Introduction
Wnt proteins regulate many stages of development,
including patterning of the embryo, initiation of axon guidance,
and synaptic formation (1). Wnt
signaling plays a major role in bone development and its defects
can lead to skeletal diseases such as osteoporosis (2,3). Loss of
Wnt leads to tetra-amelia syndrome, a severe congenital defect in
which all four limbs fail to form, as well as to craniofacial
defects (4). During skeletogenesis,
conditional removal of β-catenin in mesenchymal progenitor cells
leads to decreased osteoblast differentiation (5,6). In
contrast, constitutive activation of β-catenin under the same
conditions results in dramatically increased bone deposition with
reduced osteoclast formation (7).
The Wnt/β-catenin signaling pathway is activated by binding of a
Wnt ligand to Frizzled (Fz), a seven transmembrane protein
receptor, which mediates subsequent downstream disruption of the
β-catenin degradation complex (8).
This allows β-catenin to be accumulated in the nucleus, where it
binds to members of the T cell factor, lymphoid enhancer factor
(Lef/Tcf)/transcription factor family, and activates gene
transcription necessary for the development of multicellular
organisms and regulation of adult tissue homeostasis, including
growth and renewal of bones (9–11). Wnt
proteins are glycosylated in the endoplasmic reticulum and
palmitoylated (12). Experiments
using tunicamycin (an inhibitor of asparagine-linked glycosylation)
demonstrated that Wnt3a is modified with N-linked glycans
(13). However, Wnt proteins are
inefficiently secreted and tend to linger in the endoplasmic
reticulum. Although the molecular pathways activated by
Wnt3a/β-catenin have been extensively studied, as summarized above,
the mechanisms underlying Wnt production are unclear.
Transmembrane protein 64 (Tmem64) is a seven
transmembrane protein that is localized to the endoplasmic
reticulum and modulates the nuclear localization of β-catenin,
resulting in the activation of β-catenin-mediated transcription
(14). We hypothesized that Tmem64
has a role in Wnt3a secretion. Upon investigating this hypothesis,
we found that introduction of Tmem64 markedly inhibited the
secretion of Wnt3a by triggering its aggregation as an insoluble
cellular fraction. A deletion mutation of Tmem64 which deleted a
transmembrane region did not affect Wnt3a activity and secretion,
suggesting that Tmem64 may participate in the modulation of
Wnt3a/β-catenin signaling through Wnt3a secretion. Interestingly,
Tmem64 was strongly expressed in human prostate cancer cells,
especially PC3 cells. In a metastatic mouse model created by the
intracardiac injection of PC3 cells, Tmem64 expression was
down-regulated in metastatic spine and mandible lesions.
Collectively, these findings suggest that Tmem64 is
involved in the metastatic progression of prostate cancer cells.
These data expand our understanding of the role of Tmem64 in Wnt
secretion and provide further support for the potential of Tmem64
as a therapeutic target against bone metastasis of prostate cancer
cells via dysregulation of Wnt signaling.
Materials and methods
Plasmids
The TOPFlash reporter constructs were kindly
provided by Dr Kyung-Keun Kim (Chonnam National University). Mutant
constructs of Tmem64 were generated using a Site-Directed
Mutagenesis Kit (Invitrogen; Thermo Fisher Scientific, Inc.).
Cell culture and transfection
Human embryonic kidney 293T cells were cultured in
Dulbecco's modified Eagle's medium (Gibco-BRL; Life Technologies,
Inc.) supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin, and 100 µg/ml streptomycin. The DU145, LNCaP and PC3
human prostate cancer cell lines were obtained from the American
Type Culture Collection (ATCC) and cultured in RPMI-1640
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS and 1% penicillin-streptomycin (Life Technologies). Cells were
transfected with the indicated amounts of expression plasmids using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. As an internal
control, a cytomegalovirus β-galactosidase (CMV) plasmid was
co-transfected in each transfection experiment. Forty-eight hours
after transfection, the cells were lysed and assayed with the Dual
Luciferase Reporter Assay System (Promega). Luciferase activity was
normalized to β-galactosidase activity.
Western blotting
Cells were harvested in lysis buffer (Cell Signaling
Technology) and centrifuged for 15 min at 4°C. The Protein
concentration in the supernatant was determined using a DC Protein
Assay kit (Bio-Rad Laboratories). Cellular proteins were separated
by sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred to a polyvinylidene difluoride membrane.
After blocking in Tris-buffered saline with 5% milk and 0.1%
Tween-20, the membrane was incubated with primary antibodies for
active β-catenin (anti-ABC, 1:1,000; Merck Millipore), β-catenin
(anti-β-catenin 1:1,000; Cell Signaling Technology), Tmem64
(anti-Tmem64, 1:1,000; MyBioSource), and β-actin (anti-β-actin
1:2,000; Cell Signaling Technology). Signals were visualized using
an enhanced chemiluminescence reagent (Santa Cruz Biotechnology) in
a LAS-4000 Lumino Image Analyzer system (Fujifilm). The band
intensities were quantified using ImageJ (http://rsbweb.nih.gov/ij/) under the Gel Analysis
Tool. The intensities for each lane was taken as a ratio of the
Tmem64 protein over total protein and then normalized to bands for
the control group, whose intensity was set to one.
Conditioned medium
293T cells were transfected with the Wnt3a vector.
Twenty-four hours later, the medium was replaced with a serum-free
medium. Samples were collected after another 24 h and equal volumes
were loaded and analyzed by SDS-PAGE.
Luciferase reporter assay
Luciferase activity was measured using the
Luciferase Assay System (Promega) and detected using a GENios Plus
luminometer (Tecan Group Ltd.). The pCMV-β-galactosidase (pCMV-gal)
expression vector was added to each transfection, and the
β-galactosidase assay was carried out as described previously to
normalize transfection efficiency (15).
Quantitative PCR
Total cellular RNA was extracted using the
TRIzol-trichloromethane method, and RNA quantity was determined
spectrophotometrically. Synthesis of cDNA was performed using the
Takara PrimeScript® RT reagent Kit (Takara Bio).
Quantitative PCR was performed using the SYBR Premix Ex Taq system
(Takara Bio) and using the following primers: Tmem64 forward,
5′-GGCGTGGCTGAGGTGAGAAA-3′ and reverse, 5′-ATGAAGCCCACGACGAAGAG-3′;
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward,
5′-CCAGTCAGCTTCCCGTTCA-3′ and reverse, 5′-GAACATCATCCCTGCATCCA-3′.
The relative messenger RNA (mRNA) levels were calculated based on
the threshold cycle (Cq) values normalized to the Cq value of
β-actin using the following formula: 2−ΔCq, where
ΔCq=Cqtarget gene-Cqβ-actin. All tests were
performed in triplicate.
Animals
Five-week-old male athymic nude mice (BL-6/Nu;
Orient Bio Co., Ltd.) were housed under controlled light conditions
and fed ad libitum. All experimental procedures involving
animals were performed in compliance with institutional and
government requirements and were approved by the Institutional
Animal Care and Use Committee (CIACUC2015-A0032), Chosun
University, Gwangju, Korea.
Primary or metastatic tumor-bearing
mouse model
Primary tumors were generated by injection of PC3
cells into the flanks of mice. In addition, intracardiac injections
of PC3 cells were administered to examine the ability of tumor
cells to metastasize. Male severe combined immunodeficient mice
(aged 5–6 weeks) were anesthetized using isoflurane gas. PC3 cells
(1×105 per mouse) were injected into the flanks of mice
or the left cardiac ventricle following a modification of a
previously described technique (16). After 4 weeks, mice were sacrificed by
intraperitoneal administration of 0.4% sodium pentobarbital (1
ml/kg) and tumor-bearing tissues were excised and fixed in cold 10%
buffered formalin (Merck). Bone tissue was decalcified in sodium
citrate solution. Decalcified bones were cut at the midpoint and
embedded in paraffin blocks. Tissue sections were obtained and
stained with hematoxylin and eosin (H&E), and images were
captured using a microscope slide scanner (3DHISTECH Ltd.).
Immunohistochemical analysis of bone
specimens
Sections (3 µM thick) were deparaffinized in three
changes of xylene and rehydrated in a graded series of ethanol
solutions (ending with distilled water). For antigen retrieval,
slides were placed in 0.01 M citrate-buffer (pH 6) and heated in a
steamer for 30 min. Endogenous peroxidases were quenched by
incubating with 3% hydrogen peroxide for 20 min at room
temperature. Sections were incubated overnight at 4°C with a 1:50
dilution of primary antibody (anti-Tmem64; Cell Signaling
Technology). Subsequently, sections were incubated for 30 min with
a biotinylated secondary antibody (LSAB; Dako Cytomation), washed
in phosphate buffered saline, and incubated for 30 min with a
streptavidin-peroxidase conjugate (LSAB; Dako Cytomation). The
reaction was developed for 5 min using 3,3′-diaminobenzidine
tetrahydrochloride (Sigma-Aldrich). Slides were briefly
counterstained in hematoxylin, dehydrated, and cover slipped.
Negative and positive controls were run simultaneously. Positive
controls comprised mammary tissue. The slides were captured using a
microscope slide scanner (3D-HISTECH Ltd.).
Statistical analyses
All experiments were repeated at least twice, and
qualitatively identical results were obtained. Statistical analyses
were performed using GraphPad Prism version 5.0 software (GraphPad
Software, Inc.). A two-tailed, paired Student's t-test and one-way
analysis of variance followed by Sidak's multiple comparison test
(unless specifically mentioned otherwise) were used when comparing
more than two groups. Results are reported as means ± standard
deviation of triplicate independent experiments. A P-value <0.05
was considered statistically significant.
Results
Tmem64 is involved in Wnt3a
secretion
To investigate the relationship between Tmem64 and
Wnt3a, we co-transfected the Tmem64 expression vectors with a Wnt3a
construct and analyzed the ability of the transfectants to secrete
Wnt3a. Introduction of Tmem64 significantly reduced the secretion
of Wnt3a into the culture medium in a dose-dependent manner
(Fig. 1A). Next, the transcriptional
response to Wnt/β-catenin signaling was measured using a
Lef/Tcf-responsive promoter driving the luciferase reporter
(TOPFlash), which specifically measures the transcriptional
activation of β-catenin using conditioned medium (CM) containing
the secreted Wnt3a protein. Treatment of cells with
Wnt3a-transfected CM (Wnt3a-CM) stimulated the TOPFlash luciferase
activity (Fig. 1B). However,
Wnt3a-CM with co-transfection of Tmem64 did not affect the
luciferase activity (Fig. 1B). Taken
together, these results suggest the involvement of Tmem64 in Wnt3a
secretion.
 | Figure 1.Effects of Tmem64 on Wnt3a secretion.
(A) HA-tagged Wnt3a-expression vector was co-transfected with
Tmem64 constructs (+, 100 ng; ++, 200 ng) into 293T cells. At 48 h
later, culture medium was collected and cells were harvested. The
medium was used for an immunoprecipitation assay using
HA-conjugated agarose beads. Western blot analysis was performed
using Wnt3a antibody (upper panel). Soluble and insoluble fractions
were analyzed by immunoblotting with Wnt3a and Tmem64 antibodies
(lower panel). Actin was used as a loading control. (B) 293T cells
were transfected with TOPFlash constructs and then treated with the
indicated medium. Luciferase values were measured. Data represent
the mean ± standard deviation of samples in triplicates.
*P<0.05, as indicated. HA, hemagglutinin; Tmem64, transmembrane
protein 64; Rel. Luc, relative luciferase; IP, immunoprecipitation;
WB, western blotting; CM, conditioned medium. |
Tmem64 mutation does not affect Wnt3a
activity and secretion
Tmem64 was predicted as a seven transmembrane
protein by the TMpred transmembrane prediction software.
Specifically, transmembrane sites five and six of Tmem64 were
predicted as important functional regions. To further examine the
role of Tmem64 in Wnt3a secretion, we constructed truncated mutants
of Tmem64 by deletion of transmembrane regions (Fig. 2A). We have previously shown that
Tmem64 modulates the activation of β-catenin-mediated transcription
(14). Therefore, we first examined
the possible control of the transcriptional activity of β-catenin
by Tmem64 mutant constructs. Mutations of Tmem64 showed a marginal
effect in the inhibition of transcriptional activity of β-catenin
compared to the wild-type construct. Among others, the Tmem64-M3
construct (deletion of 181–295 region of Tmem64) strongly relieved
the inhibitory effect of Tmem64 (Fig.
2A). Therefore, we further examined the effect of Tmem64-M3 on
Wnt3a secretion. Tmem64-M3 did not affect the secretion of Wnt3a in
the culture medium and β-catenin protein levels compared to the
wild-type (Fig. 2B). Furthermore,
supernatant of Wnt3a-CM with co-transfection of Tmem64-M3 also did
not affect luciferase activity of TOPFlash (Fig. 2C), suggesting that Tmem64 mediates
the secretion of Wnt3a. In addition, Tmem64 modulates the nuclear
localization of β-catenin, thus inducing β-catenin-mediated
transcriptional activity. We then examined the effect of Tmem64-M3
on β-catenin nuclear accumulation. The results revealed that
β-catenin was located in the nuclei of the cells treated with Wnt3a
(Fig. 2D, upper panel). In contrast,
β-catenin failed to accumulate in the nuclei of the cells
expressing Tmem64. Interestingly, the introduction of Tmem64-M3 did
not affect β-catenin nuclear accumulation (Fig. 2D, lower panel). Thus, the nuclear
localization of β-catenin depends on the association of β-catenin
with Tmem64.
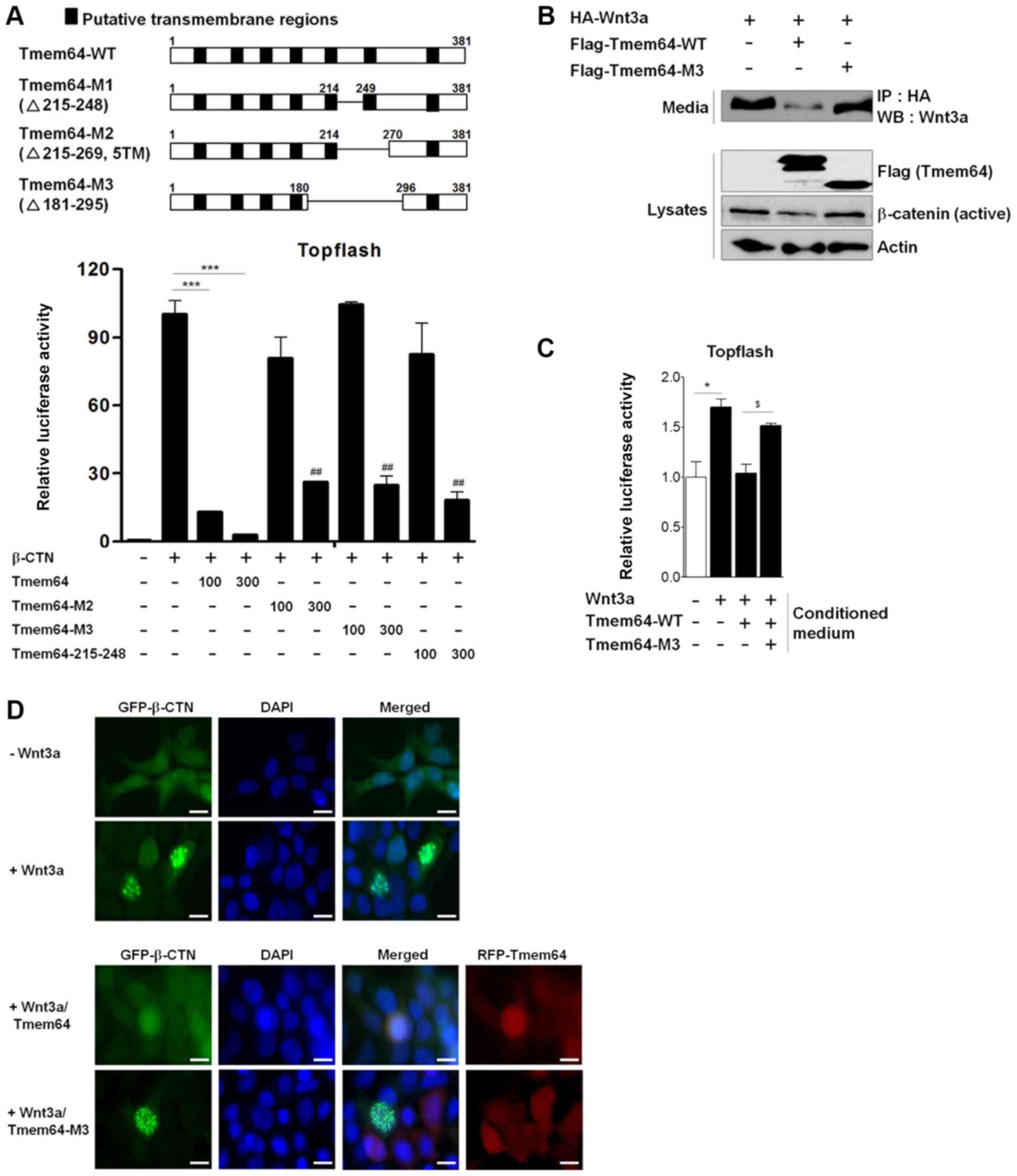 | Figure 2.Tmem64 mutant attenuates Wnt/β-catenin
signaling. (A) Illustration of Tmem64-WT and mutant constructs
(upper panel). 293T cells were co-transfected with TOPFlash
constructs and the indicated constructs, and luciferase values were
measured (lower panel). ***P<0.001, as indicated;
##P<0.01 vs. β-catenin-treated group. (B) HA-tagged
Wnt3a expression construct and Tmem64-WT or Tmem64-M3 were
co-transfected into 293T cells. At 48 h later, the culture medium
was collected and cells were harvested. Medium was subjected to an
IP assay using HA-conjugated agarose beads. Western blot analysis
was performed using a Wnt3a antibody. Lysates were analyzed by
immunoblotting with β-catenin and Tmem64 antibodies (lower panel).
Actin was used as a loading control. (C) 293T cells were
transfected with TOPFlash constructs and the indicated CM was used
as treatment. Luciferase values were measured. Data represent the
mean ± standard deviation of samples in triplicates. *P<0.05 vs.
control group; $P<0.05 vs. Tmem64-WT group. (D) 293T
cells were transfected with β-catenin (GFP) and/or Tmem64 (RFP)
expression vectors and stimulated with Wnt3a for 12 h. Cells were
stained for β-catenin (green immunofluorescence) and Tmem64 (red
immunofluorescence) and counterstained with DAPI (blue). Scale
bars, 20 µm. WT, wild-type; IP, immunoprecipitation; WB, western
blotting; HA, hemagglutinin; Tmem64, transmembrane protein 64; CM,
conditioned medium; GFP, green fluorescent protein; DAPI,
4′,6-diamidino-2-phenylindole; β-CTN, β-catenin. |
Tmem64 is not involved in Wnt3a
glycosylation
As Wnt3a is prominently modified with N-linked
glycans (12), we examined whether
the glycosylation status of Wnt3a is modulated by Tmem64. Tmem64
overexpression was confirmed by western blotting (data not shown).
When Wnt3a was completely de-glycosylated with N-glycosidase F, a
peptide that cleaves asparagine-linked glycans, a single rapidly
migrating 42 kDa band of Wnt3a was observed (Fig. 3A). Moreover, Tmem64-modified Wnt3a
still migrated at 42 kDa, revealing that Tmem64 modification does
not involve an N-glycan. In addition, a series of experiments using
tunicamycin, which inhibits the synthesis of N-linked
glycoprotein, demonstrated that Tmem64 did not affect Wnt3a
expression with asparagine-linked glycans (Fig. 3B).
Involvement of Tmem64 in prostate
cancer progression
Dysregulation of β-catenin is associated with the
development of a number of types of cancers, including prostate
cancer (17). In addition,
inhibition of STMN1, which is overexpressed in aggressive prostate
cancer, significantly downregulates the expression of Tmem64 in
prostate cancer cells (18).
Therefore, we sought to determine the possible involvement of
Tmem64 in prostate cancer cell lines. To explore this possibility,
we first examined the endogenous expression of Tmem64 in various
prostate cancer cell lines derived from human bone metastases
(PC3), lymph node metastases (LNCaP), and brain metastases (DU145).
Interestingly, PC3 cells expressed markedly higher mRNA levels of
Tmem64, but lower levels in both LNCaP and DU145 cells (Fig. 4A). Consistently, western blotting
experiments showed that these changes also occurred at the protein
level in these cells (Fig. 4B). We
next examined whether Tmem64 could participate in the progression
of prostate cancer following subcutaneous implantation of cells or
in the metastasis of prostate cancer cells to bone in vivo
by intracardiac injection of PC3 tumor cells, a well-established
model of experimental bone metastasis (19). An apparent margin between tumor cells
and other stromal cells was observed in PC3-inoculated mice
(Fig. 4C). Tumor cells were
well-distinguished from stromal and other functional cells by the
presence of nuclear atypia with prominent nucleoli, increased
nuclear size, and reduced cytoplasmic-to-nuclear ratio. In case of
mandibular metastatic tumor generated by PC3 inoculation, the tumor
cells showed small irregular acinar configuration and an
infiltrative growth pattern, as revealed by H&E staining.
 | Figure 4.Tmem64 expression in prostate cancer
cells and bone metastatic progression. (A) Total RNA was isolated
from the indicated cell lysates as described and the mRNA levels of
Tmem64 were assessed by reverse transcription-quantitative
polymerase chain reaction. Data are presented as the mean ±
standard deviation of samples in triplicate. (B) Protein levels of
Tmem64 in various prostate cancers were measured by immunoblotting
(left panel). GAPDH was used as the loading control. A
representative image of three independent experiments is shown. The
bar graph was generated by quantifying band intensities from three
independent experiments using ImageJ and normalizing the intensity
of the bands to that of the total protein, which was set to one
(right panel). *P<0.05 and **P<0.01, as indicated (C)
Representative histological sections of tissues bearing
subcutaneous tumors generated by PC3 inoculation, and tumors
generated after intracardiac injection in the mandibular and spine
are shown at both low magnification (magnification, ×10; scale bar,
2,000 µm, upper panel) and high magnification (magnification, ×40;
scale bar, 100 µm, lower panel). (D) Immunohistochemical staining
of Tmem64 and Wnt3a in the subcutaneous primary, mandibular and
spinal tumors generated by PC3 cells are shown. Positive staining
in the subcutaneous nuclei and cytoplasm of tumors (magnification,
×400; scale bar, 50 µm). Tmem64, transmembrane protein 64; H&E,
hematoxylin and eosin. |
In addition, expression patterns of Tmem64 in serial
tissue samples of primary and mandibular metastatic tumors formed
upon PC3-cell inoculation were determined. A prominent Tmem64
signal was observed in the PC3 tumor mass that later developed in a
primary tumor following subcutaneous injection of cells. However,
the expression of Tmem64 was significantly down-regulated in
mandibular and bone metastatic tumors generated by PC3 cell
inoculation (Fig. 4D, upper panel).
In contrast, lower Wnt3a expression was observed in the
PC3-inoculated primary tumor masses and prominent expression was
observed in metastatic and mandibular metastatic tumors generated
by PC3 inoculation (Fig. 4D, lower
panel). Taken together, these results implicate Tmem64 in prostate
tumor progression via its modulation of Wnt3a secretion.
Discussion
Wnt signaling is important in the prostate tumor
microenvironment, where Wnt proteins secreted by the tumor stroma
promote resistance to therapy (20).
Wnt3a has a seminal role in prostate cancer invasion and metastasis
(21). Intracellular accumulation of
β-catenin in response to Wnt3a is a hallmark of the canonical Wnt
signaling pathway and can be used as a reliable marker of Wnt3a
signaling activity. We previously showed that Tmem64 modulates the
Wnt signaling pathway by association with β-catenin to decrease the
protein level and nuclear localization of the latter (22). To assess the effect of Tmem64
overexpression or inhibition on the Wnt/β-catenin signaling
pathway, we utilized a Lef/Tcf reporter that can be activated in
response to Wnt3a or transfection with constitutively active Wnt
signaling components. Overexpression of Tmem64 inhibited β-catenin
accumulation in response to Wnt3a. This result is consistent with
the transcriptional readout and is suggestive of negative
modulation of Wnt/β-catenin signaling by Tmem64.
Several studies have demonstrated that Wnt signaling
is positively correlated with prostate cancer progression, with a
direct role reported in the induction of bone metastasis (23,24). The
expression of β-catenin in bone metastases appears down-regulated
compared to the expression in corresponding primary tumors in
patients with untreated prostate cancer (25). Aberrant mutations in the components
of Wnt signaling have been linked to multiple growth-related
pathologies, particularly to cancer metastasis (26). Several recent studies reported that
aberrantly activated Wnt/β-catenin signaling may induce tumor
formation and progression (27,28).
β-Catenin is a multifunctional transcription factor that is
involved in the Wnt signaling pathway and serves an important role
in oncogenesis in combination with T cell factor and protein kinase
D1 (29). The dysregulation of
β-catenin is associated with development of several types of
cancer, including prostate cancer (17), and the shuttling of β-catenin between
the cytoplasm and the nucleus is pivotal for its pro- or anti-tumor
function (17,30). Therefore, a study focusing on the
regulation of β-catenin would highlight the pathological mechanism
underlying prostate cancer and suggest novel treatment targets. The
results of the present study revealed that Tmem64 decreases the
expression of β-catenin and further support the potential
application of Tmem64 as a therapeutic target in prostate
cancer.
PC3 cells showed stronger expression of Tmem64 in
vitro than the other prostate cancer cell lines. The expression
was prominent in the tumor masses that developed following the
subcutaneous injection of PC3 cells. The intracardiac injection in
mice introduced the tumor cells directly into the circulation, thus
facilitating the survival and growth of prostate cancer cells in
the other organs (31). We used this
mouse model to evaluate the effect of Tmem64 in metastatic prostate
cancer. Elevated Tmem64 expression levels were negatively
correlated with mandibular and spine metastasis after intracardiac
injection. Together, our findings implicate Tmem64 in the
metastasis of prostate cancer to the bone.
Bone metastasis of PCa requires a series of specific
interactions between the cancer and host cells, such as bone marrow
stromal cells (BMSCs), at the metastatic sites (32). Cell-cell interactions between PCa
cells and cells in the bone microenvironment are important and
contribute to metastatic cell behavior. This process results in the
formation of osteosclerotic lesions filled with metastatic prostate
cancer cells. We found that Tmem64 was expressed in prostate cancer
cells and prostate cancer-induced metastatic bone lesions. However,
our study has some limitations that must be mentioned. The
expression level of Tmem64 in bone metastases of prostate cancer
was not measured, and the role of Tmem64 in osteoblastic lesions
induced by prostate cancer was not assessed. The membrane protein
Tmem64 has been reported to restrain bone degradation and promote
bone formation in knockout mice (22). In addition, Tmem64 also inhibited
osteoblast differentiation and stimulated adipogenesis through the
Wnt/β-catenin signaling pathway (14). Therefore, Tmem64 is probably involved
in the formation of osteosclerotic lesions by regulating β-catenin
production. How Tmem64 participates in this metastatic bone erosion
process remains unclear. One possibility is that Tmem64 decreases
the potential of prostate cancer to metastasize to bone by
modulating the secretion of Wnt3a or expression of β-catenin. If
so, primary cancers with high Tmem64 expression levels would show a
poorer prognosis. In addition, mRNA and protein analyses have
indicated that Tmem64 expression is a significant independent
predictor of poorer prognosis in prostate cancer (14). In terms of clinical applications,
Tmem64 may be a candidate marker for the diagnosis of bone
metastatic progression in prostate cancer patients. Further studies
involving larger number of mice or patient cohorts would be needed
to verify this potential.
In conclusion, the present study demonstrated a
novel molecular mechanism involving the inhibition of β-catenin
signaling, through which the regulation of Wnt3a secretion by
Tmem64 is mediated. Tmem64 may act as a modulator of prostate
cancer to inhibit or delay its progression by inhibiting the
transcriptional activity of β-catenin. These findings indicate that
Tmem64 has a role in the metastatic progression of prostate cancer
to the bone. Further studies on use of Tmem64 as a therapeutic
target in prostate cancer bone metastasis in the clinical setting
is warranted.
Acknowledgements
Not applicable.
Funding
This research was supported by Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(grant nos. 2012R1A1A2041418 and 2016R1D1A3B03930719).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YHM, WL and BCJ performed the experiments. YHM and
WL reviewed, analyzed and interpreted the data. BCJ wrote the
paper. All authors discussed the results and commented on the
manuscript. YHM, WL and BCJ take responsibility for the integrity
of the data analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental procedures involving animals were
performed in compliance with institutional and government
requirements and were approved by the Institutional Animal Care and
Use Committee (CIACUC2015-A0032), Chosun University, Gwangju,
Korea.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ciani L and Salinas PC: WNTs in the
vertebrate nervous system: From patterning to neuronal
connectivity. Nat Rev Neurosci. 6:351–362. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y, Li YP, Paulson C, Shao JZ, Zhang
X, Wu M and Chen W: Wnt and the Wnt signaling pathway in bone
development and disease. Front Biosci (Landmark Ed). 19:379–407.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Regard JB, Zhong Z, Williams BO and Yang
Y: Wnt signaling in bone development and disease: Making stronger
bone with Wnts. Cold Spring Harb Perspect Biol. 4:a0079972012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Niemann S, Zhao C, Pascu F, Stahl U,
Aulepp U, Niswander L, Weber JL and Müller U: Homozygous WNT3
mutation causes tetra-amelia in a large consanguineous family. Am J
Hum Genet. 74:558–563. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Day TF, Guo X, Garrett-Beal L and Yang Y:
Wnt/beta-catenin signaling in mesenchymal progenitors controls
osteoblast and chondrocyte differentiation during vertebrate
skeletogenesis. Dev Cell. 8:739–750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rodda SJ and McMahon AP: Distinct roles
for Hedgehog and canonical Wnt signaling in specification,
differentiation and maintenance of osteoblast progenitors.
Development. 133:3231–3244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holmen SL, Zylstra CR, Mukherjee A, Sigler
RE, Faugere MC, Bouxsein ML, Deng L, Clemens TL and Williams BO:
Essential role of beta-catenin in postnatal bone acquisition. J
Biol Chem. 280:21162–21168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Xu Z, Duan C, Liu W, Sun J and Han
B: Role of TCF/LEF transcription factors in bone development and
osteogenesis. Int J Med Sci. 15:1415–1422. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krishnan V, Bryant HU and Macdougald OA:
Regulation of bone mass by Wnt signaling. J Clin Invest.
116:1202–1209. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Komiya Y and Habas R: Wnt signal
transduction pathways. Organogenesis. 4:68–75. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smolich BD, McMahon JA, McMahon AP and
Papkoff J: Wnt family proteins are secreted and associated with the
cell surface. Mol Biol Cell. 4:1267–1275. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Komekado H, Yamamoto H, Chiba T and
Kikuchi A: Glycosylation and palmitoylation of Wnt-3a are coupled
to produce an active form of Wnt-3a. Genes Cells. 12:521–534. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeong BC, Kim TS, Kim HS, Lee SH and Choi
Y: Transmembrane protein 64 reciprocally regulates osteoblast and
adipocyte differentiation by modulating Wnt/β-catenin signaling.
Bone. 78:165–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lim W, Kim J, Kim S, Karna S, Won J, Jeon
SM, Kim SY, Choi Y, Choi H and Kim O: Modulation of
lipopolysaccharide-induced NF-κB signaling pathway by 635 nm
irradiation via heat shock protein 27 in human gingival fibroblast
cells. Photochem Photobiol. 89:199–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arguello F, Baggs RB and Frantz CN: A
murine model of experimental metastasis to bone and bone marrow.
Cancer Res. 48:6876–6881. 1988.PubMed/NCBI
|
|
17
|
He X, Semenov M, Tamai K and Zeng X: LDL
receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling:
Arrows point the way. Development. 131:1663–1677. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Huang Y, Zhang M, Zhang X, Tang X
and Kang Y: Bioinformatic analysis of the possible regulative
network of miR-30a/e in Cardiomyocytes 2 Days post myocardial
infarction. Acta Cardiol Sin. 34:175–188. 2018.PubMed/NCBI
|
|
19
|
Chu K, Cheng CJ, Ye X, Lee YC, Zurita AJ,
Chen DT, Yu-Lee LY, Zhang S, Yeh ET, Hu MC, et al: Cadherin-11
promotes the metastasis of prostate cancer cells to bone. Mol
Cancer Res. 6:1259–1267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murillo-Garzón V and Kypta R: WNT
signalling in prostate cancer. Nat Rev Urol. 14:683–696. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nandana S, Tripathi M, Duan P, Chu CY,
Mishra R, Liu C, Jin R, Yamashita H, Zayzafoon M, Bhowmick NA, et
al: Bone metastasis of prostate cancer can be therapeutically
targeted at the TBX2-WNT signaling axis. Cancer Res. 77:1331–1344.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim H, Kim T, Jeong BC, Cho IT, Han D,
Takegahara N, Negishi-Koga T, Takayanagi H, Lee JH, Sul JY, et al:
Tmem64 modulates calcium signaling during RANKL-mediated osteoclast
differentiation. Cell Metab. 17:249–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Verras M and Sun Z: Roles and regulation
of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett.
237:22–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu X, Wang Y, DeGraff DJ, Wills ML and
Matusik RJ: Wnt/β-catenin activation promotes prostate tumor
progression in a mouse model. Oncogene. 30:1868–1879. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bryden AA, Hoyland JA, Freemont AJ, Clarke
NW, Schembri Wismayer D and George NJ: E-cadherin and beta-catenin
are down-regulated in prostatic bone metastases. BJU Int.
89:400–403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Y, Ye YC, Chen Y, Zhao JL, Gao CC,
Han H, Liu WC and Qin HY: Crosstalk between hepatic tumor cells and
macrophages via Wnt/β-catenin signaling promotes M2-like macrophage
polarization and reinforces tumor malignant behaviors. Cell Death
Dis. 9:7932018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kishida S, Yamamoto H, Ikeda S, Kishida M,
Sakamoto I, Koyama S and Kikuchi A: Axin, a negative regulator of
the wnt signaling pathway, directly interacts with adenomatous
polyposis coli and regulates the stabilization of beta-catenin. J
Biol Chem. 273:10823–10826. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Novellasdemunt L, Antas P and Li VS:
Targeting Wnt signaling in colorectal cancer. A review in the
theme: Cell signaling: Proteins, pathways and mechanisms. Am J
Physiol Cell Physiol. 309:C511–C521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Powell SM, Zilz N, Beazer-Barclay Y, Bryan
TM, Hamilton SR, Thibodeau SN, Vogelstein B and Kinzler KW: APC
mutations occur early during colorectal tumorigenesis. Nature.
359:235–237. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee HJ, Li J, Vickman RE, Li J, Liu R,
Durkes AC, Elzey BD, Yue S, Liu X, Ratliff TL and Cheng JX:
Cholesterol esterification inhibition suppresses prostate cancer
metastasis by impairing the Wnt/β-catenin Pathway. Mol Cancer Res.
16:974–985. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suva LJ, Washam C, Nicholas RW and Griffin
RJ: Bone metastasis: Mechanisms and therapeutic opportunities. Nat
Rev Endocrinol. 7:208–218. 2011. View Article : Google Scholar : PubMed/NCBI
|


















