Introduction
Despite improvements due to an earlier diagnosis and
improved treatment methods, cervical cancer remains a leading cause
of cancer-associated mortality in women worldwide (1). Cervical cancer is preventable and often
curable if it is detected early. However, a high proportion of
patients exhibit a poor prognosis, as they are diagnosed with
advanced stage, recurrent or persistent cervical cancer (2,3).
Therefore, there is a requirement for the development of novel
treatment strategies.
Neovascularization serves an important role in the
tumor progression, invasion and metastasis of cervical cancer
(2). Although specific inhibitors of
angiogenesis have been clinically approved, toxicity and disruption
of the normal vascular bed limit their application (4). Traditional surgery often causes serious
harm to the reproductive organs and may affect sexual function and
fertility (5–7). Therefore, it is important to effectively
treat and reduce the side effects of traditional cervical cancer
treatments.
Picosecond pulsed electric field (psPEF) technology
involves use of an ultra-broadband spectrum with a high time and
spatial resolution, and low signal distortion. psPEF may be used to
non-invasively and precisely target deep tissue (8). To the best of our knowledge, the
biological effects of psPEF are not fully understood. Our
preliminary study demonstrated that psPEF exhibits necrotic and
anti-angiogenic effects in cervical cancer xenograft models by
exerting direct effects on cancer cells and vascular endothelial
cells, and indirect effects on angiogenesis-associated factors
(9). However, to the best of our
knowledge, the effects of psPEF on cervical cancer angiogenesis
in vitro remain unknown. To investigate the effects and
possible mechanism of psPEF on angiogenesis in cervical cancer
in vitro, human umbilical vein endothelial cells (HUVECs)
and HeLa cells were exposed to psPEF. The proliferation, cell
motility and tube formation capabilities of HUVECs were analyzed.
In addition, the protein and mRNA expression levels of
angiogenesis-associated factors, including vascular endothelial
growth factor (VEGF) and hypoxia-inducible factor 1α (HIF-1α), were
measured in HeLa cells following psPEF treatment. By studying the
underlying mechanism, the antitumor effect of psPEF may be
improved, which may enhance its clinical application.
Materials and methods
Cell culture and materials
HeLa cells and HUVECs were purchased from The Cell
Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China) and cultured in high glucose Dulbecco's modified
Eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (GE
Healthcare Life Sciences, Logan, UT, USA) and 1%
penicillin-streptomycin at 37°C in a 5% CO2 incubator.
Cell Counting kit-8 (CCK-8; Guangzhou Yiyuan Biological Technology
Co., Ltd., Guangzhou, China), the Annexin V-fluorescein
isothiocyanate (FITC) apoptosis detection kit (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China), the anti-VEGF polyclonal
antibody (no. bs-0279R; 1:200 dilution; Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China) and the anti-HIF-1α
monoclonal antibody (cat. no. sc-13515; 1:500 dilution; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) were used in the current
study.
Field stimulation protocol
The following parameters were fixed: i) Frequency, 3
hertz; ii) duration, 800 picosec; and iii) pulse number, 2,000.
Cells were randomly divided into four groups: The 200, 400 and 600
kV/cm psPEF treatment groups, and a control group that was
administered no treatment. Following three washes with PBS, cells
were combined with 0.125% trypsin-EDTA, and then centrifuged at 800
× g for 5 min at room temperature. Cells were then resuspended in
fresh high glucose DMEM at a concentration of 1×106
cells/ml. Subsequently, the cell suspension (200 µl) was placed
into a cuvette and power was supplied. The electric field amplitude
and pulse width of psPEF was monitored throughout the experiment
using a DP04054 oscilloscope (Tektronix, Inc., Beaverton, OR, USA).
The control group was not connected to the power supply.
CCK-8 assay
Following psPEF treatment, the cell viability was
investigated. HUVECs were transferred to 96-well plates containing
150 µl DMEM and 1×104 cells per well, and cultured for
2, 4, 6, 8, 10, 12, 14 or 16 h in a 5% CO2 humidified
incubator at 37°C. Normal control (without psPEF treatment) and
blank groups (without cells) were also included. Following
incubation, 20 µl CCK-8 was added to each well and incubated for a
further 2 h at 37°C. The optical density was measured at 470 nm
using an ELx800 absorbance microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA).
Apoptosis analysis
HUVECs were grown in 25-cm2 culture
flasks for 12 h after treatment in a 5% CO2 humidified
incubator at 37°C. Cells were double-stained using an Annexin
V-FITC apoptosis detection kit, according to the manufacturer's
protocol. The cellular fluorescence was measured at an emission
wavelength of 530 nm and an excitation wavelength of 488 nm using
BD FACScan system (BD Biosciences, Franklin Lakes, NJ, USA)
equipped with CellQuest Pro software version 5.1 (BD Biosciences,
Franklin Lakes, NJ, USA).
In vitro migration assay
Transwell invasions assay were conducted in 24-well
cell culture inserts The upper chamber with polycarbonate membrane
(8 mm pore size) (Corning Incorporated, Corning, NY, USA) were
covered with 40 µl Matrigel (1:4 dilution; BD Biosciences, Franklin
Lakes, NJ, USA) and incubated for 24 h at 37°C. The lower chamber
was filled with 750 µl DMEM with 10% FBS. Following psPEF
treatment, the HUVECs cells were harvested and plated in complete
medium on top of the culture insert at 5×104 cells per
insert in 0.5 ml. The inserts were incubated at 37°C, 5%
CO2 for 18 h. Non-invading cells were removed. Cells
that had migrated through the pores were fixed with 4%
paraformaldehyde for 30 min, stained in 0.5% crystal violet
(Beyotime Institute of Biotechnology, Shanghai, China) for 10 min
at room temperature and counted on a Leica CME microscope at total
magnification, ×40. Three inserts were counted for each group and
the experiment was repeated a minimum of three times.
In vitro wound-healing assay
Following psPEF treatment and incubation for 12 h in
a 5% CO2 humidified incubator at 37°C, HUVECs were
harvested, counted, plated at 4×105 cells/ml in 12-well
dishes and incubated overnight at 37°C. Images were captured with a
Leica CME microscope at ×20 total magnification immediately after
wounds had been made with a pipette tip and following 24 h, and the
distance migrated by the cells during this period was measured. The
distance migrated by the psPEF treatment groups was calculated
relative to the control group and expressed as the migration index.
The experiment was repeated a minimum of three times.
Lumen formation test
The lumen formation assay was performed as described
previously by Arnaoutova et al (10). Briefly, following psPEF treatment,
HUVEC suspensions from the four treatment groups were added to the
top of the gel at a density of 15,000 cells/well, incubated for 3 h
in a 5% CO2 humidified incubator at 37°C and then imaged
with a Leica CME microscope at total magnification, ×20.
Western blot analysis and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
HeLa cells from four groups (control, 200, 400 and
600 kV/cm psPEF tretment groups) were used. Western blot analysis
and RT-qPCR were performed as described previously (11). The internal loading control was
β-tubulin and bands were analyzed using Quantity One 4.6.2 software
(Bio-Rad laboratories, Inc., Hercules, CA, USA). The primers used
for RT-qPCR were as follows: VEGF forward,
5′-GTCCCAGGCTGCACCCATG-3′ and reverse, 5′-AGGAAGCTCATCTCTCCTA-3′;
and HIF-1α forward, 5′-TCCATGTGACCATGAGGAAA-3′ and reverse,
5′-CCAAGCAGGTCATAGGTGGT-3′. β-tubulin forward,
5′-CCAAGGGTCACTACACG-3′ and reverse, 5′-GCAGTCGCAGTTTTCACACTC-3′;
all data were normalized to the tubulin expression levels. Each
experiment was performed in triplicate.
Statistical analysis
All data were analyzed using SPSS software (version
10.0; SPSS, Inc., Chicago, IL, USA) and expressed as the mean ±
standard deviation of a minimum of three independent experiments.
Statistical analysis was carried out by one-way analysis of
variance (ANOVA), followed by Tukey's post-hoc test for multiple
group comparisons, P<0.01 was considered to indicate a
statistically significant difference.
Results
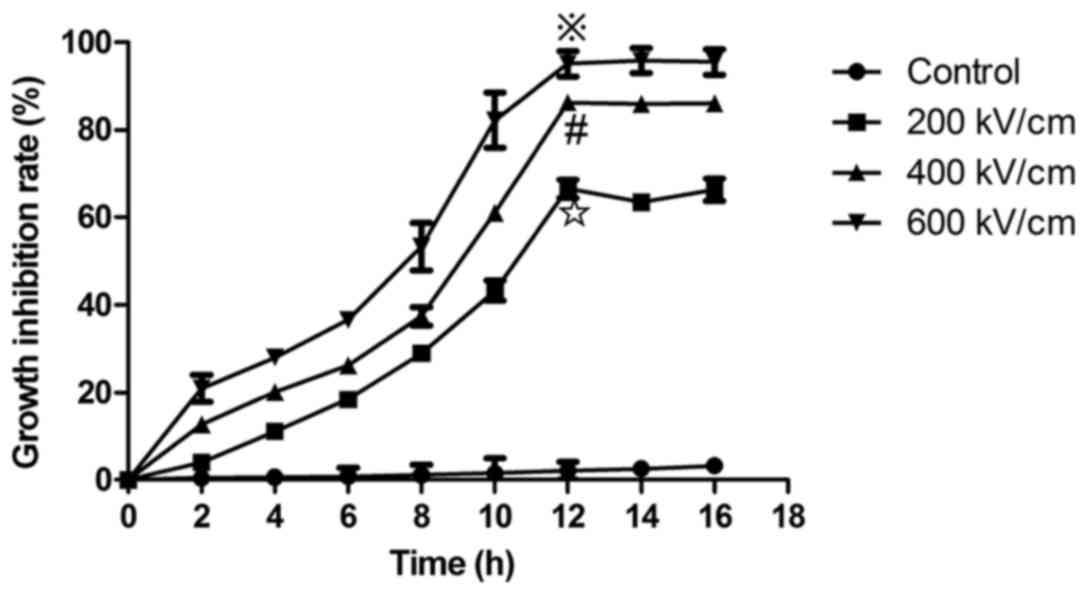
psPEF is associated with reduced cell
survival
HUVECs were exposed to psPEF and cultured for 2, 4,
6, 8, 10, 12, 14 or 16 h. The CCK-8 assay was then used to analyze
cell viability. The cell survival rate was taken as 100% for the
control group. The cell growth inhibition rate was determined as:
(The absorbance of normal control cells-the absorbance of psPEF
treatment cells)/(the absorbance of normal control cells-the
absorbance of the blank group) × 100. The results indicated that
psPEF inhibited the growth of HUVECs in an electric field
amplitude-dependent manner. An increased growth inhibition rate was
associated with higher electric field amplitude and the maximum
cell inhibition was observed at 12 h after psPEF treatment. The
cells growth inhibition rate of the control, and the 200, 400 and
600 kV/cm psPEF treatment groups at 12 h following treatment with
psPEF were 2.14±1.98, 66.53±0.2.12, 86.32±1.14 and 95.14±2.93%,
respectively (※P<0.01, vs. the control, 200 and 400
kV/cm groups; #P<0.01, vs. the control and 200 kV/cm
groups; ✩P<0.01, vs. the control group; Fig. 1).
psPEF induces apoptosis and necrosis
of HUVECs
As demonstrated in Fig.
2, the apoptosis and necrosis rates of HUVECs following psPEF
treatment were analyzed by flow cytometry. The mean initial
apoptosis rates of the control, and the 200, 400 and 600 kV/cm
psPEF treatment groups were 1.91±0.69, 2.74±0.42, 5.86±1.37 and
7.29±0.61%, respectively. The mean late apoptosis rates of the four
groups were 3.00±0.81, 8.17±0.57, 24.71±2.39 and 35.83±3.65%,
respectively. The mean necrosis rates of the four groups were
1.43±0.87, 2.49±0.80, 5.86±1.37 and 9.40±0.61%, respectively. The
initial apoptosis, late apoptosis and necrosis rates were
significantly increased in the 400 and 600 kV/cm treatment groups
compared with the control group. In summary, the results indicated
that psPEF could induce apoptosis and necrosis of HUVECs.
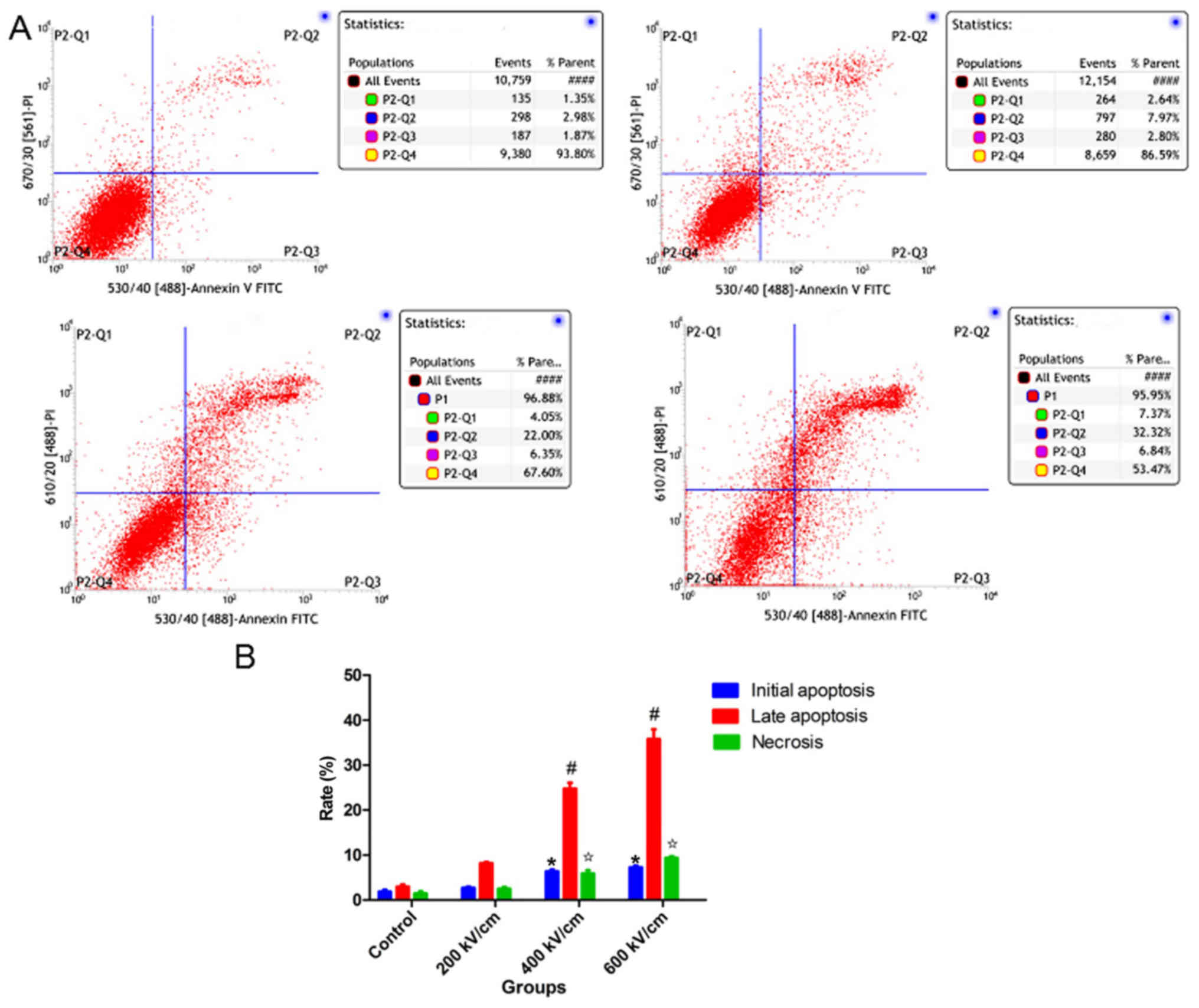 | Figure 2.Apoptosis and necrosis rates of HUVECs
following psPEF treatment analyzed by flow cytometry. (A)
Representative flow cytometry plots of the four groups (top left,
top right, bottom left and bottom right represented Control, 200,
400 and 600 kV/cm psPEF treatment group, respectively). P2-Q1
represents necrosis, P2-Q2 represents late apoptosis, P2-Q3
represents initial apoptosis and P2-Q4 represents normal cells. (B)
The mean initial apoptosis rate, late apoptosis rate and necrosis
rate of HUVECs following psPEF treatment. The apoptosis and
necrosis rates were significantly increased in the 400 and 600
kV/cm treatment groups compared with those in the control group.
Data are presented as the mean ± standard deviation. *P<0.01 vs.
control initial apoptosis rate, #P<0.01 vs. control
late apoptosis rate, ✩P<0.01 vs. control necrosis
rate. HUVEC, human umbilical vein endothelial cell; psPEF,
picosecond pulsed electric field; FITC, fluorescein
isothiocyanate. |
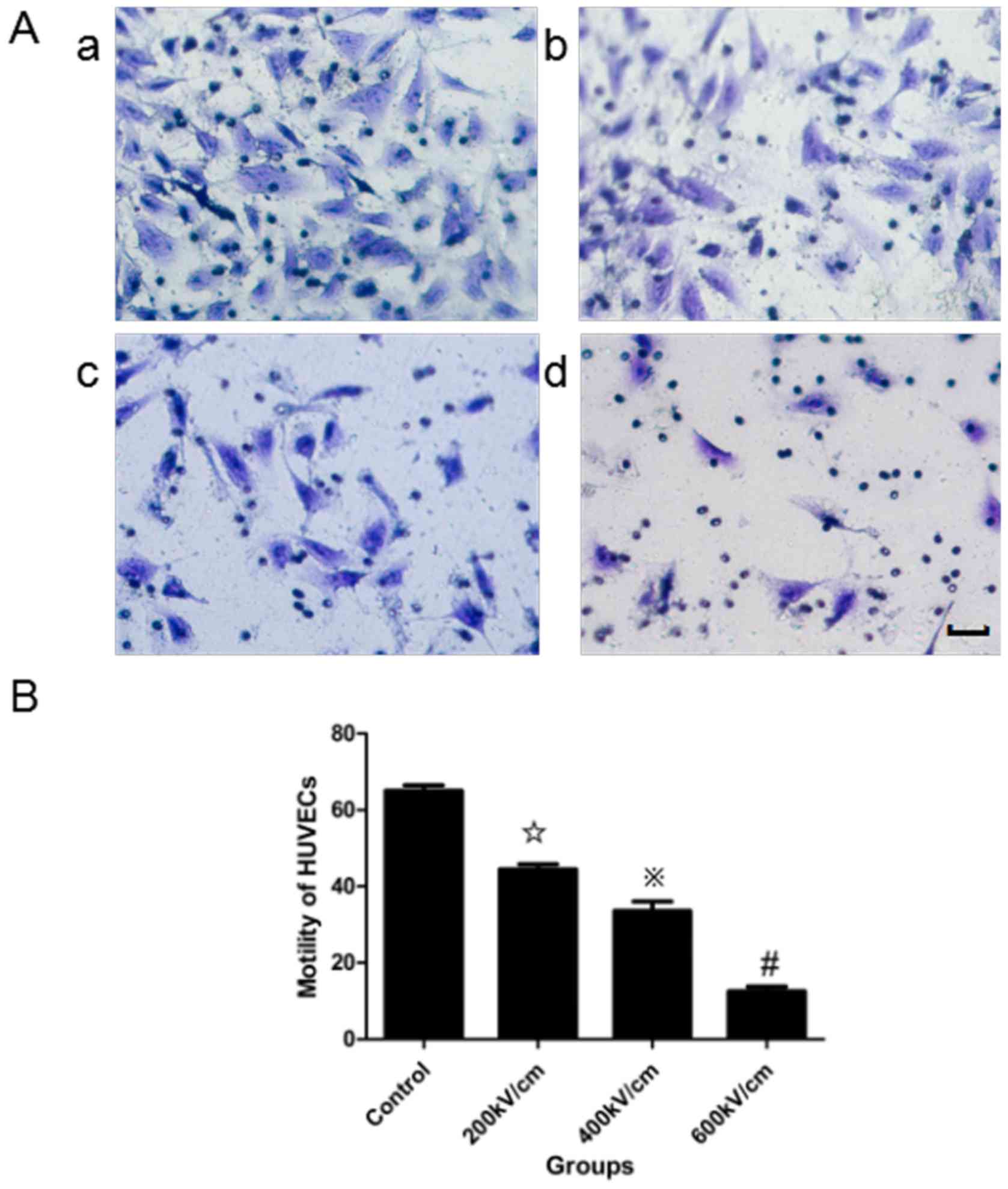
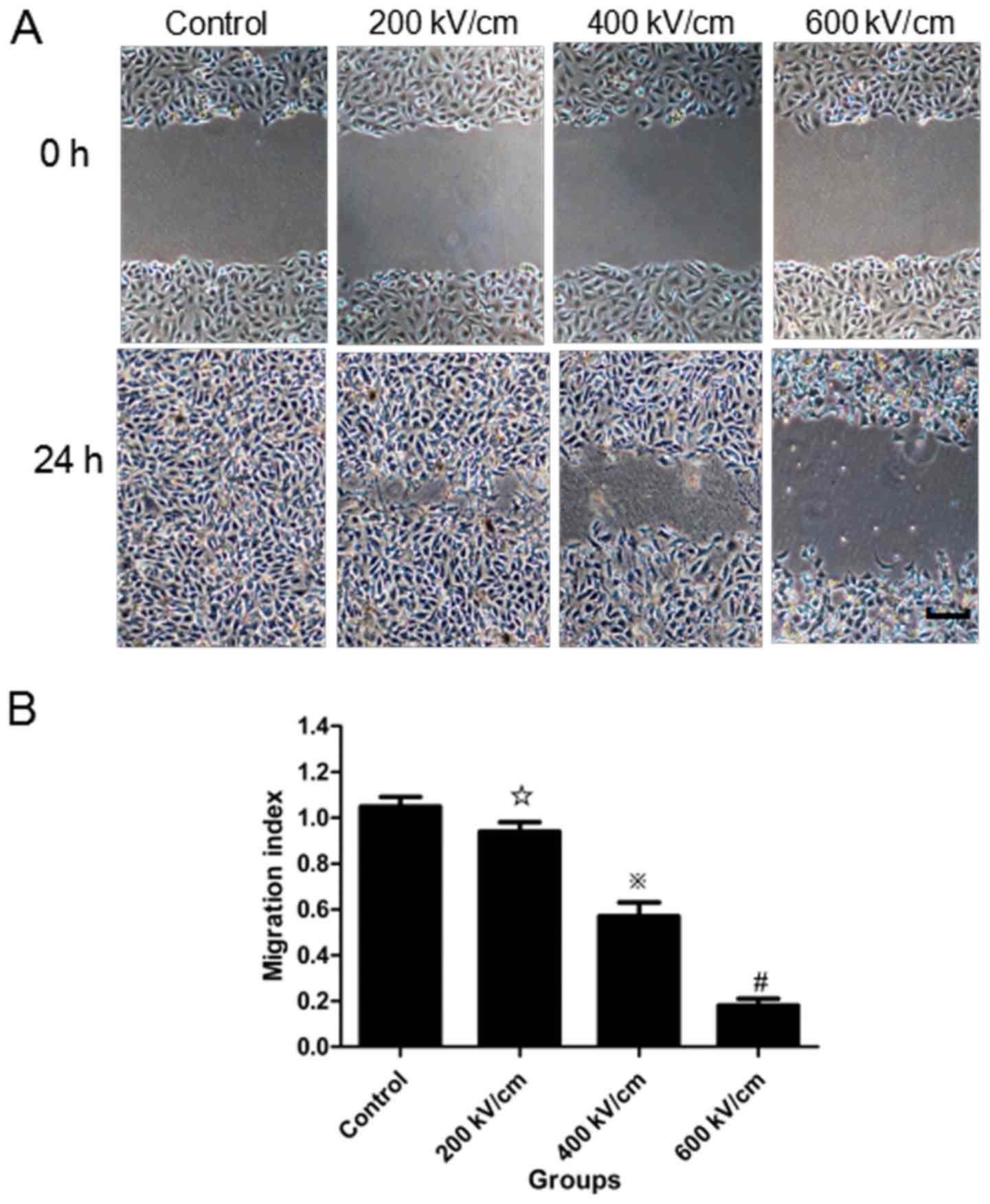
psPEF treatment impairs cell
motility
A migration assay (Fig.
3) and in vitro wound-healing assay (Fig. 4) were used to assess the effects of
psPEF on the motility of HUVECs. In the migration assay, the number
of cells that moved across a microporous membrane following psPEF
treatment was recorded. The data are expressed as motility indexes,
which represent the number of cells that moved across the membranes
relative to the control. The motility index of the control, and the
200, 400 and 600 kV/cm psPEF treatment groups was 65.11±2.43,
44.52±2.26, 33.63±4.17 and 12.52±2.13%, respectively. A
statistically significant difference was identified in the motility
index of the 400 and 600 kV/cm psPEF treatment groups compared with
the control group (P<0.01). In the wound-healing assay, the
distance moved by the wounded cells following treatment with
different psPEF intensities was measured. The migration index for
the control, and the 200, 400 and 600 kV/cm psPEF treatment groups
was 1.05±0.04, 0.94±0.04, 0.57±0.06 and 0.18±0.03%, respectively. A
statistically significant difference was identified in the 400 and
600 kV/cm psPEF treatment groups compared with the control group
(P<0.01).
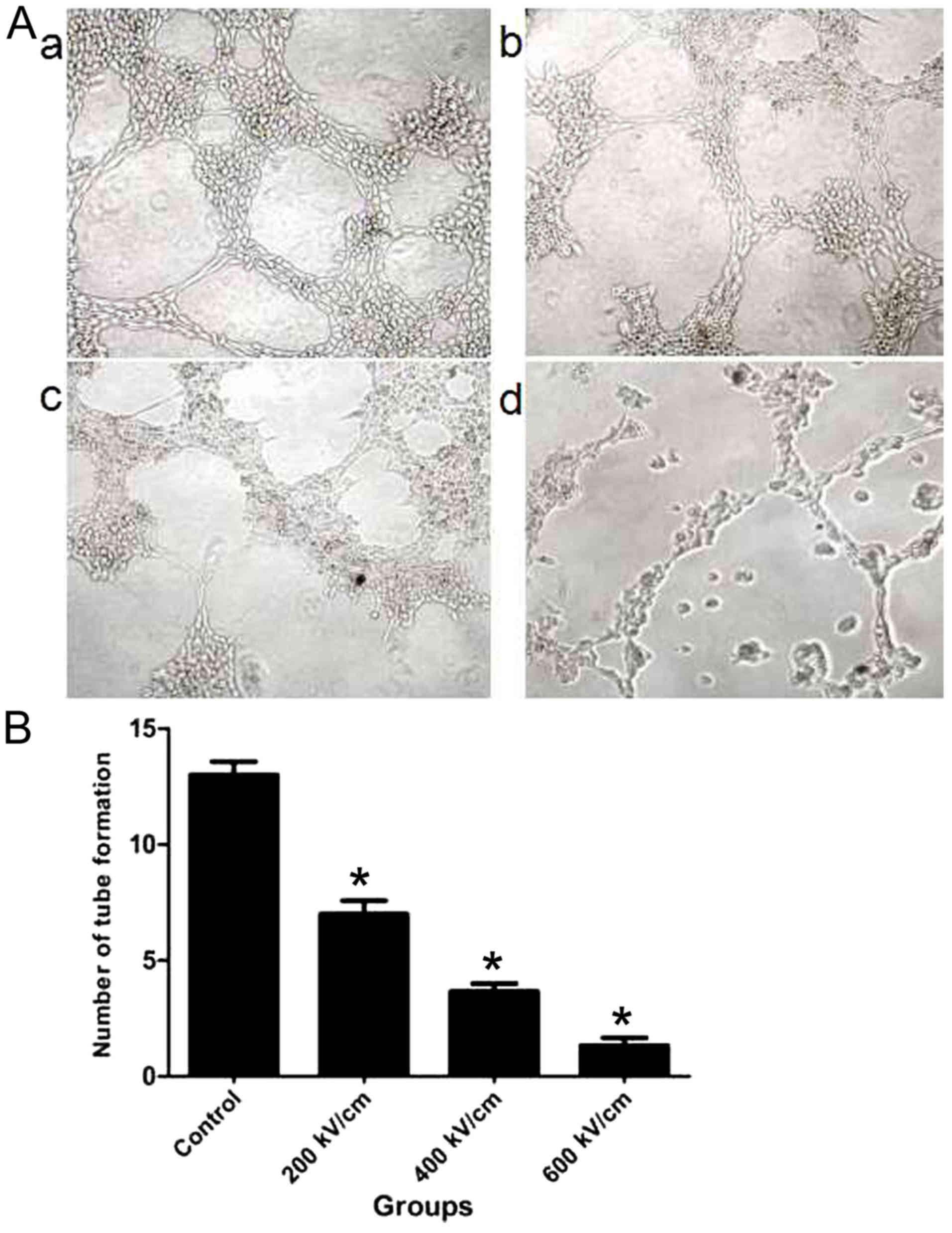
psPEF inhibits tube formation in
HUVECs
Differences in tube formation were identified
between the control and psPEF treatment groups (Fig. 5). HUVECs in the control group
demonstrated adhesion and alignment (Fig.
5Aa). However, the psPEF-treated cells appeared less elongated
and an inhibition of tube formation was identified (Fig. 5Ab-d). An increase in the psPF electric
field intensity was associated with a decreased number of tubes.
The mean number of tubes formed for the control, and the 200, 400
and 600 kV/cm psPEF treatment groups was 13.31±1.53, 7.23±0.58,
3.42±0.75 and 1.02±0.44, respectively (Fig. 5B). A statistically significant
difference was identified between the control group and all psPEF
treatment groups (P<0.01). These results indicated that psPEF
inhibited tube formation in HUVECs.
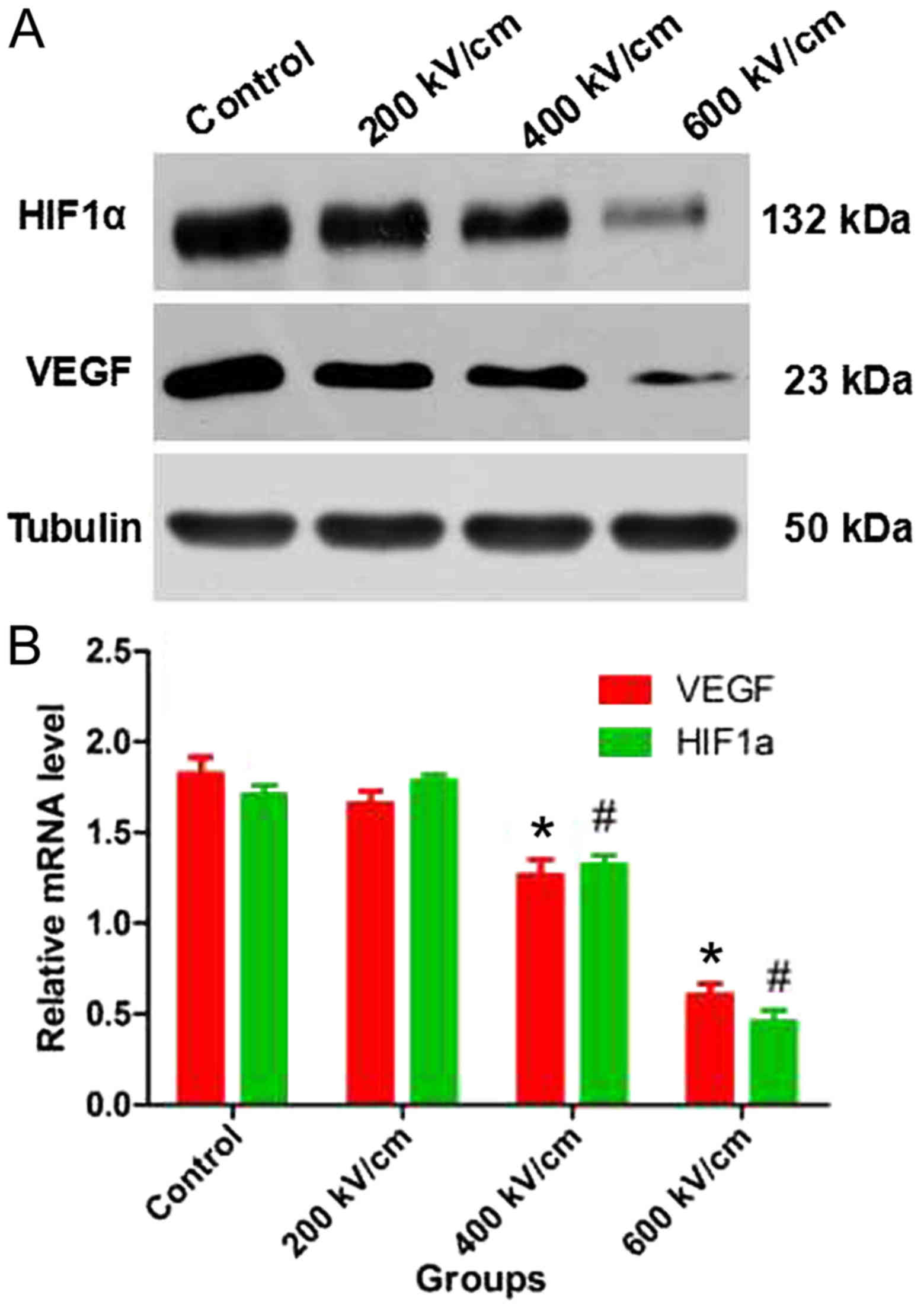
psPEF reduces the protein and mRNA
levels of VEGF and HIF-1α in HeLa cells
The protein and mRNA levels of VEGF and HIF-1α in
HeLa cells were measured following psPEF treatment. Western blot
analysis revealed that the protein levels of VEGF and HIF-1α were
decreased in the 400 and 600 kV/cm groups compared with those in
the control group (Fig. 6A). RT-qPCR
identified that the mRNA levels of VEGF and HIF-1α were
significantly decreased in the 400 and 600 kV/cm groups compared
with those in the control group (P<0.01; Fig. 6B).
Discussion
Angiogenesis serves an important role in tumor
invasion and metastasis, and is essential for tumor growth >1–2
mm3 (12,13). Folkman et al (12) reported that tumor growth requires
tumor cell proliferation and angiogenesis. The basic requirement
for angiogenesis is the proliferation and migration of vascular
endothelial cells (14–17). Therefore, blocking these processes in
vascular endothelial cells may inhibit tumor vascularization.
In the current study, psPEF inhibited the
proliferation of HUVECs in a dose- and time-dependent manner.
Furthermore, psPEF impaired the motility of HUVECs in a
dose-dependent manner. Tumor blood vessels are predominantly
composed of vascular endothelial cells; therefore, direct
inhibition of tumor vascular endothelial cell growth is an
important target to inhibit tumor growth, invasion and metastasis
(13). The current study investigated
whether psPEF may inhibit the angiogenesis of HUVECs using the
lumen formation test. The results demonstrated that a decrease in
the number of tubes was associated with an increase in the psPEF
electric field intensity. Our previous study indicated that psPEF
could induce apoptosis through a mitochondrial-mediated pathway in
HeLa cells (11). In the current
study, the apoptosis rate of HUVECs, particularly the late
apoptosis rate, increased significantly compared with that of the
control group. However, to the best of our knowledge, the
underlying mechanism of this remains unknown.
Impairment of blood flow leads to tumor cell death
due to a lack of nutrients and an accumulation of catabolite
products (18,19). The inhibition of
angiogenesis-associated factors inhibits blood flow, which leads to
tumor growth inhibition (20).
Hypoxia is considered to serve as a driving force for tumor
angiogenesis (21). Tumor cells can
adapt to hypoxia by altering the transcription of genes associated
with angiogenesis, including VEGF and HIF-1α (22–25). In
the current study, the protein and mRNA levels of VEGF and HIF-1α
in HeLa cells were measured following treatment with psPEF. The
results revealed that psPEF treatment is associated with decreased
protein and mRNA levels of VEGF and HIF-1α. Therefore, we
hypothesize that psPEF may indirectly decrease angiogenic activity
in vitro by downregulating angiogenesis-associated factors
in HeLa cells, which is consistent with our previous animal study
(9).
In summary, the current study demonstrated that
psPEF exhibited anti-angiogenesis effects in cervical cancer cells
by two mechanisms. Firstly, psPEF exhibited a direct
anti-angiogenic effect in vitro on HUVECs. Secondly, psPEF
treatment was associated with a downregulation of
angiogenesis-associated factors secreted by cancer cells, which
suggests that psPEF could indirectly inhibit the formation of tumor
vessels in vitro.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81301928) and the
Project Foundation of Chongqing Municipal Education Committee
(grant no. KJ1500210).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZX, CY and YH designed the experiments. LW, YW, MZ
and RZ performed the experiments and collected data. CY, YH, LW, YW
and RZ analyzed and interpreted the data. LW and YW drafted the
manuscript. YH, ZX, LW and MZ revised the paper critically for
important intellectual content. LW YW and YH agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the study are
appropriately investigated and resolved. All authors read and gave
final approval of the version to be published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Landt S, Wehling M, Heidecke H, Jeschke S,
Korlach S, Stöblen F, Schmid P, Blohmer JU, Lichtenegger W, Sehouli
J and Kümmel S: Prognostic significance of angiogenic factors in
uterine cervical cancer. Anticancer Res. 31:2589–2595.
2011.PubMed/NCBI
|
|
2
|
Monk BJ, Willmott LJ and Sumner DA:
Anti-angiogenesis agents in metastatic or recurrent cervical
cancer. Gynecol Oncol. 116:181–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Monk BJ and Herzog TJ: The evolution of
cost-effective screening and prevention of cervical carcinoma:
Implications of the 2006 consensus guidelines and human
papillomavirus vaccination. Am J Obstet Gynecol. 197:337–339. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ackermann M, Carvajal IM, Morse BA, Moreta
M, O'Neil S, Kossodo S, Peterson JD, Delventhal V, Marsh HN,
Furfine ES and Konerding MA: Adnectin CT-322 inhibits tumor growth
and affects microvascular architecture and function in Colo205
tumor xenografts. Int J Oncol. 38:71–80. 2011.PubMed/NCBI
|
|
5
|
Verheul HM and Pinedo HM: Possible
molecular mechanisms involved in the toxicity of angiogenesis
inhibition. Nat Rev Cancer. 7:475–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma J and Waxman DJ: Combination of
antiangiogenesis with chemotherapy for more effective cancer
treatment. Mol Cancer Ther. 7:3670–3684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seol HJ, Ulak R, Ki KD and Lee JM:
Cytotoxic and targeted systemic therapy in advanced and recurrent
cervical cancer: Experience from clinical trials. Tohoku J Exp Med.
232:269–276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baum CE, Stone AP and Tyo JS:
Ultra-wideband, short-pulse electromagnetics. 8th. New York:
Springer; 2007, View Article : Google Scholar
|
|
9
|
Wu L, Yao C, Xiong Z, Zhang R, Wang Z, Wu
Y, Qin Q and Hua Y: The effects of a picosecond pulsed electric
field on angiogenesis in the cervical cancer xenograft models.
Gynecol Oncol. 141:175–181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arnaoutova I, George J, Kleinman HK and
Benton G: The endothelial cell tube formation assay on basement
membrane turns 20: State of the science and the art. Angiogenesis.
12:267–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hua YY, Wang XS, Zhang Y, Yao CG, Zhang XM
and Xiong ZA: Intense picosecond pulsed electric fields induce
apoptosis through a mitochondrial-mediated pathway in HeLa cells.
Mol Med Rep. 5:981–987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Folkman J: The role of angiogenesis in
tumor growth. Semin Cancer Biol. 3:65–71. 1992.PubMed/NCBI
|
|
13
|
Eskander RN and Tewari KS: Targeting
angiogenesis in advanced cervical cancer. Ther Adv Med Oncol.
6:280–292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kerbel RS: Tumor angiogenesis. N Engl J
Med. 358:2039–2049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Erös de Bethlenfalva-Hora C, Mertens JC,
Piguet AC, Kettenbach J, Schmitt J, Terracciano L, Weimann R,
Dufour JF and Geier A: Radiofrequency ablation suppresses distant
tumour growth in a novel rat model of multifocal hepatocellular
carcinoma. Clin Sci (Lond). 126:243–252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Groblewska M, Siewko M, Mroczko B and
Szmitkowski M: The role of matrix metalloproteinases (MMPs) and
their inhibitors (TIMPs) in the development of esophageal cancer.
Folia Histochem Cytobiol. 50:12–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ribatti D: Genetic and epigenetic
mechanisms in the early development of the vascular system. J Anat.
208:139–152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Denekamp J, Hill SA and Hobson B: Vascular
occlusion and tumour cell death. Eur J Cancer Clin Oncol.
19:271–275. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hajitou A, Grignet C, Devy L, Berndt S,
Blacher S, Deroanne CF, Bajou K, Fong T, Chiang Y, Foidart JM and
Noël A: The antitumoral effect of endostatin and angiostatin is
associated with a down-regulation of vascular endothelial growth
factor expression in tumor cells. FASEB J. 16:1802–1804. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaur B, Khwaja FW, Severson EA, Matheny
SL, Brat DJ and Van Meir EG: Hypoxia and the
hypoxia-inducible-factor pathway in glioma growth and angiogenesis.
Neuro Oncol. 7:134–153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chaplin DJ and Horsman MR: The influence
of tumour temperature on ischemia-induced cell death: Potential
implications for the evaluation of vascular mediated therapies.
Radiother Oncol. 30:59–65. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Noguera R, Fredlund E, Piqueras M, Pietras
A, Beckman S, Navarro S and Påhlman S: HIF-1alpha and HIF-2alpha
are differentially regulated in vivo in neuroblastoma: High
HIF-1alpha correlates negatively to advanced clinical stage and
tumor vascularization. Clin Cancer Res. 15:7130–7136. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brown JM and Wilson WR: Exploiting tumour
hypoxia in cancer treatment. Nat Rev Cancer. 4:437–447. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vaupel P and Mayer A: Hypoxia in tumors:
Pathogenesis-related classification, characterization of hypoxia
subtypes, and associated biological and clinical implications. Adv
Exp Med Biol. 812:19–24. 2014. View Article : Google Scholar : PubMed/NCBI
|