Introduction
Pancreatic cancer is one of the most fatal tumors,
with a 5-year survival rate of 3–6% (1). An estimated 48,960 new cases of
pancreatic cancer and 40,560 mortalities from pancreatic cancer
occurred in the United States of America in 2015, making this
cancer type the fourth leading cause of cancer-associated mortality
(2). Surgery is the only reported
cure for pancreatic cancer; however, the resectability rate is only
18% due to its high rate of vascular invasion and metastasis
(3). Radiotherapy and chemotherapy
may extend survival time to some extent, but their overall
effectiveness remains limited (4).
Major histocompatibility complex (MHC) class II
expression has previously been demonstrated in a proportion of
patients with pancreatic cancer, and has been shown to be
correlated with better prognosis (5).
Class II MHC transactivator-induced expression of MHC class II can
inhibit pancreatic cancer metastasis and improve prognosis
(6); however, the specific molecular
mechanisms underlying MHC class II expression in pancreatic cancer
remain unclear. Previous studies have demonstrated that regulatory
factor X-associated protein (RFXAP) is a key transcription factor
for the synthesis of MHC class II molecules in dendritic cells
(DCs) (7). Exosomal microRNA
(miRNA/miR)-212-3p secreted by pancreatic cancer cells can inhibit
RFXAP expression in DCs, and thus suppress MHC class II expression
and promote the immune escape of pancreatic tumors (8). This may explain why MHC class II
expression is downregulated in pancreatic cancer, which leads to
poor outcomes. Interferon (IFN)-γ has been demonstrated to induce
MHC class II expression in immune cells, including DCs (9); however, whether IFN-γ can stimulate
pancreatic cancer cells to express RFXAP and MHC class II molecules
has not been reported, to the best of our knowledge. Therefore, we
hypothesized that IFN-γ may inhibit the expression of miR-212-3p in
pancreatic cancer and thereby increase the expression of RFXAP and
MHC class II molecules. To the best of our knowledge, this is a
novel mechanism for the effect of IFN-γ on pancreatic cancer, and
is the first observed correlation between RFXAP deficiency and
tumor progression. The data from the present study may aid with the
development of immunological and biological treatments for patients
with pancreatic cancer, and improve their prognosis.
Materials and methods
Cell culture
The human pancreatic ductal adenocarcinoma (PDAC)
cell lines, PANC-1, CFPAC-1, SW1990, BxPC-3 and MIAPaCa-2 were
obtained from the Chinese Academy of Sciences (Shanghai, China).
The cell lines were cultured in 25-cm2 cell culture
flask at 37°C in a humidified atmosphere of 5% CO2 with
RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The cell lines were harvested
following treatment with IFN-γ (1,000 IU/ml; Peprotech, Inc., Rocky
Hill, NJ, USA) or equal amounts of PBS for 24 h at 37°C in a
humidified atmosphere of 5% CO2. Human embryonic kidney
293T cells obtained from the Chinese Academy of Sciences (Shanghai,
China) were maintained in Dulbecco's modified Eagle's medium
(Corning Incorporated, Corning, New York, USA) supplemented with
10% fetal bovine serum under the aforementioned conditions.
Cell transfection
Cells were washed with PBS and switched to FBS-free
growth medium for 24–48 h prior to transfection. Cells were
transiently transfected with human miR-212-3p mimics or inhibitors,
or negative controls (NC) (cat. nos. miR10000269-1-5,
miR20000269-1-5, miR01201-1-5 and miR02201-1-5; Guangzhou Ribo Bio
Co., Ltd., Guangzhou, China). Briefly, 2×105 cells were
seeded in 6-well plates. miR-212-3p mimics (final concentration, 50
nM) or inhibitors (final concentration, 100 nM) were transfected
into the cells using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. At 48 h after transfection, the cells of each group were
harvested for polymerase chain reaction (PCR) and western blot
analyses.
Luciferase assay
293T cells were co-transfected with pLMP vectors
(Invitrogen; Thermo Fisher Scientific, Inc.) containing the
wild-type RFXAP3′-untranslated region (CUG AAA ACU GUU) or mutant
sequences (AAU GAC GUA UCU), and miRNA mimics or inhibitors. The
potential binding sequences of miR-212-3p on the RFXAP 3′UTR were
mutated using the QuikChange™ Site-Directed Mutagenesis Kit
(Stratagene, La Jolla, CA, United States). Cells were harvested and
subjected to lysis at 48 h following transfection. Renilla
luciferase activity was used for normalization, and firefly
luciferase activity was detected with a Dual-Luciferase Reporter
Assay kit (Promega Corporation Madison, WI, USA), according to the
manufacturer's protocol.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA from the cells was isolated and purified
using an RNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA),
according to the manufacturer's protocol. The concentration,
purity, and amount of total RNA were quantified using a Nano Drop
spectrophotometer (ND-1000 version 3.5.2 software; Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). Reverse transcriptase
reactions contained RNA samples, 50 nM stem-loop RT primer
(miRQ0000269-1-2, Ribo Bio Co., Ltd., Guangzhou, China), 1× RT
buffer (Applied Biosystems; Thermo Fisher Scientific, Inc.), 0.25
mM each of dNTPs, 3.33 U/ml MultiScribe reverse transcriptase
(Applied Biosystems; Thermo Fisher Scientific, Inc.), and 0.25 U/ml
RNase inhibitor (P/N:N8080119; Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reverse transcriptase reactions conditions
were as follows: 16°C for 30 min, 42°C for 30 min, and 85°C for 5
min. qPCR was performed on an Applied Biosystems® 7500
Fast Real-Time PCR System (Thermo Fisher Scientific, Inc.),
including 2 µl of RT product, 10 µl of Power SYBR Green PCR Master
Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.), 4 µl of
RNase-free water, and 2 µl forward and reverse primers. The qPCR
thermocycling conditions were as follows: 95°C for 10 min, followed
by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The relative
expression ratio of miR-212-3p and RFXAP was presented as
the fold change, which was normalized to an endogenous reference
(U6 RNA) using the 2−ΔΔCq method (10). The primers were purchased from
Guangzhou Ribo Bio Co., Ltd., and the sequences were as follows:
miR-212-3p forward, 5′-GGTAACAGTCTCCAGTCA-3′, and reverse,
5′-GCAATTGCACTGGATACG-3′; RFXAP forward,
5′-CAGTAGAATTCGGCCAAGCAGGTGCTAAAAG-3′, and reverse,
5′-CAGAGGATCCATGTAGATGTTCTTGGTAAG-3′. Experiments were performed in
triplicate.
Western blot analysis
Cells were lysed with RIPA buffer (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) containing a protease inhibitor
cocktail (Thermo Fisher Scientific, Inc.). The lysates were cleared
by centrifugation at 1.2×104 × g at 4°C for 15 min, and
the concentrations of proteins were measured using a BCA protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.). The proteins
were denatured in 2X SDS buffer at 95°C and 10 µl of proteins were
loaded per well to a 10% SDS-PAGE gel, then transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% skim milk powder for 1 h
at room temperature and probed with the following antibodies
overnight at 4°C: RFXAP (dilution, 1:200; cat. no. ab172281; Abcam,
Cambridge, UK), or MHC class II (dilution, 1:500; cat. no. ab17101;
Abcam), or β-actin (dilution, 1:1,000; cat. no. ab8226; Abcam). The
samples were incubated with secondary goat anti-mouse antibodies
conjugated to horseradish peroxidase (dilution, 1:5,000; ab6789;
Abcam.) for 1 h at room temperature. The blots were visualized by
GE Healthcare enhanced chemiluminescence kit (GE Healthcare,
Chicago, IL, USA) using Kodak X-OMATLS film (Eastman Kodak,
Rochester, NY, USA). Each sample was measured three times.
Statistical analysis
All data are presented as mean ± standard deviation
and were analyzed using a Student's t-test. P<0.05 was
considered to indicate a statistically significant difference. All
data were analyzed using SPSS software (version 19.0; IBM Corp.,
Armonk, NY, USA) and GraphPad Prism 6.0 software (GraphPad
Software, Inc., La Jolla, CA, USA).
Results
miR-212-3p is down regulated by IFN-γ
in PDAC cell lines
RT-qPCR analysis demonstrated that the expression of
miR-212-3p was markedly reduced in IFN-γ-stimulated PDAC cells
compared with the control, particularly in PANC-1 cells (Fig. 1A, P<0.05). A time-course analysis
of miR-212-3p expression in PANC-1 cells following IFN-γ treatment
revealed that miR-212-3p expression decreased over time during the
first 24 h after treatment (Fig. 1B,
P<0.05). In addition, the decrease in miR-212-3p expression was
dose-dependent between 1 and 1,000 U/ml IFN-γ (Fig. 1C, P<0.05).
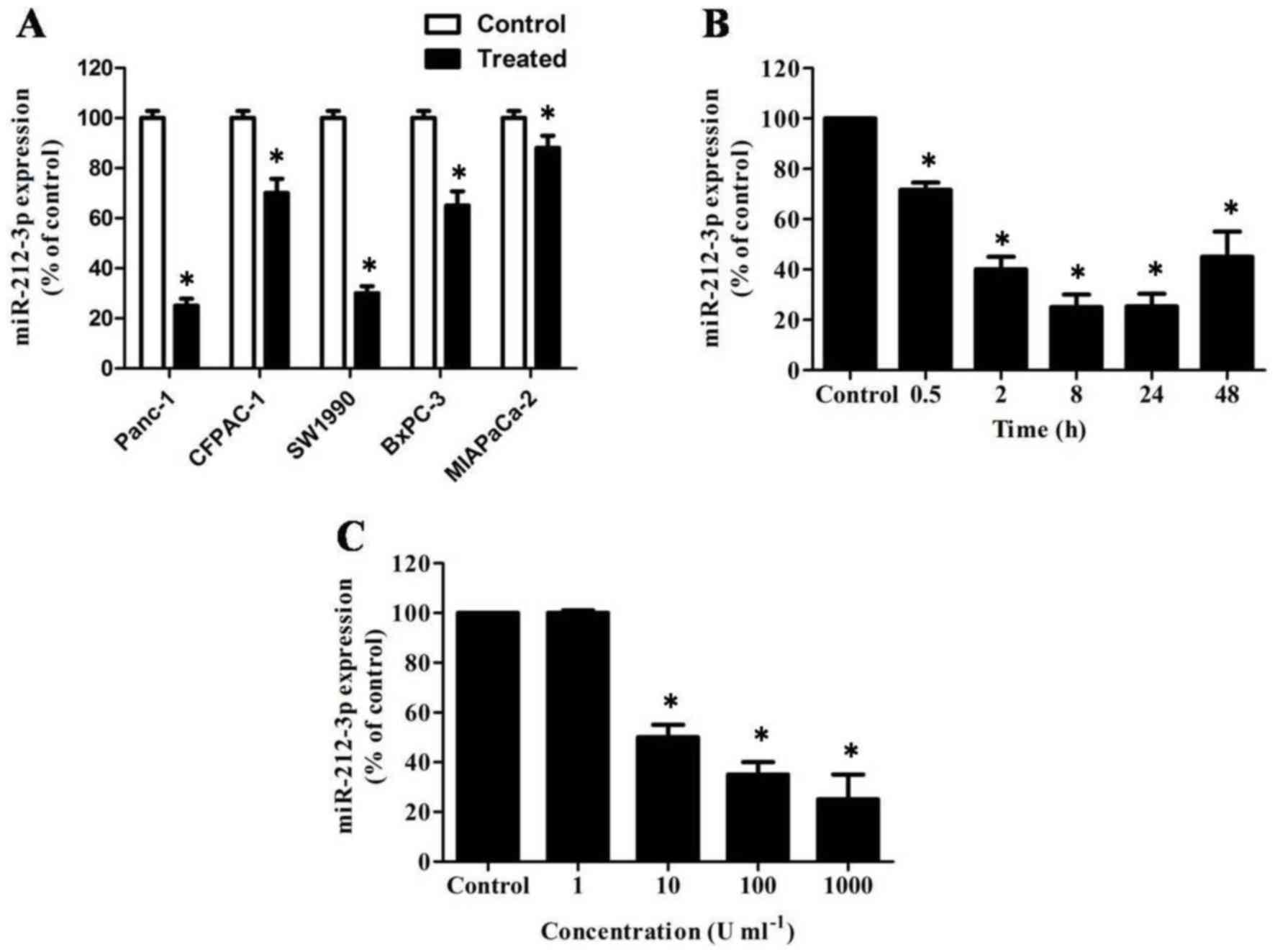 | Figure 1.miR-212-3p is downregulated by IFN-γ
in PDAC cell lines. (A) RT-qPCR analysis of relative miR-212-3p
expression in IFN-γ- or PBS-treated PDAC cell lines, including
PANC-1, CFPAC-1, SW1990, BxPC-3 and MIAPaCa-2. miR-212-3p was
markedly reduced in IFN-γ-stimulated PDAC cells. Transcript levels
were normalized to U6 expression. (B) Time-course of miR-212-3p
induction by IFN-γ. PANC-1 cells were stimulated with 1,000 U/ml
IFN-γ for the indicated times and miR-212-3p expression was
quantified by RT-qPCR. miR-212-3p expression decreased over time
during the first 24 h after treatment. (C) Dose-response analysis
of miR-212-3p induction by IFN-γ. Decreased expression of
miR-212-3p was observed between 1 and 1,000 U/ml IFN-γ. PANC-1
cells were stimulated with the indicated doses of IFN-γ for 24 h
and miR-212-3p expression was quantified by RT-qPCR. miR, microRNA;
IFN-γ, interferon-γ; PDAC, pancreatic ductal adenocarcinoma;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction. *P<0.05 vs. PBS stimulated PDAC cells. |
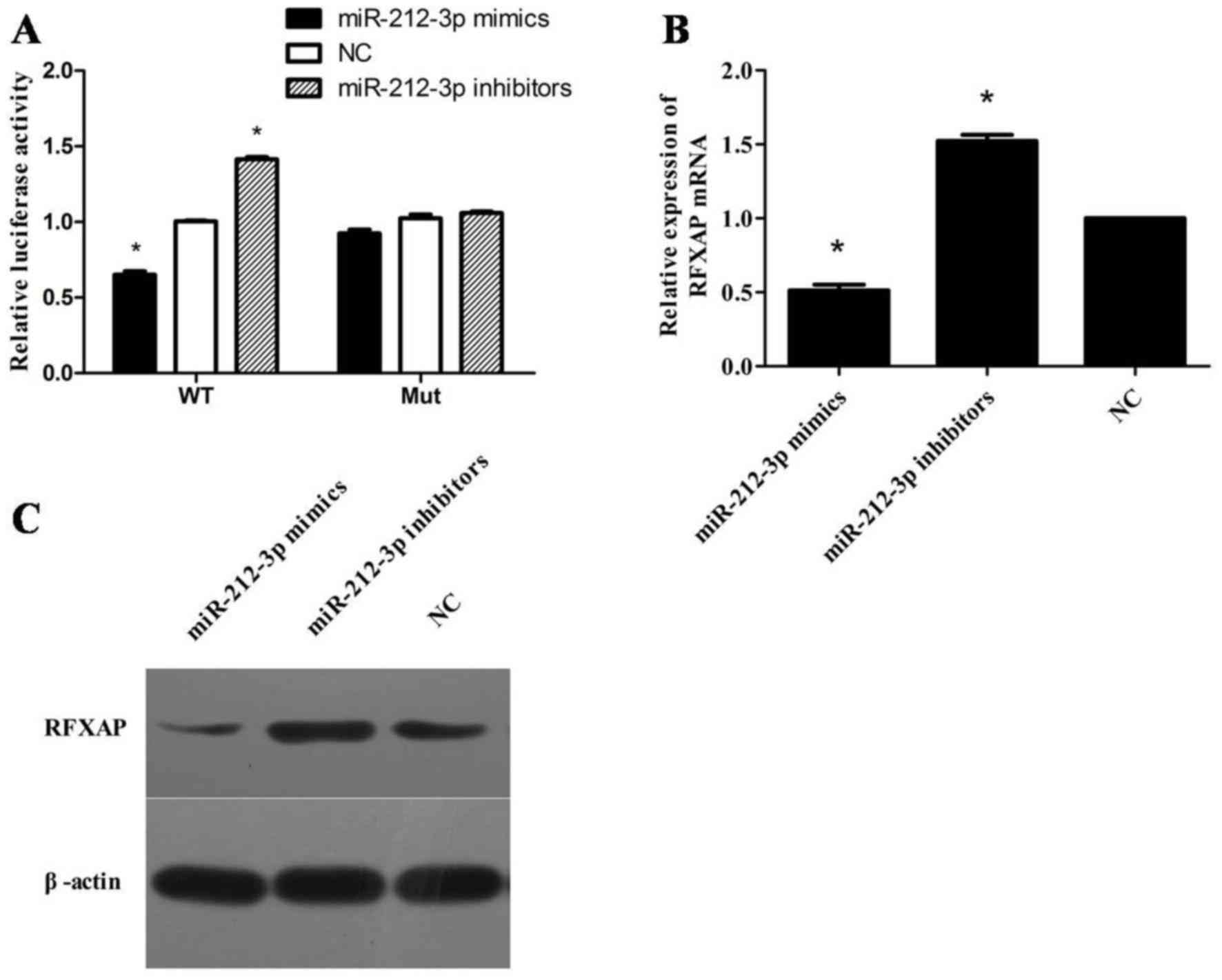
RFXAP is a direct target of miR-212-3p
in PANC-1 cells
A dual-luciferase activity assay demonstrated that
miR-212-3p mimics significantly suppressed the luciferase activity
of reporter vectors containing the wild-type RFXAP 3′-UTR
but not in the mutant (P<0.05; Fig.
2A). To assess whether miR-212-3p has a functional role in the
downregulation of RFXAP, the expression level of miR-212-3p
was manipulated in in PANC-1 cells. RT-qPCR analysis demonstrated
that overexpression of miR-212-3p significantly inhibited
RFXAP and MHC class II mRNA expression in PANC-1 cells,
while inhibition of miR-212-3p exhibited opposite effects
(P<0.05; Fig. 2B). Western blot
analysis demonstrated that overexpression of miR-212-3-p resulted
in a marked decrease in RFXAP protein levels, whereas inhibition of
miR-212-3p resulted in a marked increase in RFXAP protein levels
(Fig. 2C).
IFN-γ increases RFXAP and MHC class II
expression by inhibiting miR-212-3p in PANC-1 cells
Western blot analysis revealed that RFXAP was
markedly up regulated following treatment with IFN-γ, which was
concomitant with an upregulation in MHC class II expression
(Fig. 3A). However, following
transfection withmiR-212-3pmimics, IFN-γ stimulation did not
increase the expression of RFXAP or MHC class II (Fig. 3B). These results suggest that IFN-γ
increases RFXAP and MHC class II expression by inhibiting the
expression of miR-212-3p in PANC-1 cells.
Discussion
As an immune adjuvant, IFN-γ has demonstrated some
efficacy in treating pancreatic cancer (11,12). It
can enhance the immunogenicity of tumor vaccines and promote the
immune response of antigen-specific helper T-cells (13). When used in combination with
chemotherapeutic drugs, including gemcitabine, it can effectively
prolong survival time for patients with pancreatic cancer (14). Thus far, few studies have explored the
molecular mechanisms by which IFN-γ suppresses the proliferation
and metastasis of pancreatic cancer cells. Detjen et al
(15) demonstrated that IFN-γ
suppressed the proliferation of pancreatic cancer cells through the
caspase-1-mediated apoptosis pathway. In the present study, IFN-γ
upregulated RFXAP and MHC class II expression in the PDAC cell line
PANC-1. This novel mechanism provides experimental evidence for the
use of IFN-γ in adjuvant treatment for pancreatic cancer, which may
improve patient prognosis.
Human MHC class II molecules mainly comprise human
leukocyte antigen-DP, -DQ, and -DR antigens, which are primarily
expressed in antigen-presenting cells, including DCs and
monocytes/macrophages (16). The
regulatory factor X (RFX) complex, which contains regulatory factor
X-associated ankyrin-containing protein, RFXAP and regulatory
factor X 5, is a key initiating component that regulates MHC class
II transcription (17). RFXAP is the
core transcription factor of the RFX complex, and its normal
expression and functioning are essential for the transcription of
MHC class II molecules (18).
RFXAP mutation or deletion may result in bare lymphocyte
syndrome (18). As tumor cells
typically lack the specific mechanism to directly activate cluster
of differentiation (CD) 8+ T-cells via MHC class I
molecules, the MHC class II molecule-activated CD4+
T-cells serve a key role in anti-tumor immunity (19,20).
Certain tumor cells, including pancreatic cancer cells, can also
express MHC class II molecules, which is associated with relatively
good prognosis, suggesting that activation of MHC class II
expression could inhibit pancreatic cancer metastasis and improve
prognosis (21). The present study
confirmed that IFN-γ upregulates MHC class II expression in
pancreatic cancer cells, and thus provided evidence for use of
IFN-γ as an immunological and biological therapy for pancreatic
cancer.
A previous study demonstrated miR-212-3p involved in
the generation of pancreatic cancer-derived exosomes and could
suppress RFXAP protein expression in DCs and thus elicit DC immune
tolerance (8). Other studies have
also demonstrated the key function of miR-212-3p in pancreatic
cancer metastasis; Park et al (22) demonstrated that miR-212 expression
increased in pancreatic cancer cells and promoted their
proliferation by inhibiting Retinoblastoma 1 gene expression in a
targeted manner. In addition, Ma et al (23) demonstrated that miR-212 promoted tumor
cell proliferation and infiltration by the targeted inhibition of
Patch-1 gene expression. Data from the present study confirmed that
IFN-γ could inhibit miR-212-3p expression in pancreatic cancer
cells, and thus upregulate the expression of RFXAP and MHC class
II. Following transfection with miR-212-3p mimics, IFN-γ could not
stimulate expression of RFXAP and MHC class II, which implies that
IFN-γ stimulates MHC class II expression by suppressing miR-212-3p
and thereby upregulating RFXAP expression. To the best of our
knowledge, this function of IFN-γ had not been reported previously.
IFN-γ treatment in patients may inhibit miR-212-3p expression,
promote RFXAP and MHC class II expression, and thus lead to immune
effects on pancreatic cancer cells and improve the prognosis of
patients.
Surgery is currently the only curative treatment for
patients with pancreatic cancer. Together with post-operative
chemotherapy, it may achieve a 5-year survival rate of 15–40%.
However, only 10–15% of patients with pancreatic cancer are
candidates for radical resection as ~85% of patients with
pancreatic cancer initially present with local major vessel
invasion or distant metastasis (24).
Although chemotherapy, radiotherapy and interventional treatment
have extended the survival time of patients with pancreatic cancer,
the overall effectiveness of these approaches remains limited
(25). Immunotherapies, biological
therapies and adjuvant therapies may extend survival times for
pancreatic cancer. The present study revealed that IFN-γ treatment
of tumor cells can promote RFXAP expression. RFXAP is not
only a key transcription factor for MHC class II expression but may
also promote the expression of other tumor suppressor genes. IFN-γ
may serve an important role in tumor immunotherapy by promoting
RFXAP expression and therefore the targeting of downstream
genes. However, as genes associated with RFXAP transcription
have not been identified, further studies are required to
investigate the roles of RFXAP in tumorigenesis.
In conclusion, IFN-γ may inhibit miR-212-3p
expression in pancreatic cancer, leading to upregulation of RFXAP
and MHC class II. This may reflect a novel molecular mechanism
underlying the effects of IFN-γ on pancreatic cancer.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (grant no. 81772548), Scientific Research Fund
of Zhejiang Provincial Education Department (grant no. Y201534694)
and the Natural Science Foundation of Zhejiang Province (grant no.
LY16H160007).
Glossary
Abbreviations
Abbreviations:
|
RFXAP
|
regulatory factor X-associated
protein
|
|
MHC
|
major histocompatibility complex
|
|
IFN
|
interferon
|
|
DCs
|
dendritic cells
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
References
|
1
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morganti AG, Massaccesi M, La Torre G,
Caravatta L, Piscopo A, Tambaro R, Sofo L, Sallustio G, Ingrosso M,
Macchia G, et al: A systematic review of resectability and survival
after concurrent chemoradiation in primarily unresectable
pancreatic cancer. Ann Surg Oncol. 17:194–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liao WC, Chien KL, Lin YL, Wu MS, Lin JT,
Wang HP and Tu YK: Adjuvant treatments for resected pancreatic
adenocarcinoma: A systematic review and network meta-analysis.
Lancet Oncol. 14:1095–1103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gaida MM, Welsch T and Herpel E: MHC class
II expression in pancreatic tumors: A link to intratumoral
inflammation. Virchows Arch. 460:47–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sartoris S, Valle MT, Barbaro AL, Tosi G,
Cestari T, D'Agostino A, Megiovanni AM, Manca F and Accolla RS: HLA
class II expression in uninduciblehepatocarcinoma cells after
transfection of AIR-1 gene product CIITA: Acquisition of antigen
processing and presentation capacity. J Immunol. 161:814–820.
1998.PubMed/NCBI
|
|
7
|
van Eggermond MC, Tezcan I, Heemskerk MH
and van den Elsen PJ: Transcriptional silencing of RFXAP in MHC
class II-deficiency. Mol Immunol. 45:2920–2928. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding G, Zhou L, Qian Y, Fu M, Chen J, Chen
J, Xiang J, Wu Z, Jiang G and Cao L: Pancreatic cancer-derived
exosomes transfer miRNAs to dendritic cells and inhibit RFXAP
expression via miR-212-3p. Oncotarget. 6:29877–29888. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blanck G: Components of the IFN-gamma
signaling pathway in tumorigenesis. Arch Immunol Ther Exp.
50:151–158. 2002.
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Einhorn S and Strander H: Interferon
treatment of human malignancies-a short review. Med Oncol Tumor
Pharmacother. 10:25–29. 1993.PubMed/NCBI
|
|
12
|
Vihinen P, Tervahartiala T, Sorsa T,
Hansson J, Bastholt L, Aamdal S, Stierner U, Pyrhönen S, Syrjänen
K, Lundin J and Hernberg M: Benefit of adjuvant interferon alfa-2b
(IFN-α) therapy in melanoma patients with high serum MMP-8 levels.
Cancer Immunol Immunother. 64:173–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boehm U, Klamp T, Groot M and Howard JC:
Cellular responses to interferon-gamma. Annu Rev Immunol.
15:749–795. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Endou M, Mizuno M, Nagata T, Tsukada K,
Nakahara N, Tsuno T, Osawa H, Kuno T, Fujita M, Hatano M and
Yoshida J: Growth inhibition of human pancreatic cancer cells by
human interferon-beta gene combined with gemcitabine. Int J Mol
Med. 15:277–283. 2005.PubMed/NCBI
|
|
15
|
Detjen KM, Farwig K, Welzel M, Wiedenmann
B and Rosewicz S: Interferon gamma inhibits growth of human
pancreatic carcinoma cells via caspase-1 dependent induction of
apoptosis. Gut. 49:251–262. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Accolla RS, Scupoli MT, Cambiaggi C, Tosi
G and Sartoris S: Cell lineage-specific and developmental
stage-specific controls of MHC class-II-antigen expression. Int J
Cancer Suppl. 6:20–25. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garvie CW and Boss JM: Assembly of the RFX
complex on the MHCII promoter: Role of RFXAP and RFXB in relieving
autoinhibition of RFX5. Biochim Biophys Acta. 1779:797–804. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hanna S and Etzioni A: MHC class I and II
deficiencies. J Allergy Clin Immunol. 134:269–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scupoli MT, Sartoris S, Tosi G, Ennas MG,
Nicolis M, Cestari T, Zamboni G, Martignoni G, Lemoine NR, Scarpa A
and Accolla RS: Expression of MHC class I and class II antigens in
pancreatic adenocarcinomas. Tissue Antigens. 48:301–311. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Surmann EM, Voigt AY, Michel S, Bauer K,
Reuschenbach M, Ferrone S, von Knebel Doeberitz M and Kloor M:
Association of high CD4-positive T cell infiltration with mutations
in HLA class II-regulatory genes in microsatellite-unstable
colorectal cancer. Cancer Immunol Immunother. 64:357–366. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gaida MM, Welsch T, Herpel E,
Tschaharganeh DF, Fischer L, Schirmacher P, Hänsch GM and Bergmann
F: MHC class II expression in pancreatic tumors: A link to
intratumoral inflammation. Virchows Arch. 460:47–60. 2015.
View Article : Google Scholar
|
|
22
|
Park JK, Henry JC, Jiang J, Esau C, Gusev
Y, Lerner MR, Postier RG, Brackett DJ and Schmittgen TD: miR-132
and miR-212 are increased in pancreatic cancer and target the
retinoblastoma tumor suppressor. Biochem Biophys Res Commun.
406:518–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma C, Nong K, Wu B, Dong B, Bai Y, Zhu H,
Wang W, Huang X, Yuan Z and Ai K: miR-212 promotes pancreatic
cancer cell growth and invasion by targeting the hedgehog signaling
pathway receptor patched-1. J Exp Clin Cancer Res. 33:542014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:2140–2141. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karakhanova S, Ryschich E, Mosl B, Harig
S, Jäger D, Schmidt J, Hartwig W, Werner J and Bazhin AV:
Prognostic and predictive value of immunological parameters for
chemoradioimmunotherapy in patients with pancreatic adenocarcinoma.
Br J Cancer. 112:1027–1036. 2015. View Article : Google Scholar : PubMed/NCBI
|