Introduction
Calophyllum inophyllum L. (C.
inophyllum) of the family Clusiaceae is an associated mangrove
species and is located in the tropical regions of Asia. In addition
to its traditional use in treating skin problems, including rashes,
ulcers, pimples, cuts, wounds and sores, the leaf extracts function
as antioxidant, antidyslipidemic and anti-inflammatory agents
(1,2).
The seeds (tonka beans) of C. cerasiferum Vesque and C.
inophyllum contain the highest levels of coumarins
(2H-1-benzopyran-2-one) (3),
which are phenolic substances composed of fused benzene and
α-pyrone rings that are secondary metabolites found in plants,
bacteria and fungi. Environmental conditions and seasonal changes
may affect the incidence of coumarins in various parts of the plant
(4). Among coumarins, a number of
compounds have been reported to exhibit anticancer properties,
including imperatorin (5), esculetin
(6) and osthole, which inhibits the
migration and invasion of breast cancer cells, potentially via the
inhibition of matrix metalloproteinase promoter and enzymatic
activity (7). Another type of
4-phenylcoumarins isolated from C. inophyllum is
calocoumarin A (8).
Colon cancer and small-cell and non-small-cell lung
cancer (NSCLC) are important types of human cancer. Colon cancer
arises in the colon or rectum due to the abnormal growth of cells
with the ability to invade or spread to other tissue sites
(9). In 2012, there were 1.4 million
new cases of colon cancer and 694,000 associated mortalities from
the disease (10). Therefore, it is
necessary to develop novel strategies to increase the anticancer
effects of colon cancer treatments. Lung cancer is considered to be
the most common type of cancer in the world (11) and remains a major global health
problem, accounting for 1.8 million annual mortalities worldwide in
2012 (12) and 17.8% of all
cancer-associated mortalities in India in 2002 (13). Activation of the epidermal growth
factor receptor (EGFR) signaling pathway in cancer cells was
reported to able to inhibit apoptosis and induce cell
proliferation, angiogenesis and metastasis, leading to a poor
disease prognosis (14).
The seed oil from C. inophyllum changes in
color from yellow to green due to processing and storage
conditions. The seed oil contains numerous compounds. The present
study investigated the effects of the yellow and green pigments
from the seed oil of C. inophyllum for the treatment of
colon cancer and NSCLC in combination with gefitinib. These results
may provide a rationale to utilize the pigments with or without
gefitinib for the treatment of colon cancer and lung cancer.
Materials and methods
Chemicals and reagents
Trifluoroacetic acid (TFA) and deuterated methanol
were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
The LiChrospher 100 RP-18e column (4.0 mm i.d. ×250 mm; 5 µm) was
purchased from Merck KGaA. Methanol and acetonitrile were Liquid
Chromatography LiChrosolv® and were purchased from Merck
KGaA. High-performance liquid chromatography (HPLC) was performed
using Waters 1525 binary HPLC pump, Waters in-line degasser AF,
Waters 717 plus autosampler, and Waters 2487 dual λ absorbance
detector (Waters Corporation, Milford, MA, USA). The proton nuclear
magnetic resonance (1H-NMR) spectrum was evaluated using
a Bruker Avance DRX500 instrument (Bruker Corporation, Billerica,
MA, USA) at 500 MHz.
Analysis of seed oil pigments from C.
inophyllum L
The seeds were collected from the coast of Chiayi
County and heated to a range of temperatures from 70 to 105°C for
at least 1 h to obtain green and yellow seed oil. The preliminary
analysis indicated that, aside from fatty acids, the pigments were
the major compounds in the seed oils, as determined by infrared
spectrum analysis (Agilent 6890 N GC-FID; Agilent Technologies,
Inc., Santa Clara, CA, USA). The pigment solution (10 µl) was
dissolved in 990 µl absolute methanol. The solution was centrifuged
at 9,400 × g for 10 min at room temperature. The supernatant was
separated using a LiChrospher 100 RP-18e (4 mm i.d. ×250 mm, 5 µm;
Merck KGaA) column with the following conditions: A mobile phase of
0.05% TFA-CH3CN (23:77), a flow rate of 1.0 ml/min and a
column temperature maintained at 40°C and detected at 280 nm. An
aliquot of sample (10 µl) was dissolved in 600 µl of
methanol-d4 completely and poured into the NMR tube. The
1H-NMR spectrum was measured using a Bruker Avance
DRX500 instrument (Bruker Corporation) at 500 MHz.
Effects of the pigments on the DLD-1
human colon cancer cell line
The DLD-1 human colon cancer cell line was obtained
from the Bioresource Collection and Research Center (Hsinchu,
Taiwan). RPMI-1640 was obtained from Hyclone (GE Healthcare, Logan,
UT, USA). Fetal bovine serum (FBS) was purchased from Gibco (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Propidium iodide (PI),
MTT, trypan blue and other chemicals were purchased from
Sigma-Aldrich; Merck KGaA.
The DLD-1 cells were cultured in RPMI-1640 medium
supplemented with 10% FBS, 100 U/ml penicillin G and 100 µg/ml
streptomycin. The cells were maintained at 37°C in humidified air
containing 5% CO2. The culture medium was replaced every
two days (15). The cells were plated
in 60 mm culture dishes for all experiments. The culture medium was
replaced with new medium when the cells reached 80% confluence, and
then the cells were exposed to 0, 0.19, 0.38, 0.75, 1.50 and
3.00×10−3% of the yellow pigment and 0, 1.6, 3.1, 6.3,
12.5 and 25.0×10−3% of the green pigment for 24, 48 and
72 h at 37°C. Following treatment, the cells were treated with MTT
solution to determine cell viability and propidium iodide solution
to determine cell cycle.
Cell viability was evaluated by MTT assay, as
previously described (16). The
yellow MTT was reduced to purple formazan by dehydrogenase in the
mitochondria of living cells. A solubilization agent, dimethyl
sulfoxide (DMSO), was added to dissolve the insoluble purple
formazan product and to form a colored solution. The absorbance of
this colored solution can be quantified using a specific wavelength
(550–600 nm) and a spectrophotometer. The cells (1×106)
were cultured in 60 mm tissue culture dishes at 37°C for 24 h. The
culture medium was replaced with new medium, and the cells were
exposed to various concentrations, as above, of the green or yellow
pigments at 37°C for 24, 48 and 72 h. Following treatment, the
cells were incubated with 0.5 mg/ml MTT for 2 h at room temperature
and then lysed with DMSO at room temperature for 5 min. A 200 µl
aliquot of the resulting solution was then removed from the 60 mm
culture dishes and transferred to 96-well plates. The absorbance of
the solution in the 96-well plates was evaluated at 595 nm using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
The analyses of DNA damage and the cell cycle were
performed by staining with PI and flow cytometry, as previously
described (17,18). The cells (1×106) were
cultured in 60 mm tissue culture dishes at 37°C for 24 h. The RPMI
1640 culture medium (Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA) was replaced with fresh medium, and the cells were exposed
to 0.60, 0.75 and 1.50×10−3% of the yellow pigment and
5.0, 12.5 and 25.0×10−3% of the green pigment at 37°C
for 48 h. Subsequently, the cells were pooled, washed with
phosphate-buffered saline (PBS), fixed in a PBS-methanol
(volume/volume, 1:2) solution at room temperature for 5 min and
then incubated at 4°C for at least 18 h. Following washing with PBS
once, the cell pellets were stained with the PI solution
supplemented with PBS, 40 µg/ml PI and 40 µg/ml of DNase-free RNase
A (Sigma-Aldrich; Merck KGaA) for 30 min at room temperature in the
dark and then analyzed using a Becton-Dickinson FACScan flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA). A total of
≥10,000 cells were counted per sample, and the DNA histograms were
further evaluated using the Modfit software version no. 3.2 (BD
Biosciences, Franklin Lakes, NJ, USA) on a PC workstation to
determine the percentage of cells in various phases of the cell
cycle and to quantify cells with DNA damage.
Effects of the pigments on the A549
and H1975 human lung carcinoma cell lines
The A549 and H1975 human lung carcinoma cell lines
were obtained from the American Type Culture Collection (Manassas,
VA, USA) and the cells were cultured at 37°C in a humidified
atmosphere containing 5% CO2 in RPMI-1640 complete
medium supplemented with sodium bicarbonate (w/v, 2.2%),
L-glutamine (w/v, 0.03%), penicillin (100 U/ml), streptomycin (100
µg/ml) and fetal calf serum (10%; Gibco; Thermo Fisher Scientific,
Inc.). The cell lines were routinely analyzed to confirm that the
cells were free of Mycoplasma. The cells were cultured at
5,000 cells per well in 96-well tissue culture plates at 37°C for
24 h in a humidified atmosphere with 5% CO2. To
determine cell viability, 0.05, 0.10, 0.25, 0.50 and 1.00% of the
green and yellow pigments of C. inophyllum alone or
gefitinib (IressaR, ZD1839; AstraZeneca, London, UK) in
combination with 0.00 and 0.05% green pigment, respectively, were
introduced to the cells following plating. At the end of the
culture period, 20 µl MTS solution (CellTiter 96 Aqueous One
Solution Cell Proliferation Assay; Promega, Madison, WI, USA) was
added per well, and the cells were incubated for an additional 2 h
at 37°C. The absorbance was then evaluated at 490 nm using an ELISA
plate reader (Bio-Rad Laboratories, Inc.).
Statistical analyses
For each protocol, three to four independent
experiments were performed. The results are presented as the mean ±
standard deviation. The statistical analyses were performed using
the SigmaPlot 2000 software version 11.0 (Systat Software, San
Jose, CA, USA). The differences in the evaluated variables between
the experimental and control groups were assessed by the unpaired
t-test, and comparisons between multiple groups were assessed using
two-way analysis of variance and Tukey's honest significant
difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Differences in compounds contained in
yellow and green pigments obtained from seed oil of C.
inophyllum
The green and yellow pigments were obtained from the
same batch of seeds using different processing methods. The present
study hypothesized that both pigments contained identical compounds
but with differing concentrations. Therefore, the pigments were
dissolved in methanol and the nuclear magnetic resonance spectra
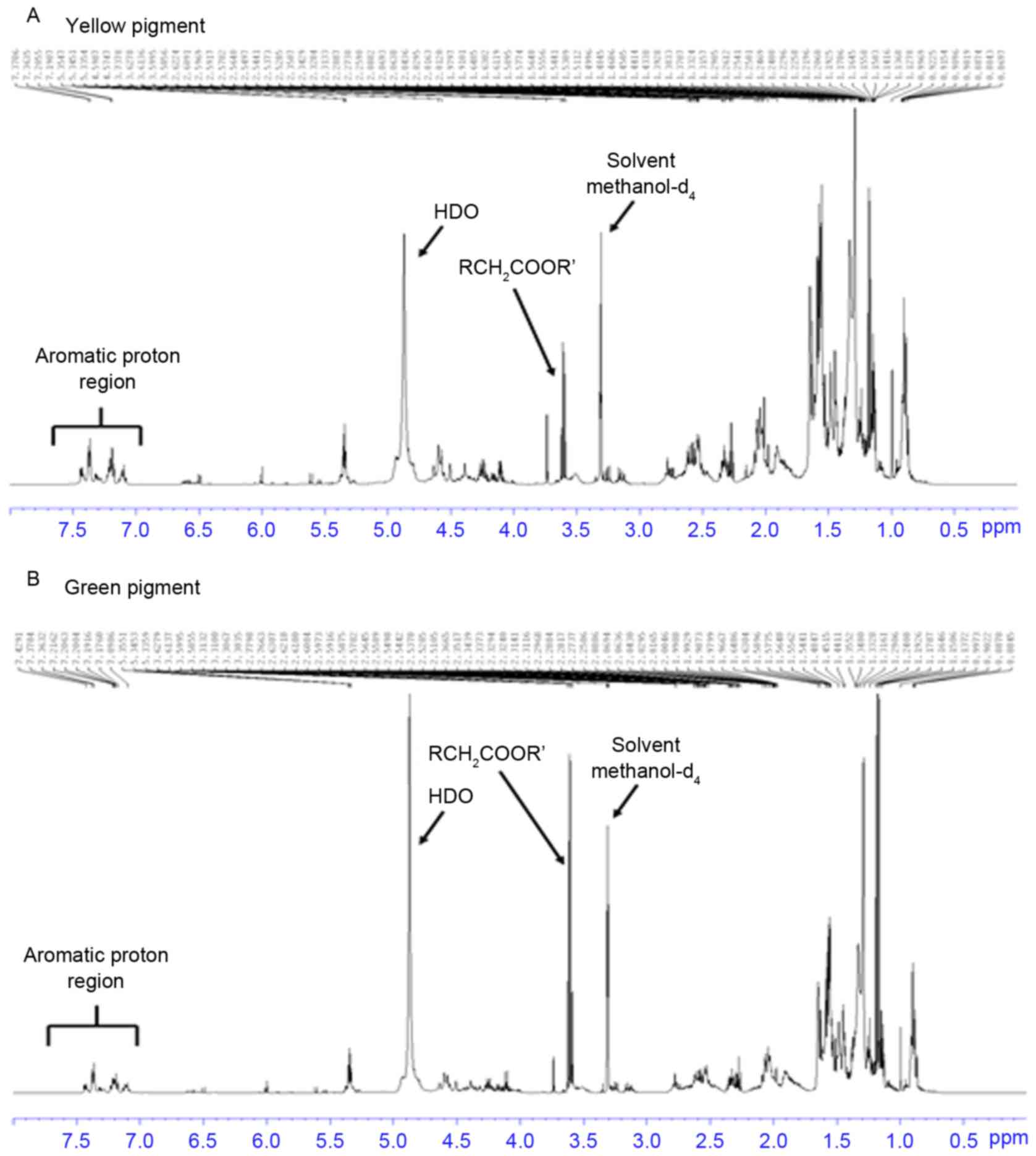
were valuated. The results demonstrated that the pigments contained
similar aromatic protons at 7.0–7.5 ppm (Fig. 1). HPLC analysis at 280 nm indicated
multiple types of aromatic compounds in the pigments. The green and
yellow pigments exhibited identical banding patterns (Fig. 2). The analysis of 9 major compounds
determined that the concentrations were the same for peaks 8 and 9
in green and yellow pigments. The concentrations were higher for
peaks 4 and 5 in the yellow pigment compared with the same peaks in
the green pigment. By contrast, the concentrations of the remaining
7 peaks were higher in the green pigment compared with the yellow
pigment (Table I). Due to these
differences, the present study evaluated the effects of the yellow
and green pigments on two major cancer cell types, including the
DLD-1 human colon carcinoma cell line and the A549 and H1975 human
NSCLC cell lines.
 | Table I.Differences in the concentration of
nine major compounds between the yellow and green pigments. |
Table I.
Differences in the concentration of
nine major compounds between the yellow and green pigments.
|
|
| Yellow pigment | Green pigment |
|---|
|
|
|
|
|
|---|
| Item | Major peak | RT | Area | Area (%) | RT | Area | Area (%) |
|---|
| 1 | 8 | 6.469 | 31937683 | 16.26 | 6.500 | 13641377 | 15.74 |
| 2 | 9 | 7.046 | 11713133 | 5.96 | 7.067 | 5735710 | 6.62 |
| 3 | 11 | 7.708 | 7969980 | 4.06 | 7.731 | 5522168 | 6.37 |
| 4 | 13 | 10.223 | 42321622 | 21.54 | 10.338 | 14476792 | 16.71 |
| 5 | 14 | 11.635 | 60684041 | 30.89 | 11.788 | 24102810 | 27.82 |
| 6 | 15 | 13.354 | 6879517 | 3.50 | 13.449 | 4574100 | 5.28 |
| 7 | 16 | 14.132 | 6423519 | 3.27 | 14.228 | 5804760 | 6.70 |
| 8 | 18 | 15.078 | 4736314 | 2.41 | 15.162 | 3212602 | 3.71 |
| 9 | 20 | 16.053 | 11111070 | 5.66 | 16.105 | 9580900 | 11.06 |
Yellow and green pigments inhibit the
viability of DLD-1 cells
The treatment of the DLD-1 human colon carcinoma
cell line with the green pigment at concentrations of 0, 5, 12.5 or
25×10−3% or the yellow pigment at concentrations of 0,
0.6, 0.75, 1.5 or 3.0×10−3% for 24, 48 and 72 h resulted
in a dose-dependent decrease in cell viability with both pigments
as determined by the MTT assay (Fig.
3). The yellow and green pigments differed in their effects on
cell viability. Following 48 and 72 h, there was a 40% decrease in
cell viability as compared with the untreated cells when treated
with the green pigment at a concentration of 12.5×10−3%,
whereas there was a 30% decrease in cell viability as compared with
the untreated cells when treated with the green pigment at a
concentration of 1.5×10−3%.
Treatment with pigments from seed oil
of C
inophyllum induces apoptosis and G2/M
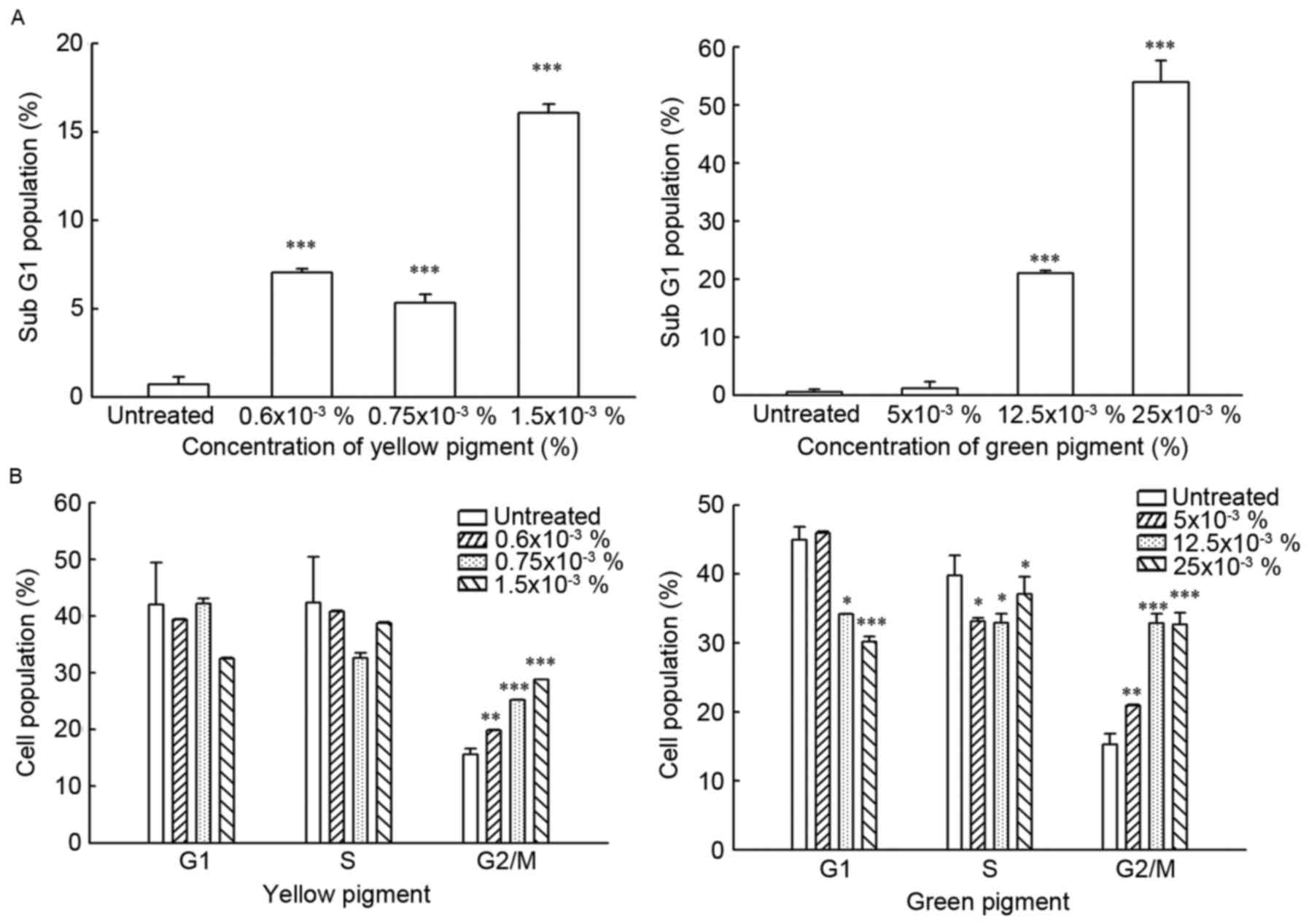
cell cycle arrest in DLD-1 cells. To evaluate whether the green or
yellow pigments are able to induce apoptosis or arrest cell cycle,
the DLD-1 cells were exposed to the green pigment at concentrations
0, 5, 12.5 and 25×10−3% or the yellow pigment at
concentrations 0, 0.6, 0.75, 1.5 and 3.0×10−3% for 24 h.
The fraction of cells associated with apoptosis and DNA damage
(sub-G1) was evaluated by PI staining and flow
cytometry. DLD-1 is a colorectal adenocarcinoma cell line, which
was isolated by D.L. Dexter and associates during 1977–1979
(19). DLD-1 cells are positive for
p53 antigen expression. DLD-1 cells also express metastatic and
invasive characteristics, and may be transplanted into nude mice.
For these reasons, the DLD-1 cells were selected in the present
study.
As presented in Fig.
4A, treatment with the yellow pigment was able to induce an
increased fraction of DLD-1 cells in the sub-G1 phase
(increased to 16% with 1.5×10−3%, compared with 0.6% in
the untreated cells). Treatment with the green pigment was able to
induce a sharp increase of DLD-1 cells in the sub-G1
phase (2 to 57% with 5×10−3 and 25×10−3%
green pigment, respectively, as compared with 0.3% in the untreated
cells) at 37°C for 24 h treatments.
Although the portion of cells in the G1
and S cell cycle phases did not change significantly when treated
with the three different concentrations of yellow
(0.6×10−3, 0.75×10−3 and
1.5×10−3%) and green pigments (5×10−3,
12.5×10−3 and 2.5×10−3%), the fraction of
cells in the G2/M cell cycle phase was increased in a
dose-dependent manner (16 to 27% with 0.6×10−3 and
1.5×10−3% yellow pigment; Fig.
4B).
However, treatment with yellow pigment induced a
different phase change. The fraction of cells in the G1
and S phases decreased when the cells were treated with
0.75×10−3% and 1.5×10−3% of yellow pigment,
but the fraction of cells in the G2/M phase increased in
a dose-dependent manner.
Yellow and green pigments inhibit the
survival of the NSCLC cells
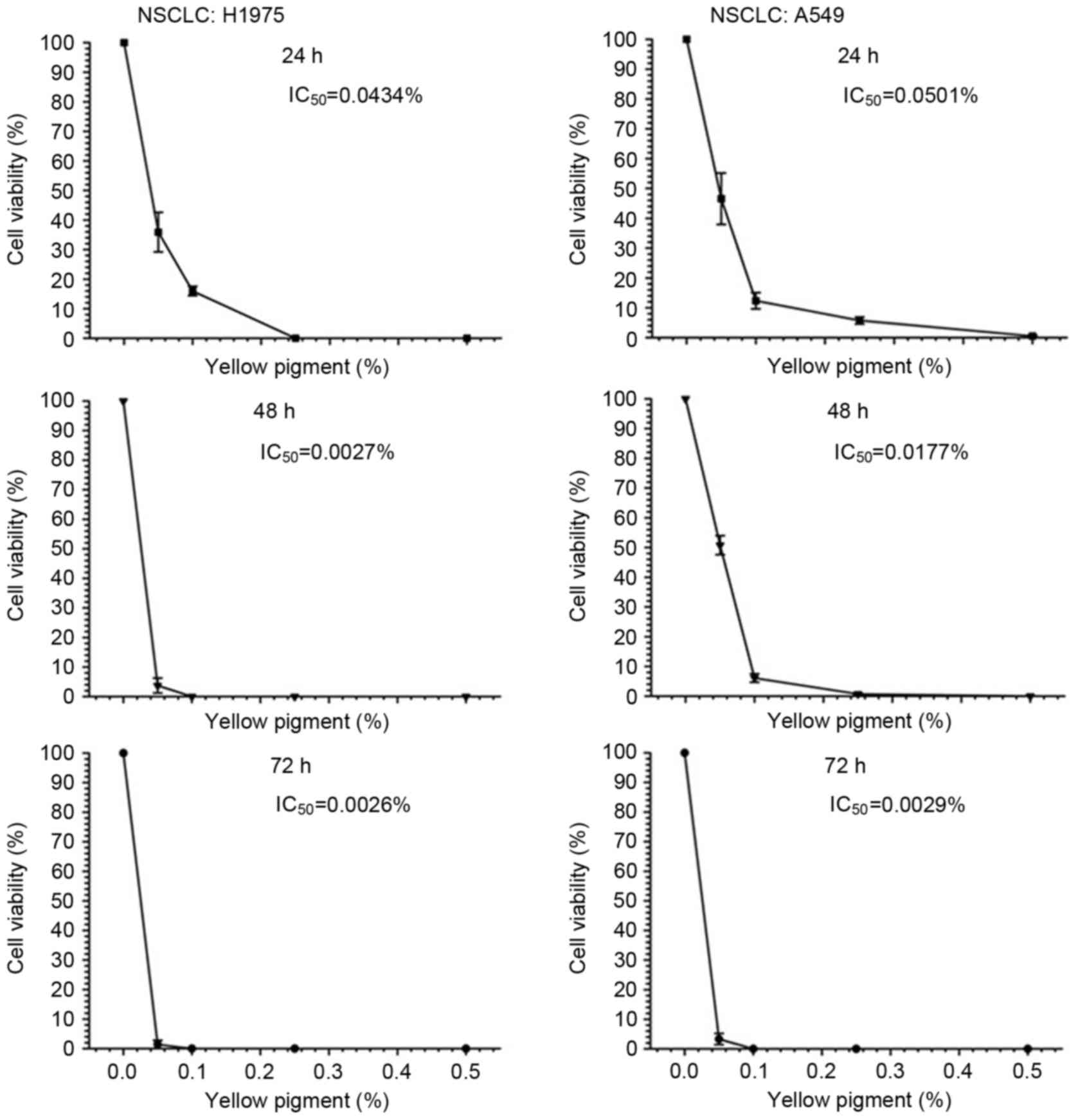
To investigate whether the pigments are able to
exhibit any cytotoxic effects on human NSCLC cells, two different
NSCLC cell types, A549 bronchioloalveolar cell carcinoma and H1975
adenocarcinoma cells, were employed in the present study. The cells
were treated with various concentrations (0.05, 0.10, 0.25, 0.50
and 1.00%) of the pigments for 24–72 h, and the cell viability was
then assessed by MTS assay. In A549 and H1975 cells, treatment with
yellow and green pigments induced a concentration-dependent
reduction in cell viability (Figs. 5
and 6). The level of cytotoxicity
induced by the green and yellow pigments was determined by
IC50 value at 24 h. The green pigment induced increased
cell death in the A549 cells compared with the H1975 cells, with
IC50 values of 0.1206 and 0.0676%, respectively
(Fig. 5). Treatment with the yellow
pigments exhibited increased cytotoxicity against H1975 and A549
cells at 24 h, with lower IC50 values of 0.0434 and
0.0501%, respectively (Fig. 6).
Treatment with green pigments
increases the cytotoxic effect of gefitinib
The present study aimed to determine whether the
green pigments are able to increase the cytotoxic effects of
gefitinib in NSCLC cells. The effect of combined treatment of green
pigments and gefitinib on cell viability was investigated by MTS
assay. Treatment with gefitinib alone was able to reduce the
viability of A549 and H1975 cells to a similar extent and decrease
cell viability with increasing concentrations from 2.5 to 5 µM
(Table II). By contrast, combined
treatment with green pigments and gefitinib for 24 h resulted in a
greater decrease in viability in the H1975 cells compared with the
A549 cells at concentrations 1 to 5 µM. Compared with the treatment
with 0.05% green pigment alone, the treatment with 1 µM gefitinib
and 0.05% green pigment was able to markedly decrease cell
viability by 14.6% (from 57.6 to 43.0%) for the H1975 cells and
7.22% (from 58.02 to 50.80%) for the A549 cells, with a difference
in viability of 51.52 and 41.23% between cells treated with or
without gefitinib for H1975 and A549 cells, respectively.
 | Table II.Co-treatment of green pigments and
gefitinib increases cytotoxicity. |
Table II.
Co-treatment of green pigments and
gefitinib increases cytotoxicity.
| A, A549 cells |
|---|
|
|---|
|
| Green pigment |
|
|---|
|
|
|
|
|---|
| Gefitinib (µM) | 0%a | 0.05%b–d | Probability | Differences in cell
viability between 0–0.05% green pigment |
|---|
| 0 | 100 | 58.02±1.14 | P<0.001 |
41.98±1.03a |
| 1 | 92.03±1.52 | 50.80±1.56 | P=0.022 |
41.23±1.24a |
| 2.5 | 88.65±1.00 | 48.28±2.00 | P=0.007 |
40.37±1.45a |
| 5 | 75.23±1.20 | 46.13±1.58 | P=0.021 |
29.10±1.41a |
| 10 | 68.05±2.00 | 31.14±0.98 | P<0.001 |
36.91±1.17a |
|
| B, H1975
cells |
|
|
| Green
pigment |
|
|
|
|
|
|
| Gefitinib
(µM) | 0%a |
0.05%b–d |
Probability | Differences in
cell viability between 0–0.05% green pigment |
| 0 | 100±0 | 57.61±2.00 | P<0.001 |
42.39±1.11a |
| 1 | 94.55±1.23 | 43.03±1.25 | P=0.002 |
51.52±1.16a |
| 2.5 | 89.12±2.10 | 42.75±1.77 | P=0.003 |
46.37±1.24a |
| 5 | 76.23±0.56 | 39.08±1.20 | P<0.001 |
37.15±1.35a |
| 10 | 69.00±1.78 | 32.72±0.56 | P<0.001 |
36.28±1.74a |
Discussion
Natural medicines have been a target for the
development of cancer drugs over the last 20 years. The leaf and
seed extracts of C. inophyllum have been revealed to exhibit
anti-inflammatory and antioxidant functions (1,2). The seed
oil has been demonstrated to promote wound healing and to exhibit
antibacterial activity (8).
Furthermore, coumarins are present in the seed extracts, with
calophyllolide 3 as the major compound along with inophyllum B, C,
P and E (3). A number of coumarins
have been revealed to have anticancer activities (5–7). The
present study did not purify the compounds and instead used the
crude extracts from the seed oil, which differed in color. The HPLC
analysis demonstrated no differences in the identity of the nine
major compounds present, with major distinction between the green
and yellow pigments being the difference in concentrations
(Fig. 2 and Table I), implying a possible change in the
structure of the yellow and green pigments occurring during the
isolation process. Therefore, the present study evaluated whether
the pigments function differently in their treatment of cancer
cells.
Colon cancer and lung cancer have been the most
prevalent types of cancer worldwide over the last 15 years
(10,11). Despite the observation that both
pigments are able to induce the apoptosis of the DLD-1 colon cancer
cells and increase the fraction of cells in the G2/M
phase in a concentration-dependent manner (Fig. 4), the yellow pigments were more toxic
with a ~10-fold concentration difference compared with the green
pigment (Fig. 3). The difference in
the concentrations required of each pigment for the same level of
toxicity indicated that the pigments differed in the induction of
apoptosis of DLD-1 colon cancer cells (Fig. 3). Numerous anticancer agents function
via the induction of apoptosis (20).
However, these anticancer agents may also trigger cell cycle
checkpoints, which induce cell cycle arrest and thus protects cells
from apoptosis (21,22). As malignant cells often have defects
at the G1 checkpoint, a common strategy of anticancer
treatment is to activate the G2 checkpoint in cancer
cells. Therefore, the G2 checkpoint represents a
survival mechanism for cancer cells to avoid therapy-induced
apoptosis (23). Numerous pigments
that are extracted from plants exert anticancer effects. For
example, curcumin, a naturally occurring yellow pigment isolated
from the turmeric plant, is able to induce apoptosis in various
cancer cell lines (24). Wang et
al (25) demonstrated that
curcumin treatment was able to induce the activation of the
checkpoint kinase 1-mediated G2 checkpoint, which was
associated with the induction of G2/M cell cycle arrest
and the resistance of hepatoma cells to curcumin-induced apoptosis.
These findings are also consistent with the present study, where
the yellow and green pigments were revealed to potentiate the
effects of chemotherapy on DLD-1 colon cancer cells via
G2/M cell cycle arrest and the induction of
apoptosis.
Different types of cancer cells differ in their
response to treatment with gefitinib (26). Subsequently, the present study
investigated the effects of the pigments on A549 and H1975 NSCLC
cells, respectively. With regards to the treatment of NSCLC,
gefitinib, a small molecule EGFR tyrosine kinase inhibitor, has
been used to prolong the survival of patients with advanced NSCLC
following first- and second-line chemotherapies (26). A previous study examined the effect of
NKG2-D-activating natural killer cell receptor expression in NSCLC
cells, and revealed that gefitinib attenuated NK cell-mediated cell
death (27). However, the use of
gefitinib may induce gefitinib-resistant NSCLC via the
overexpression of cathepsin L (28).
Therefore, the combination of gefitinib with other drugs may
increase the killing of NSCLC cells. Recently, a synergistic effect
of high concentrations of gefitinib and celecoxib on A549 cells
significantly increased the anti-proliferative and pro-apoptotic
effects of the drugs by downregulation of cyclooxygenase-2 and
phosphorylated-EGFR (29).
The synergistic effect of gefitinib and 0.05% green
pigments was investigated in the present study. Green pigment was
selected for this part of the study as a lower cytotoxicity of
green pigments was observed in A549 and H1975 cell lines compared
with yellow pigments. Treatment with green pigments alone or in
combination with gefitinib was able to increase the cell death of
A549 and H1975 NSCLC cell lines (Table
II). A synergistic effect was observed with 10 µM gefitinib and
0.05% green pigments for A549 cells, and with 1 µM gefitinib and
0.05% green pigments for H1975 cells, indicating that the two cell
lines differed in their response to the combination treatment of
gefitinib and the green pigments. In conclusion, treatment with
yellow and green pigments was able to increase the rate of
apoptosis and the percentage of colon cancer cells in the
sub-G2/M phase. It was also indicated that the yellow
pigment was more toxic to the colon cancer cells and the NSCLC
cells compared with the green pigment. Variable synergistic effects
of gefitinib and the green pigment were observed in the two NSCLC
cell lines (A549 and H1975).
Acknowledgements
The present study was supported by the Ministry of
Economy (grant no. 104-EC-17-A-18-S1-226) and the Taiwan Forestry
Research Institute (grant no. 102AS-13.1.3-FI-G6), the Council of
Agriculture (Executive Yuan, Taiwan, R.O.C).
References
|
1
|
Prasad J, Shrivastava A, Khanna AK, Bhatia
G, Awasthi SK and Narendera T: Antidyslipidemic and antioxidant
activity of the constituents isolated from the leaves of
Calophyllum inophyllum. Phytomedicine. 19:1245–1249. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsai SC, Liang YH, Chiang JH, Liu FC, Lin
WH, Chang SJ, Lin WY, Wu CH and Weng JR: Anti-inflammatory effects
of Calophyllum inophyllum L. in RAW264.7 cells. Oncol Rep.
28:1096–1102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spino C, Dodier M and Sotheeswaran S:
Anti-HIV coumarins from calophyllum seed oil. Bioorg Med Chem Lett.
8:3475–3478. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Venugopala KN, Rashmi V and Odhav B:
Review on natural coumarin lead compounds for their pharmacological
activity. Biomed Res Int. 2013:9632482013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo KW, Sun JG, Chan JY, Yang L, Wu SH,
Fung KP and Liu FY: Anticancer effects of imperatorin isolated from
Angelica dahurica: Induction of apoptosis in HepG2 cells through
both death-receptor and mitochondria-mediated pathways.
Chemotherapy. 57:449–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yun ES, Park SS, Shin HC, Choi YH, Kim WJ
and Moon SK: p38 MAPK activation is required for esculetin-induced
inhibition of vascular smooth muscle cells proliferation. Toxicol
In Vitro. 25:1335–1342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang D, Gu T, Wang T, Tang Q and Ma C:
Effects of osthole on migration and invasion in breast cancer
cells. Biosci Biotechnol Biochem. 74:1430–1434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Itoigawa M, Ito C, Tan HT, Kuchide M,
Tokuda H, Nishino H and Furukawa H: Cancer chemopreventive agents,
4-phenylcoumarins from Calophyllum inophyllum. Cancer Lett.
169:15–19. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cooper GM: The Development and Causes of
Cancer. The Cell: A Molecular Approach. 2nd. Sinauer Associates;
Sunderland, MA: 2000
|
|
10
|
World Health Organization, . Cancer
worldwide = World Cancer Report 2014. Stewart BW and Wild CP: IARC
Nonserial Publication; pp. 16–81. 2014
|
|
11
|
Parkinn DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
World Health Organization, . GLOBOCAN
2012: Estimated Cancer Incidence, Mortality and Prevalence
Worldwide in 2012. Cancer fact sheets: Lung. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspxDec
27–2013
|
|
13
|
Dikshit R, Gupta PC, Ramasundarahettige C,
Gajalakshmi V, Aleksandrowicz L, Badwe R, Kumar R, Roy S, Suraweera
W, Bray F, et al: Cancer mortality in India: A nationally
representative survey. Lancet. 379:1807–1816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Earp HS, Dawson TL, Li X and Yu H:
Heterodimerization and functional interaction between EGF receptor
family members: A new signaling paradigm with implications for
breast cancer research. Breast Cancer Res Treat. 35:115–132. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yeh CH, Yang ST and Chen CH: Calvatia
lilacina protein extract induces apoptosis through endoplasmic
reticulum stress in human colon carcinoma cells. Process Biochem.
46:1599–1606. 2011. View Article : Google Scholar
|
|
16
|
Chen CH, Lin WC, Kuo CN and Lu FJ: Role of
redox signaling regulation in propyl gallate-induced apoptosis of
human leukemia cells. Food Chem Toxicol. 49:494–501. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang JT, Li ZL, Wu JY, Lu FJ and Chen CH:
An oxidative stress mechanism of shikonin in human glioma cells.
PLoS One. 9:e941802014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen CY, Li ZL, Chung KT, Lu FJ and Chen
CH: Liriodenine enhances the apoptosis effect of valproic acid in
human colon cancer cells through oxidative stress upregulation and
Akt inhibition. Process Biochem. 49:1990–2000. 2014. View Article : Google Scholar
|
|
19
|
Chen TR, Dorotinsky CS, McGuire LJ, Macy
ML and Hay RJ: DLD-1 and HCT-15 cell lines derived separately from
colorectal carcinomas have totally different chromosome changes but
the same genetic origin. Cancer Genet Cytogenet. 81:103–108. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gartel AL: Mechanisms of apoptosis induced
by anticancer compounds in melanoma cells. Curr Top Med Chem.
12:50–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Z, Xiao Z, Gu WZ, Xue J, Bui MH,
Kovar P, Li G, Wang G, Tao ZF, Tong Y, et al: Selective Chk1
inhibitors differentially sensitize p53-deficient cancer cells to
cancer therapeutics. Int J Cancer. 119:2784–2794. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hirose Y, Berger MS and Pieper RO:
Abrogation of the Chk1-mediated G(2) checkpoint pathway potentiates
temozolomide-induced toxicity in a p53-independent manner in human
glioblastoma cells. Cancer Res. 61:5843–5849. 2001.PubMed/NCBI
|
|
23
|
Kawabe T: G2 checkpoint abrogators as
anticancer drugs. Mol Cancer Ther. 3:513–519. 2004.PubMed/NCBI
|
|
24
|
Karunagaran D, Rashmi R and Kumar TR:
Induction of apoptosis by curcumin and its implications for cancer
therapy. Curr Cancer Drug Targets. 5:117–129. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang WZ, Cheng J, Luo J and Zhuang SM:
Abrogation of G2/M arrest sensitizes curcumin-resistant hepatoma
cells to apoptosis. FEBS Lett. 582:2689–2695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sridhar SS, Seymour L and Shepherd FA:
Inhibitors of epidermal-growth-factor receptors: A review of
clinical research with a focus on non-small-cell lung cancer.
Lancet Oncol. 4:397–406. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okita R, Wolf D, Yasuda K, Maeda A, Yukawa
T, Saisho S, Shimizu K, Yamaguchi Y, Oka M, Nakayama E, et al:
Contrasting effects of the cytotoxic anticancer drug gemcitabine
and the EGFR tyrosine kinase inhibitor gefitinib on NK
cell-mediated cytotoxicity via regulation of NKG2D ligand in
non-small-cell lung cancer cells. PLoS One. 10:e01398092015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui F, Wang W, Wu D, He X, Wu J and Wang
M: Overexpression of cathepsin L is associated with gefitinib
resistance in non-small cell lung cancer. Clin Transl Oncol.
18:722–727. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li N, Li H, Su F, Li J, Ma X and Gong P:
Relationship between epidermal growth factor receptor (EGFR)
mutation and serum cyclooxygenase-2 level, and the synergistic
effect of celecoxib and gefitinib on EGFR expression in non-small
cell lung cancer cells. Int J Clin Exp Pathol. 8:9010–9020.
2015.PubMed/NCBI
|