the statistical significance of MVD.
Correlation analysis between DPC4 and VEGF/TSP-1 was conducted by
Spearman's correlations. P<0.05 was considered to indicate a
statistically significant difference.
Results
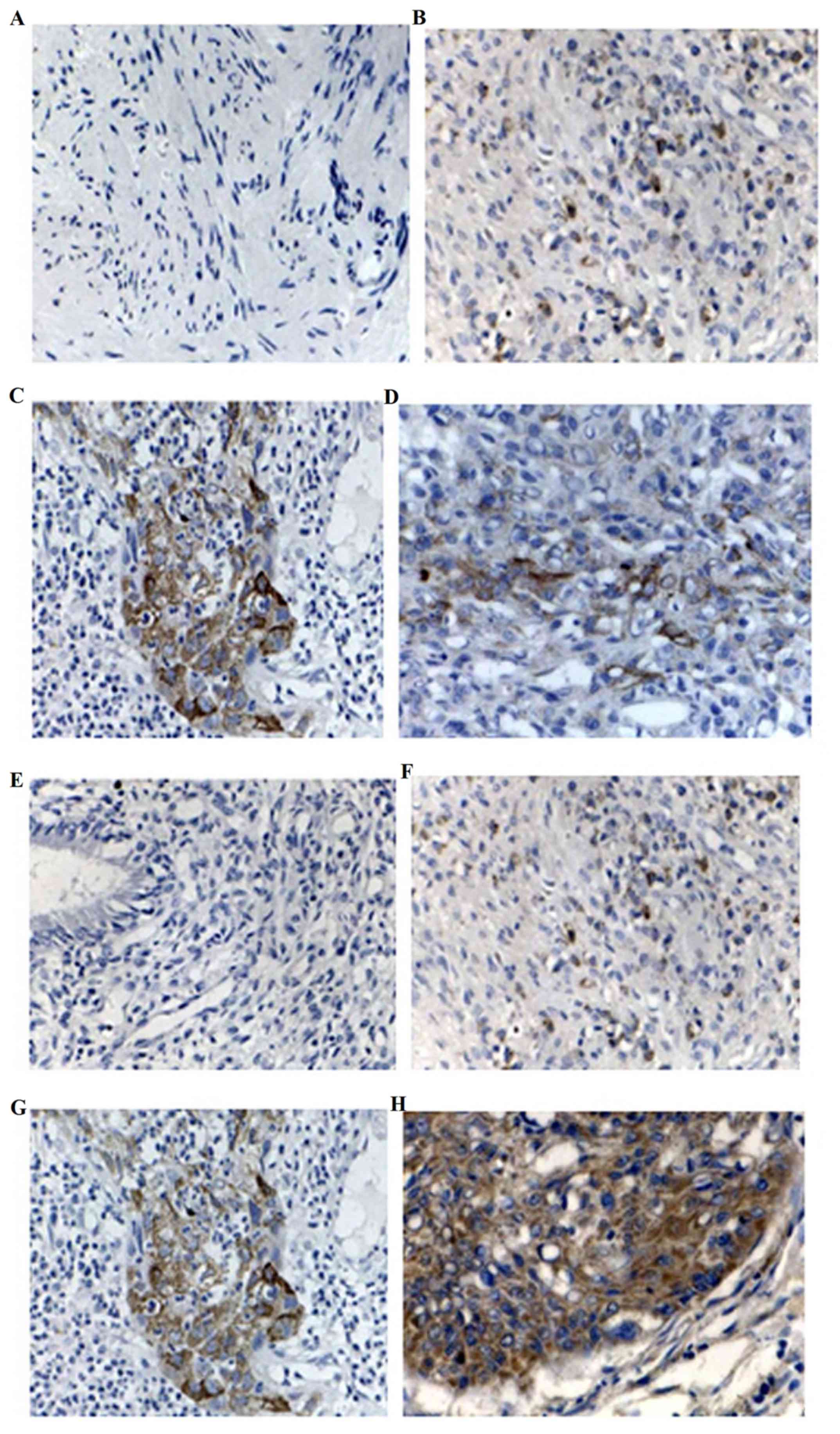
Protein expression rates of DPC4 and
VEGF detected using immunohistochemistry
The negative expression rates of DPC4 in the normal
control and chronic cervicitis groups were 0% (Fig. 1). The rates in the CIN I, CIN II and
CIN III groups were 10, 9 and 14.3% respectively, which were all
significantly lower compared with that in the CSCC group (40.9%;
P<0.05; Table I; Fig. 1). Regarding the expression of VEGF,
the positive expression rates in the CIN I (10%), CIN II (18.2%)
and CIN III (35.7%) groups were significantly lower compared with
that in the CSCC group (73.8%; P<0.05). Furthermore, no VEGF
expression was detected in the normal control and chronic
cervicitis groups.
 | Table I.Expression of DPC4 and VEGF in
cervical tissues of differential pathological types. |
Table I.
Expression of DPC4 and VEGF in
cervical tissues of differential pathological types.
|
|
| Negative expression
of DPC4 | Positive expression
of VEGF |
|---|
|
|
|
|
|
|---|
| Group | No. of cases | No. of cases | Negative rate, % | No. of cases | Positive rate, % |
|---|
| Normal cervix | 12 | 0 | 0.0a | 0 | 0.0a |
| Cervical
inflammation | 19 | 0 | 0.0a | 0 | 0.0a |
| CIN I | 10 | 1 | 10.0 | 1 | 10.0a |
| CIN II | 11 | 1 | 9.0a | 2 | 18.2a |
| CIN III | 14 | 2 | 14.3 | 5 | 35.7a |
| CCSC | 61 | 21 | 40.9 | 45 | 73.8a |
Association analysis between DPC4/VEGF
and the clinical pathology of CSCC
As presented in Fig.
1A, DPC4 protein was located in the cytoplasm and karyon of
normal cervical tissue, and irregularly distributed; however, the
protein was located primarily in the cytoplasm of CSCC tissue
(Fig. 1B) and was granulated. DPC4
protein staining exhibited claybank spot distribution. The negative
expression rate of DPC4 in the stage I group was 20%, significantly
lower compared with that in the stage II group (56.3%; P<0.05;
Table II). However, no statistical
difference was observed in pathological grade. In addition, the
DPC4 protein expression was associated with lymph node metastasis
(Table II), the negative rate in the
lymph node metastasis group (71.4%) was significantly higher
compared with that of the lymph node metastasis-negative group
(25.0%; P<0.05; Table II).
 | Table II.Expression of DPC4 and VEGF in CSCC
samples classified according to different clinicopathological
factors. |
Table II.
Expression of DPC4 and VEGF in CSCC
samples classified according to different clinicopathological
factors.
|
|
| Negative expression
of DPC4 | Positive expression
of VEGF |
|---|
|
|
|
|
|
|---|
| Clinicopathological
factor | No. of cases | No. of cases | Negative rate,
% | No. of cases | Positive rate,
% |
|---|
| Stage |
|
|
|
|
|
| I | 20 | 4 | 20.0 | 11 | 60.0b |
| II | 25 | 7 | 48.0 | 22 | 84.0 |
|
III | 16 | 8 | 56.3a | 12 | 75.0 |
| Grade |
|
|
|
|
|
| G1 | 18 | 5 | 22.2 | 13 | 72.2 |
| G2 | 26 | 10 | 38.5 | 19 | 73.1 |
| G3 | 17 | 10 | 64.7 | 13 | 76.5 |
| Lymph node
metastasis |
|
|
|
|
|
| R | 21 | 15 | 71.4c | 17 | 80.9c |
| F | 40 | 10 | 25.0 | 19 | 47.5 |
For VEGF, no expression was detected in normal
cervical tissues (Fig. 1E), but the
protein was located in the cytoplasm in CSCC (Fig. 1F) with granulated and dispersedly
distributed claybank spot staining. The positive expression rate of
VEGF in stage I was 60%, which was decreased significantly compared
with that in stage II (84%; P<0.05; Table II). However, no statistical
difference was observed in pathological grade. Furthermore, the
positive rate of VEGF protein expression in the lymph node
metastasis group (80.9%) was significantly higher compared with
that of the lymph node metastasis-negative group (47.5%; P<0.05;
Table II).
Expression levels of DPC4 and VEGF in
different clinical stages
As presented in Table
III, the protein expression level of DPC4 in stage I, II and
III were 39±2.01, 34±2.2, and 50±2.83, respectively, which were
significantly lower compared with that in the normal control group
(86±4.21; P<0.05). However, for VEGF, the expression level in
stage I (90±4.40), stage II (106±4.89) and stage III (70±3.89)
increased significantly compared with the normal control group
(26±1.83; P<0.05).
 | Table III.Expression of DPC4 and VEGF in
different stage of cervical squamous-cell carcinoma. |
Table III.
Expression of DPC4 and VEGF in
different stage of cervical squamous-cell carcinoma.
| Expression | Normal cervix | Stage I | Stage II | Stage III |
|---|
| DPC4 | 86±4.21 |
39±2.01a,b |
34±2.20a |
50±2.83a |
| VEGF | 26±1.83 |
90±4.40a,b |
106±4.89a |
70±3.89a |
Association between DPC4, VEGF, TSP-1
and tumor angiogenesis
As presented in Table
IV, in CSCC samples, the MVD of the DPC4 (−) group (8.38±3.15)
was significantly higher compared with that of the DPC4 (+) group
(11.28±3.55; P<0.05). The MVD of the VEGF (−) group (10.51±3.90)
was significantly lower compared with the VEGF (+) group
(15.37±4.59; P<0.01). In addition, the MVD of the TSP-1 (−)
group (15.37±3.10) was significantly higher compared with the TSP-1
(+) group (9.31±2.4; P<0.01).
 | Table IV.Mean MVD of cervical squamous-cell
carcinoma samples with positive/negative expression of DPC4, VEGF
or TSP-1. |
Table IV.
Mean MVD of cervical squamous-cell
carcinoma samples with positive/negative expression of DPC4, VEGF
or TSP-1.
| Expression | No. of cases | MVD (mean ±
SD) | dR |
|---|
| DPC4 |
|
| −0.76 |
|
Negative | 22 | 18.38±3.15 |
|
|
Positive | 39 |
11.28±3.55a |
|
| VEGF |
|
| 0.48 |
|
Negative | 18 | 10.51±3.90 |
|
|
Positive | 43 |
15.37±4.59a |
|
| TSP-1 |
|
| −0.58 |
|
Negative | 16 | 15.37±3.10 |
|
|
Positive | 45 |
9.31±2.40a |
|
| DPC4 and TSP-1 |
|
|
|
| DPC4
(+) TSP-1 (−) | 16 |
11.49±3.89b |
|
| DPC4
(−) TSP-1 (+) | 24 | 12.74±3.59 |
|
| DPC4
(+) TSP-1 (+) | 21 |
19.34±3.55b |
|
| DPC4 and VEGF |
|
|
|
| DPC4
(+) VEGF (−) | 18 |
10.4±3.89c |
|
| DPC4
(−) VEGF (+) | 22 | 18.4±3.25 |
|
| DPC4
(+) VEGF (+) | 21 |
11.74±3.89c |
|
Statistical analysis demonstrated that the MVD in
the DPC4 (−) TSP-1 (+) group (12.74±3.59) was significantly
increased compared with the DPC4 (+) TSP-1 (−) group (11.49±3.89),
and significantly decreased compared with the DPC4 (+) TSP-1 (+)
group (19.38±3.35; P<0.01). The MVD of DPC4 (−) VEGF (+) group
(18.38±3.25) was significantly higher compared with the DPC4 (+)
VEGF (+) group (11.74±3.89) and the DPC4 (+) VEGF (−) group
(10.39±3.89; P<0.01).
A negative correlation was identified between the
expression of DPC4 (r=−0.762) and TSP-1 (r=−0.578), and tumor
angiogenesis (both P<0.01; Table
IV). However, a positive correlation between VEGF expression
and tumor angiogenesis was reported (r=0.478; P<0.01; Table IV).
Correlation between DPC4, VEGF and
TSP-1
As presented in Table
V, the expression of DPC4 was identified to be negatively
correlated with VEGF (r=−0.486; P<0.01) and TSP-1 (r=−0.480;
P<0.01).
 | Table V.Correlation between DPC4 and VEGF or
TSP-1. |
Table V.
Correlation between DPC4 and VEGF or
TSP-1.
|
| VEGF | TSP-1 |
|---|
|
|
|
|
|---|
| DPC4 | + | − | R | P-value | + | − | R | P-value |
|---|
| + | 21 | 18 | −0.49 | <0.01 | 21 | 16 | 0.48 | <0.01 |
| − | 22 | 0 |
|
| 24 | 0 |
|
|
Discussion
Cervical carcinoma is a common type of gynecologic
cancer caused by various factors. However, the mechanism underlying
tumorigenesis remains to be elucidated. Loss of DPC4 and
overexpression of VEGF have been reported to be associated with
cervical carcinoma carcinogenesis (17,18). Thus,
DPC4 and VEGF may be candidate molecules that contribute to the
progression of cervical carcinoma. In the present study, it was
demonstrated that the negative expression rate of DPC4 and positive
expression rate of VEGF in the CSCC group were increased
significantly compared with that in the normal, inflammatory, and
CIN groups (P<0.05). In addition, DPC4 (−) and VEGF (+) were
associated with clinical stages, and lymph node metastasis.
Furthermore, the expression levels of DPC4 and TSP-1 were
identified to be negatively associated with angiogenesis. However,
the expression of VEGF and angiogenesis were positively associated
(P<0.01). The expression of DPC4 was negatively associated with
VEGF and with TSP-1 (P<0.01).
DPC4 is a tumor suppressor gene frequently
inactivated in various types of carcinoma, including cervical
carcinoma (19–22). Reduced or lost expression of DPC4 is
frequently observed during cancer progression (13). In the present study, it was revealed
that DPC4 was negatively expressed in CIN and CSCC tissues, and the
negative expression rate increased gradually along with the
progression of cervical carcinoma, corresponding with a study by
Baldus et al (17). The
results of the present study suggest that DPC4 is associated with
the tumorigenesis of cervical carcinoma. Furthermore, previous
evidence has reported that DPC4 alterations are associated with the
specific loss of TGF-β-induced growth resulting in increased
angiogenesis (23,24). In the present study, the results
demonstrated that the negative expression of DPC4 was associated
with angiogenesis. In addition, it has been reported that DPC4
restoration can inhibit angiogenesis in different carcinoma types,
including colon and pancreatic (25),
lung (26) and pancreatic (4) cancer. These results indicate that the
loss of DPC4 may contribute to cervical carcinoma progression via
angiogenesis.
VEGF is one of the most important angiogenesis
factors in regulating angiogenesis. Its expression is associated
with MVD (27) and a high expression
level has been detected in numerous human tumor types, including
lung (28), colon (29), and gastric (30) cancer. In the present study, a high
positive expression rate and high level of VEGF were detected in
cervical carcinoma. Furthermore, these results revealed that the
expression of VEGF was positively associated with angiogenesis. As
angiogenesis serves an important role in the progression of
tumorigenesis, VEGF may participate in the tumorigenesis of
cervical carcinoma by inducing angiogenesis.
As a potent inhibitor of neovascularization, TSP-1
also serves a key role in regulating angiogenesis. Reduced
expression of TSP-1 can switch normal cells to an angiogenic
phenotype, thus progressing cells towards malignancy (31). Overexpression of TSP-1 can reduce MVD
and inhibit the growth of prostate cancer cells (32). The results of the present study
reported that the MVD in the TSP-1 (−) group was significantly
higher compared with that of the TSP-1 (+) group, indicating that
angiogenesis of cervical carcinoma may be suppressed by TSP-1.
Consistent with these results, a previous study demonstrated that
microvessel counts are significantly higher when decreased TSP-1
mRNA expression is evident in cervical carcinoma (11). Therefore, TSP-1 may inhibit the
angiogenesis of cervical carcinoma.
Due to the observations of decreased DPC4 expression
and VEGF overexpression in the progression of angiogenesis, the
correlation between DPC4, and VEGF was analyzed. The results
demonstrated that they were associated. Correspondingly, it has
been reported that carcinomas with high DPC4 expression are
accompanied with low VEGF expression (26). Increased expression of DPC4 results in
decreased expression levels of VEGF, shifting cells from an
angiogenic to antiangiogenic phenotype in various cancer types,
including pancreatic (4),
gastrointestinal (19) and lung
carcinoma (10). Furthermore, the
loss of DPC4 has been demonstrated to enhance VEGF protein
expression in human ovarian cancer cells (13). DPC4 expression is negatively
associated with VEGF-C expression in colon cancer (33). The results of the aforementioned
studies are all consistent with the results of the present study.
Therefore, the loss of DPC4 may induce VEGF expression, increasing
angiogenesis and consequently promoting the progression of cervical
carcinoma. Although it is not yet clear how DPC4 modulates VEGF
expression, DPC4 may be a candidate target for inhibiting cancer
progression.
The association between DPC4 and TSP-1 was also
investigated as TSP-1 is involved in angiogenesis, and may be a
target gene of DPC4. The results demonstrated that the expression
of DPC4 and TSP-1 was negatively associated, which suggested that
the loss of DPC4 may induce the expression of TSP-1. Although
reduced DPC4 expression and high TSP-1 expression are common in
various cancer types (34–37), few studies have investigated the
association between DPC4 and TSP-1 in tumorous tissue. Furthermore,
contrary to the results of the present study, Schwarte-Waldhoff
et al (4) indicated that DPC4
restoration may increase the expression of TSP-1 in human
pancreatic adenocarcinoma cells. The correlation between DPC4 and
TSP-1 in cervical carcinoma remains to be elucidated with further
investigations required.
In conclusion, the loss of DPC4 and overexpression
of VEGF may play important roles in the cervical carcinoma
progression. The conceivable mechanism may involve the loss of
DPC4, which induces angiogenesis by increasing VEGF expression,
subsequently promoting the progression of cervical carcinoma. The
results of the present study suggest that VEGF is a target gene
regulated by DPC4. Furthermore, the negative expression of TSP-1
contributes to angiogenesis, and the correlation between DPC4 and
TSP-1 requires further investigation.
References
|
1
|
Arbyn M, Castellsagué X, de Sanjosé S,
Bruni L, Saraiya M, Bray F and Ferlay J: Worldwide burden of
cervical cancer in 2008. Ann Oncol. 22:2675–2686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: GLOBOCAN 2008 v2.0, Cancer incidence and
mortality worldwide: IARC CancerBase No. 10 [Internet].
International Agency for Research on Cancer, Lyon. 2010.29;2010.
http://globocan.iarc.fr/Default.aspxJuly
12–2012
|
|
3
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwarte-Waldhoff I, Volpert OV, Bouck NP,
Sipos B, Hahn SA, Klein-Scory S, Lüttges J, Klöppel G, Graeven U,
Eilert-Micus C, et al: Smad4/DPC4-mediated tumor suppression
through suppression of angiogenesis. Proc Natl Acad Sci USA. 97:pp.
9624–9629. 2000; View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao S, Venkatasubbarao K, Lazor JW,
Sperry J, Jin C, Cao L and Freeman JW: Inhibition of STAT3 Tyr705
phosphorylation by Smad4 suppresses transforming growth factor
beta-mediated invasion and metastasis in pancreatic cancer cells.
Cancer Res. 68:4221–4228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee S, Cho YS, Shim C, Kim J, Choi J, Oh
S, Kim J, Zhang W and Lee J: Aberrant expression of Smad4 results
in resistance against the growth-inhibitory effect of transforming
growth factor-beta in the SiHa human cervical carcinoma cell line.
Int J Cancer. 94:500–507. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carmeliet P: Mechanisms of angiogenesis
and arteriogenesis. Nat Med. 6:389–395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith-McCune K, Zhu YH, Hanahan D and
Arbeit J: Cross-species comparison of angiogenesis during the
premalignant stages of squamous carcinogenesis in the human cervix
and K14-HPV16 transgenic mice. Cancer Res. 57:1294–1300.
1997.PubMed/NCBI
|
|
9
|
Veikkola T, Karkkainen M, Claesson-Welsh L
and Alitalo K: Regulation of angiogenesis via vascular endothelial
growth factor receptors. Cancer Res. 60:203–212. 2000.PubMed/NCBI
|
|
10
|
Dai Y, Zhang X, Peng Y and Wang Z: The
expression of cyclooxygenase-2, VEGF and PGs in CIN and cervical
carcinoma. Gynecol Oncol. 97:96–103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kodama J, Hashimoto I, Seki N, Hongo A,
Yoshinouchi M, Okuda H and Kudo T: Thrombospondin-1 and-2 Messenger
RNA Expression in Invasive Cervical Cancer: Correlation with
Angiogenesis and Prognosis. Clin Cancer Res. 7:2826–2831.
2001.PubMed/NCBI
|
|
12
|
Ren B, Yee KO, Lawler J and Khosravi-Far
R: Regulation of tumor angiogenesis by thrombospondin-1. Biochim
Biophys Acta. 1765:178–188. 2006.PubMed/NCBI
|
|
13
|
Chen C, Sun MZ, Liu S, Yeh D, Yu L, Song
Y, Gong L, Hao L, Hu J and Shao S: Smad4 mediates malignant
behaviors of human ovarian carcinoma cell through the effect on
expressions of E-cadherin, plasminogen activator inhibitor-1 and
VEGF. BMB Rep. 43:554–560. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lewin SN, Herzog TJ, Medel NI Barrena,
Deutsch I, Burke WM, Sun X and Wright JD: Comparative performance
of the 2009 international Federation of gynecology and obstetrics'
staging system for uterine corpus cancer. Obstet Gynecol.
116:1141–1149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Broders AC: Squamous cell epithelioma of
the skin: A study of 256 cases. Ann Surg. 73:141–160. 1921.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baldus SE, Schwarz E, Lohrey C, Zapatka M,
Landsberg S, Hahn SA, Schmidt D, Dienes HP, Schmiegel WH and
Schwarte-Waldhoff I: Smad4 deficiency in cervical carcinoma cells.
Oncogene. 24:810–819. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Van Trappen PO, Steele D, Lowe DG, Baithun
S, Beasley N, Thiele W, Weich H, Krishnan J, Shepherd JH, Pepper
MS, et al: Expression of vascular endothelial growth factor
(VEGF)-C and VEGF-D, and their receptor VEGFR-3, during different
stages of cervical carcinogenesis. J Pathol. 201:544–554. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyaki M, Iijima T, Konishi M, Sakai K,
Ishii A, Yasuno M, Hishima T, Koike M, Shitara N, Iwama T, et al:
Higher frequency of Smad4 gene mutation in human colorectal cancer
with distant metastasis. Oncogene. 18:3098–3103. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kloth JN, Kenter GG, Spijker HS, Uljee S,
Corver WE, Jordanova ES, Fleuren GJ and Gorter A: Expression of
Smad2 and Smad4 in cervical cancer: Absent nuclear Smad4 expression
correlates with poor survival. Mod Pathol. 21:866–875. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hahn SA, Bartsch D, Schroers A, Galehdari
H, Becker M, Ramaswamy A, Schwarte-Waldhoff I, Maschek H and
Schmiegel W: Mutations of the DPC4/Smad4 gene in biliary tract
carcinoma. Cancer Res. 58:1124–1126. 1998.PubMed/NCBI
|
|
22
|
Bartsch D, Hahn SA, Danichevski KD,
Ramaswamy A, Bastian D, Galehdari H, Barth P, Schmiegel W, Simon B
and Rothmund M: Mutations of the DPC4/Smad4 gene in neuroendocrine
pancreatic tumors. Oncogene. 18:2367–2371. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elliott RL and Blobe GC: Role of
transforming growth factor Beta in human cancer. J Clin Oncol.
23:2078–2093. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyazono K, Suzuki H and Imamura T:
Regulation of TGF-beta signaling and its roles in progression of
tumors. Cancer Sci. 94:230–234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schwarte-Waldhoff I and Schmiegel W: Smad4
transcriptional pathways and angiogenesis. Int J Gastrointest
Cancer. 31:47–59. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ke Z, Zhang X, Ma L and Wang L: Expression
of DPC4/Smad4 in non-small-cell lung carcinoma and its relationship
with angiogenesis. Neoplasma. 55:323–329. 2008.PubMed/NCBI
|
|
27
|
Kim MH, Seo SS, Song YS, Kang DH, Park IA,
Kang SB and Lee HP: Expression of cyclooxygenase-1 and-2 associated
with expression of VEGF in primary cervical cancer and at
metastatic lymph nodes. Gynecol Oncol. 90:83–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hasturk S, Kemp B, Kalapurakal SK, Kurie
JM, Hong WK and Lee JS: Expression of cyclooxygenase-1 and
cyclooxygenase-2 in bronchial epithelium and nonsmall cell lung
carcinoma. Cancer. 94:1023–1031. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tokunaga T, Oshika Y, Abe Y, Ozeki Y,
Sadahiro S, Kijima H, Tsuchida T, Yamazaki H, Ueyama Y, Tamaoki N
and Nakamura M: Vascular endothelial growth factor (VEGF) mRNA
isoform expression pattern is correlated with liver metastasis and
poor prognosis in colon cancer. Br J Cancer. 77:998–1002. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maeda K, Chung YS, Ogawa Y, Takatsuka S,
Kang SM, Ogawa M, Sawada T and Sowa M: Prognostic value of vascular
endothelial growth factor expression in gastric carcinoma. Cancer.
77:858–863. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dameron KM, Volpert OV, Tainsky MA and
Bouck N: Control of angiogenesis in fibroblasts by p53 regulation
of thrombospondin-1. Science. 265:1582–1584. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin RJ, Kwak C, Lee SG, Lee CH, Soo CG,
Park MS, Lee E and Lee SE: The application of an anti-angiogenic
gene (thrombospondin-1) in the treatment of human prostate cancer
xenografts. Cancer Gene Ther. 7:1537–1542. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Liu B, Xiao J, Yuan Y, Ma J and
Zhang Y: Roles of VEGF-C and Smad4 in the lymphangiogenesis,
lymphatic metastasis, and prognosis in colon cancer. J Gastrointest
Surg. 15:2001–2010. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alvarez AA, Axelrod JR, Whitaker RS, Isner
PD, Bentley RC, Dodge RK and Rodriguez GC: Thrombospondin-1
expression in epithelial ovarian carcinoma: Association with p53
status, tumor angiogenesis, and survival in platinum-treated
patients. Gynecol Oncol. 82:273–278. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grossfeld GD, Ginsberg DA, Stein JP,
Bochner BH, Esrig D, Groshen S, Dunn M, Nichols PW, Taylor CR,
Skinner DG and Cote RJ: Thrombospondin-1 expression in bladder
cancer: Association with p53 alterations, tumor angiogenesis, and
tumor progression. J Natl Cancer Inst. 89:219–227. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grant SW, Kyshtoobayeva AS, Kurosaki T,
Jakowatz J and Fruehauf JP: Mutant p53 correlates with reduced
expression of thrombospondin-1, increased angiogenesis, and
metastatic progression in melanoma. Cancer Detect Prev. 22:185–194.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tokunaga T, Nakamura M, Oshika Y, Tsuchida
T, Kazuno M, Fukushima Y, Kawai K, Abe Y, Kijima H, Yamazaki H, et
al: Alterations in tumour suppressor gene p53 correlate with
inhibition of thrombospondin-1 gene expression in colon cancer
cells. Virchows Arch. 433:415–418. 1998. View Article : Google Scholar : PubMed/NCBI
|