Introduction
Gliomas are common malignant brain tumors, with an
incidence of 3–4 new cases per 100,000 adults each year worldwide
(1). As a result of the aggressive
nature of gliomas, prognosis is poor; the median survival time for
patients is ~1 year, with <10% of patients surviving >3 years
post-diagnosis (2). The standard
treatment for glioblastoma is radiotherapy alongside temozolomide
(TMZ) chemotherapy. However, its efficacy is limited; even with
treatment, the median survival time of patients with gliomas is
12–18 months (3).
Bevacizumab (BEV) inhibits tumor growth by binding
to vascular endothelial growth factor and preventing its
interaction with receptors on the endothelial cell surface.
Survival data from the GENOM 009 trial (a randomized phase II
trial), which compared TMZ treatment to TMZ/BEV combination therapy
in adult patients with unresected glioblastoma, are currently used
for cost-effective analysis (4). The
trial results indicated that both progression-free survival (PFS;
4.8 months vs. 2.2 months) and overall survival (OS; 10.6 months
vs. 7.7 months) were longer in patients treated with combination of
BEV and TMZ (4). Although this
increase in survival was not statistically significant, these
clinical data may provide a new option for patients and policy
makers in the treatment of unresected glioblastoma.
Considering the efficacy and high cost of BEV, it
was included in the National Health Insurance Directory (2017)
following negotiations between the government and pharmaceutical
companies. Thus, the retail price of BEV (100 mg/4 ml) was reduced
from $852.23/unit to $305.38/unit in Sichuan, a 64.17% reduction.
Considering the efficacy of BEV in glioma treatment, the rapidly
rising cost of drugs and the current lack of medical resources, a
cost-effectiveness analysis would be useful to measure the
potential economic benefit of BEV/TMZ co-treatment of patients with
glioblastoma, particularly in China (a developing country). In the
current study, a Markov model was used to estimate the
cost-effectiveness of BEV/TMZ co-therapy as a neoadjuvant treatment
option for patients with glioblastoma.
Materials and methods
Patients and therapy
The clinical data used in the present study were
retrieved from the GENOM 009 trial (4), a phase II study in which 102 patients,
including 54% (55/102) male and 46% (47/102) female, (mean, 62;
range, 36–75 years) with unresected glioblastoma were randomized to
either the TMZ control arm or the TMZ+BEV treatment arm (ratio,
1:1). The inclusion criteria included: i) Patients with unresected
glioblastoma; ii) diagnosis using biopsy (including stereotactic or
open craniotomy); iii) no prior treatment; iv) a tumor size ≥2 cm;
and v) exhibiting stable or decreasing glucocorticoid doses within
5 days of randomization. Moreover, in patients undergoing
craniotomy, post-operative magnetic resonance imaging (MRI) was
mandatory within 72 h. Other inclusion criteria including: Age ≥18
years, Eastern Cooperative Oncology Group performance status 0–2,
Barthel index ≥50%, adequate healing of craniotomy or cranial
biopsy (infection or bleeding at the wound site), normal baseline
hematology and biochemistry, and the absence of proteinuria. The
exclusion criteria were: i) Patient history of a prior malignant
infiltrating disease during the last five years; ii) uncontrolled
arterial hypertension; iii) inflammatory digestive disease; iv)
cardiac or vascular disease; and v) recent symptomatic intracranial
hemorrhage discovered using post-operative MRI or post-biopsy
computerized tomography. Patients were randomly assigned to receive
BEV (10 mg/kg/day; days 1 and 15; administrated IV drip) and
TMZ (85 mg/m2/day; days 1–21; administrated per
os), or TMZ alone (85 mg/m2/day; days 1–21;
administrated per os) for two 28-day cycles. After a 28-day
break, BEV maintenance was allowed in the BEV + TMZ group for a
maximum of six cycles, until the disease progressed or unacceptable
toxic effects developed.
Model structure
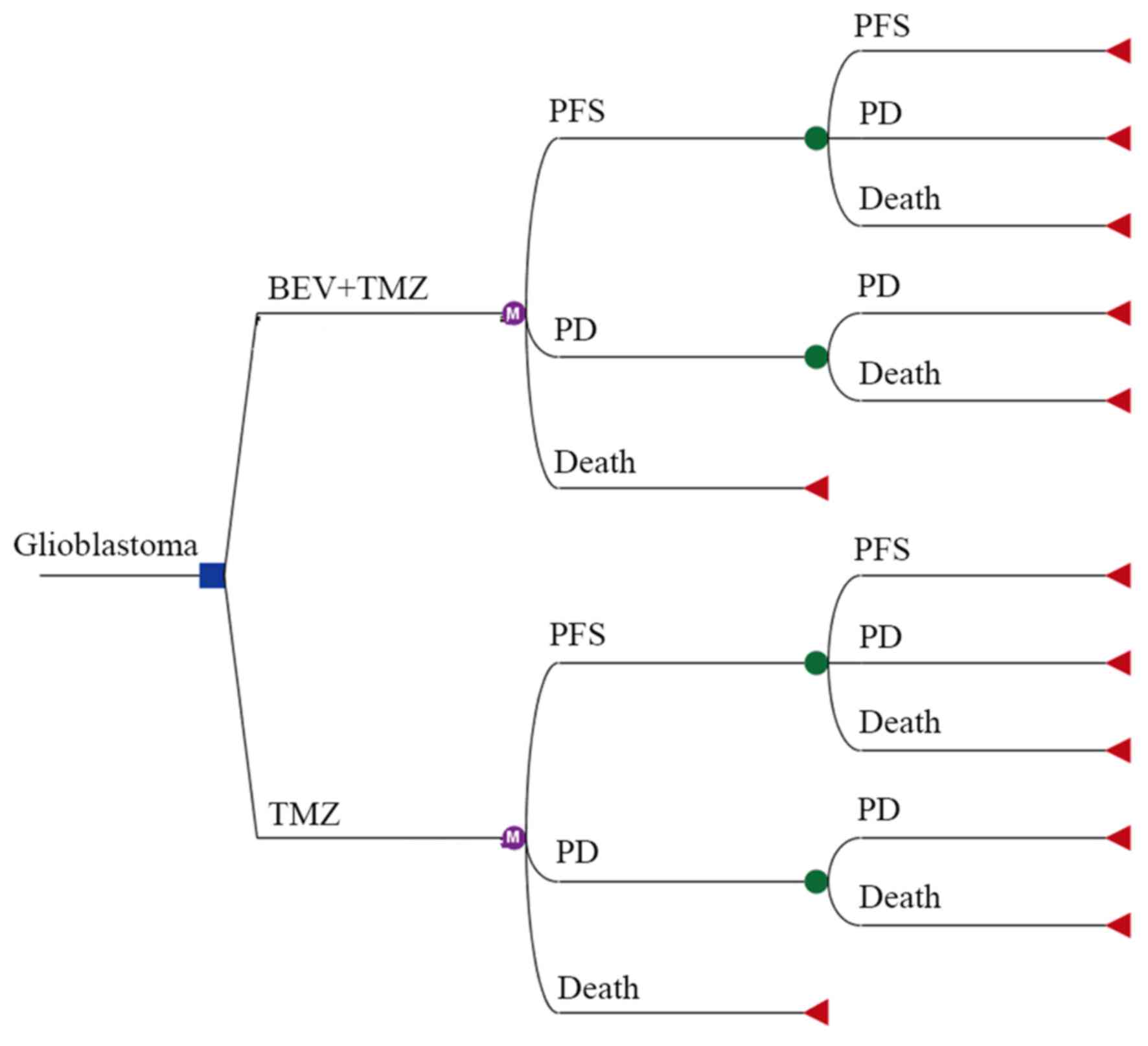
A Markov decision tree model was selected to compare
the clinical and economic data associated with the two therapeutic
strategies (Fig. 1). The model is
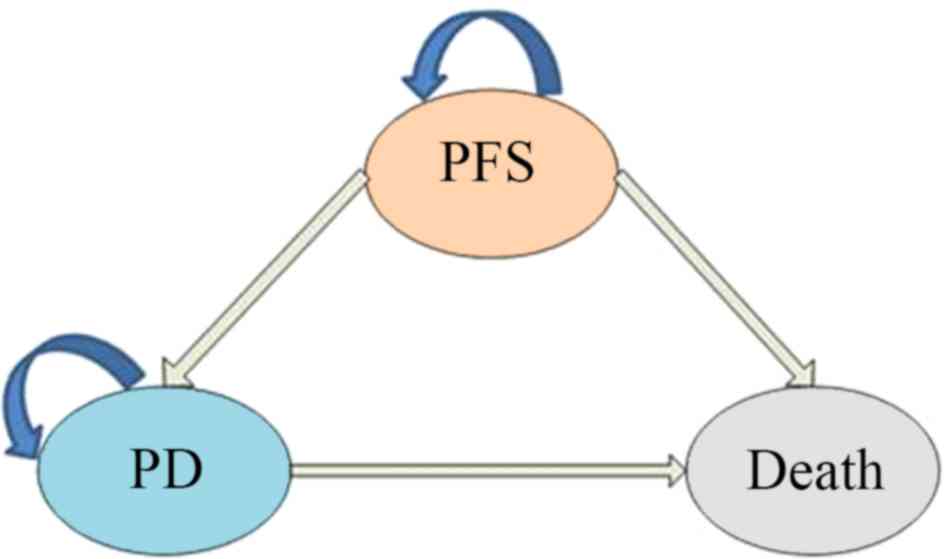
composed of mutually exclusive disease states, including
progression-free survival (PFS), progressive disease (PD) and
death. All patients with unresected glioblastoma begin treatment in
the PFS state, but they may move between health states over time
(Fig. 1 and 2). Following a Markov cycle, patients in
the PFS state may transition to the PD or death state, or may
remain in the PFS state. However, patients in the PD state cannot
return to the PFS state, and all patients may transition to the
death state (Fig. 2). For patients
with unresected tumors, a poor prognosis is common, with no
significant difference in overall survival between treatment with
standard chemoradiotherapy, and radiotherapy alone (9.4 months vs.
7.8 months; hazard ratio [HR], 0.7, 95% CI 0.5–0.9) (5). The absence of previous debulking
surgery increases the likelihood of neurological instability during
treatment (5). According to the
clinical symptoms and progression time of the disease, the
transition cycle length was adjusted to one month in the current
study. Monthly transition probabilities were converted from median
survival estimates (Table I) using
the following formula: P(1 month)=1-(0.5)^(1/median time to event),
which was derived through the formula P=1-e-R, where R=
-ln[0.5]/(time to event/number of treatment cycles) (6,7). The
time horizon chosen for this model was 10 years, at which timepoint
all patients had succumbed to the disease.
 | Table I.Transition probabilities between
glioblastoma disease states. |
Table I.
Transition probabilities between
glioblastoma disease states.
| Transition
probabilities | Baseline value | Lower limit | Upper limit |
|---|
| BEV+TMZ group |
|
|
|
|
PPFS-PFS-1 | 0.81 | 0.65 | 0.97 |
|
PPFS-PD-1 | 0.13 | 0.11 | 0.16 |
|
PPFS-death-1 | 0.06 | 0.05 | 0.08 |
|
PPD-PD-1 | 0.89 | 0.71 | 1.00 |
|
PPD-death-1 | 0.11 | 0.09 | 0.14 |
| TMZ group |
|
|
|
|
PPFS-PFS-2 | 0.64 | 0.51 | 0.77 |
|
PPFS-PD-2 | 0.27 | 0.22 | 0.32 |
|
PPFS-death-2 | 0.09 | 0.07 | 0.10 |
|
PPD-PD-2 | 0.88 | 0.71 | 1.00 |
|
PPD-death-2 | 0.12 | 0.09 | 0.14 |
Model parameters
Costs were estimated from a Chinese societal
perspective (Table II). The
following costs were considered during analysis: Anticancer drugs,
tests (enhanced head CT, blood biochemical examination), management
of grade 3–4 adverse events (AEs), absenteeism and hospitalization.
Implicit costs were ignored as a consequence of individual
differences. The dosage of anticancer drugs was calculated based on
the median reported body surface area and weight in China (8). Unit price for each drug and test was
obtained by consulting the 2017 fee standards of West China
Hospital, Sichuan University. Regarding the data retrieved from the
GENOM 009 trial, the cost of second-line treatment in the two
groups was estimated. In the control group, TMZ was administered
orally in the outpatient department, whilst patients in the
treatment group needed to be hospitalized for intravenous
administration. Consequently, loss-of-productivity was estimated to
be higher in the treatment group. The present study estimated the
cost of absenteeism by referring to the gross domestic product
(GDP) per capita of Sichuan in 2017 ($7,057.26/person/year)
(9). All costs were converted to US
dollars, with an exchange rate of $1=¥6.3339 (Jan, 2018) (10).
 | Table II.Model parameters related to costs and
effectiveness collected from the GENOM 009 trial. |
Table II.
Model parameters related to costs and
effectiveness collected from the GENOM 009 trial.
| Parameter | BEV+TMZ group | TMZ group |
|---|
| Clinical efficacy,
months |
|
|
| Median
PFS, months (95% CI) | 4.8 (4.0–5.6) | 2.2 (2.0–2.5) |
| Median
OS, months (95% CI) | 10.6 (6.9–14.3) | 7.7 (5.4–10.0) |
| Probability of grades
3–4 adverse events, % |
|
|
|
Hypertension | 4.17 | 0.00 |
|
Hemorrhage | 2.08 | 0.00 |
|
Gastrointestinal
perforation | 4.17 | 0.00 |
|
Thrombocytopenia | 2.08 | 11.11 |
|
Leucopenia | 2.08 | 4.44 |
| Febrile
neutropenia | 2.08 | 4.44 |
| Nausea
and vomiting | 2.08 | 0.00 |
|
Infection | 10.42 | 6.67 |
|
Thrombotic events | 4.17 | 6.67 |
|
Asthenia | 4.17 | 2.22 |
| Unit costs,
$/months |
|
Bevacizumab,100 mg/4 ml | 4140.22 | 0.00 |
|
Temozolomide,100 mg ×5 | 3,691.64 | 4,798.422 |
| Cost of
tests | 60.32 | 55.36 |
|
Hospitalization | 47.36 | 0.00 |
| Absence
from work | 108.35 | 78.80 |
| Cost for
adverse events | 68.71 | 132.98 |
| Cost for
the progressive disease state | 1,990.75 | 1,282.90 |
| Annual
discount rate, % | 3.00 | 3.00 |
Data on health outcomes in the present model were
derived from the GENOM 009 study. Survival time was adjusted to
quality-adjusted life years (QALY) using both utility scores and
transition probabilities of the health state. Given that no
information on utilities was available in the original literature,
health utility values were acquired from a study that used the
standard gamble to evaluate glioblastoma health states, based on a
small section of the general population of the United Kingdom
(11) Consequently, the utility
values for the progression-free disease, progressive disease, and
death states were 0.89, 0.73 and 0.00, respectively. Model
parameters related to costs and effectiveness, which were derived
from the GENOM 009 trial, are depicted in Table II. In view of the recommendations of
the 2015 China Guidelines for Pharmacoeconomic Evaluations and
Manual (12), both the costs and the
utility values were discounted at an annual rate of 3%.
Sensitivity analyses
All parameters used in the evaluation (excluding
discount rate) varied in the results of the deterministic one-way
sensitivity analysis (range=±20%). The discount rate ranged between
0 and 8%. Probabilistic sensitivity analysis was conducted using a
second-order Monte Carlo simulation by running 1,000 iterations
(13). Cost-effectiveness
acceptability analysis was conducted to evaluate optimal strategies
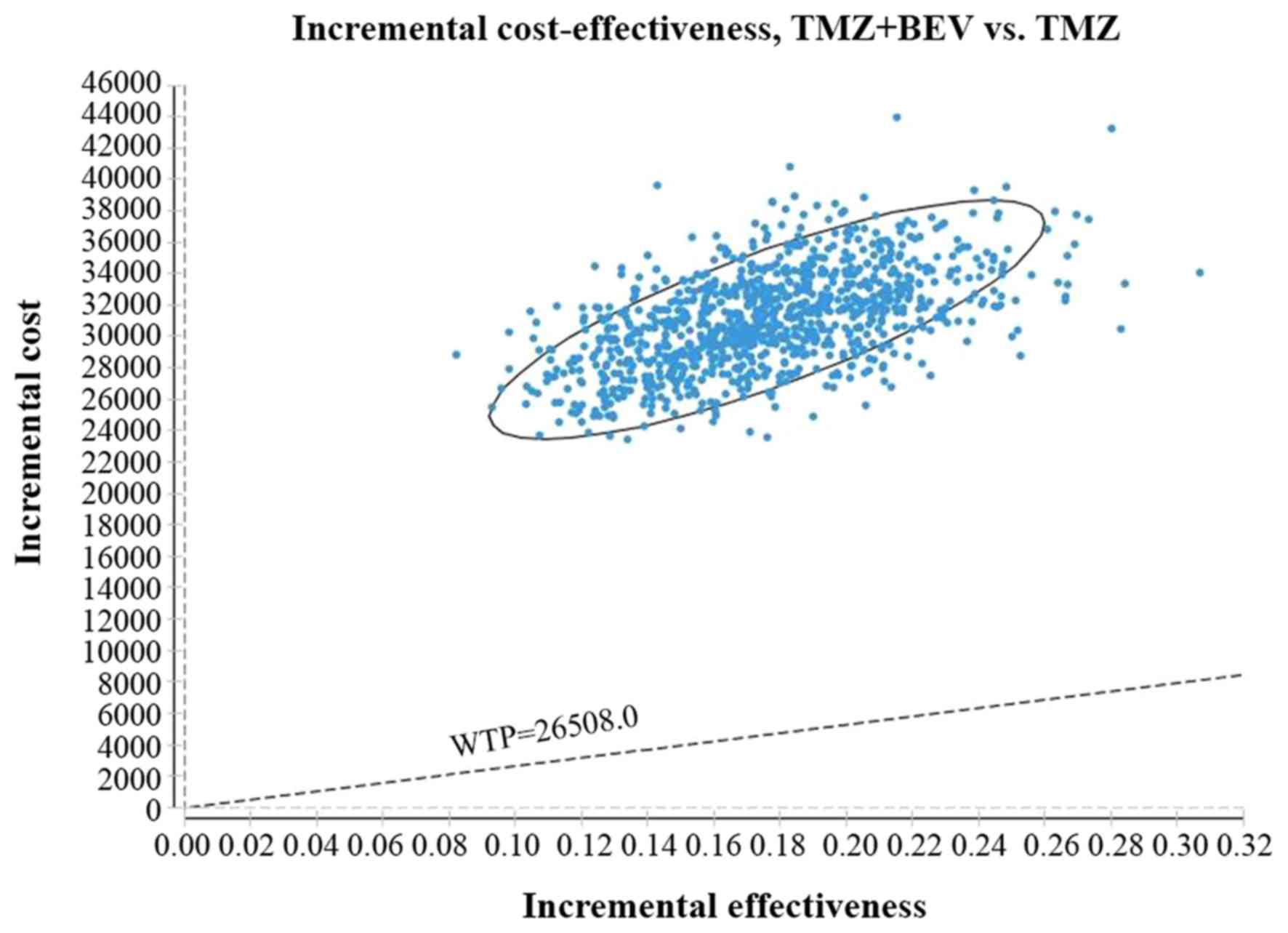
at various willingness-to-pay (WTP) thresholds. The WTP threshold
was set as three times the GDP per capita in 2017, which was
$26,508.00/QALY in China (14).
Results
Cost-effectiveness analysis
Over a 10-year time horizon, the addition of BEV to
TMZ treatment increased the total QALYs by 0.18 (0.71 vs. 0.53
QALYs). However, total costs in the BEV+TMZ group were
significantly higher than in the TMZ group ($50,190.52 vs.
$19,295.53). Therefore, the co-administration of BEV had an
incremental cost-effectiveness ratio (ICER) of $171,638.83/QALY
compared with TMZ alone, exhibiting a 0% chance of being cost
effective at the WTP threshold of $26,508.00/QALY (Table III). Accounting for the increase in
total costs, the addition of BEV to TMZ treatment was not an
economically viable treatment option for unresected glioblastoma
from a Chinese societal perspective. The cost of combination
treatment for patients in the PFS disease state was >3 times
that of TMZ treatment alone ($38,211.30 vs. $11,476.12). Moreover,
when compared with the TMZ group, the overall costs for the PD
state in the BEV+TMZ group were $4,159.81 higher per person
($11,979.22 vs. $7,819.41).
 | Table III.Results of the cost-effectiveness
analysis. |
Table III.
Results of the cost-effectiveness
analysis.
| Parameter | BEV+TMZ group | TMZ group |
|---|
| Costs for the PFS
state, $ | 38,211.30 | 11,476.12 |
| Costs for the PD
state, $ | 11,979.22 | 7,819.41 |
| Total costs, $ | 50,190.52 | 19,295.53 |
| Incremental costs,
$ | 30,894.99 | / |
| Effectiveness for
the PFS state, QALYs | 0.35 | 0.17 |
| Effectiveness for
the PD state, QALYs | 0.36 | 0.36 |
| Total
effectiveness, QALYs | 0.71 | 0.53 |
| Incremental
effectiveness, QALYs | 0.18 | / |
| Total C/E,
$/QALY | 70,690.87 | 36,406.66 |
| ICER, $/QALY | 171,638.83 | / |
Sensitivity analysis
One-way sensitivity analysis
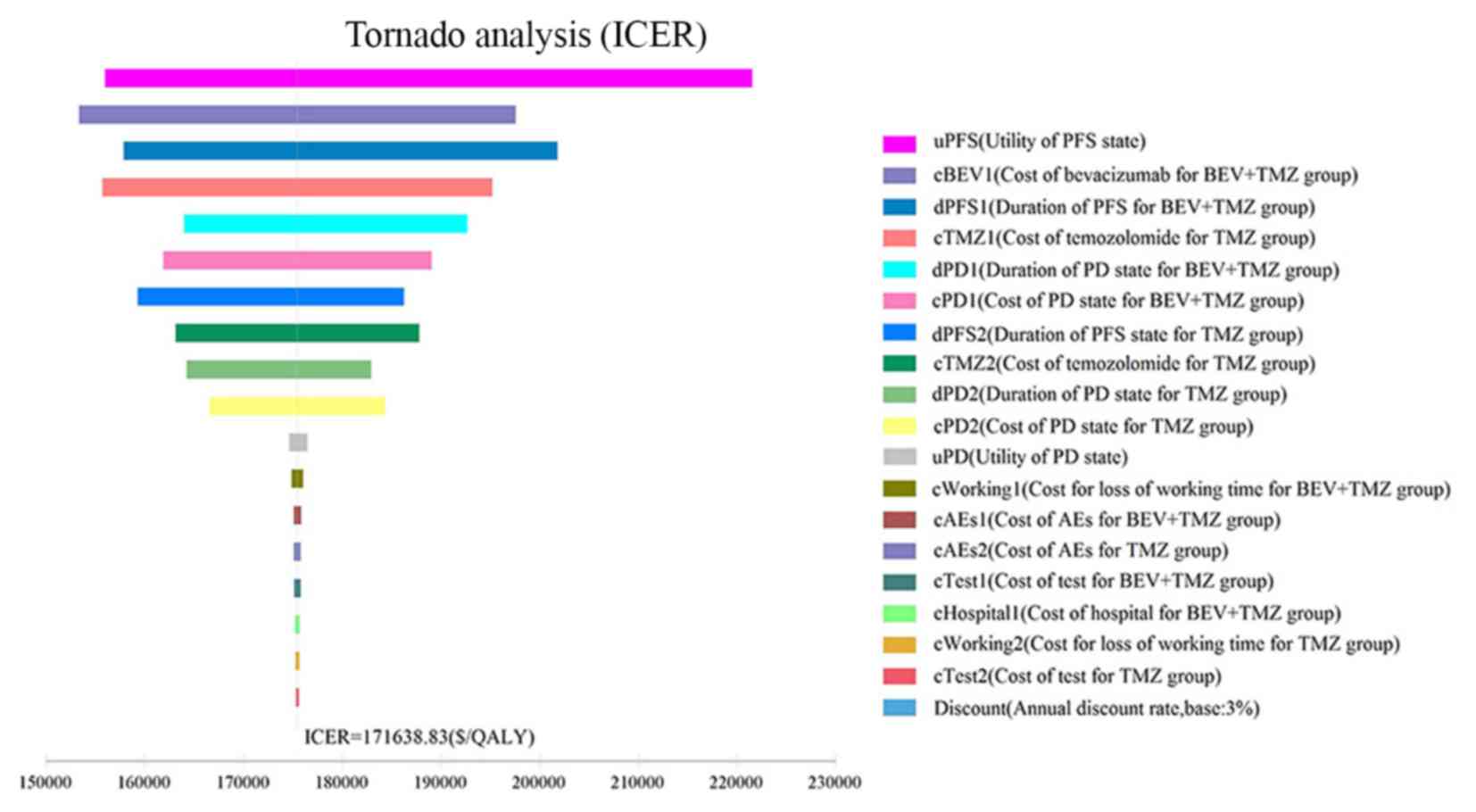
One-way sensitivity analysis was conducted to assess
the impact of individual parameters in the Markov model. The
results are illustrated using a tornado diagram (Fig. 3). The utility of the PFS state, cost
of BEV and the duration of the PFS state in the BEV+TMZ group all
showed a variation of ±20%, and were the most influential
parameters of the model. Changing the utility of PFS from 0.71 to
1.00 caused the ICER to decrease from $221,561.85 to
$155,990.85/QALY. Variation in the cost of BEV treatment from
$3,312.18/month to $4,968.27/month saw the ICER increase from
$153,324.46/QALY to $197,603.80/QALY. However, variations in the
costs related to management of grade 3–4 AEs, tests used or
hospital fees incurred, had a smaller impact on the ICER values
predicted by sensitivity analysis.
Probabilistic sensitivity
analysis
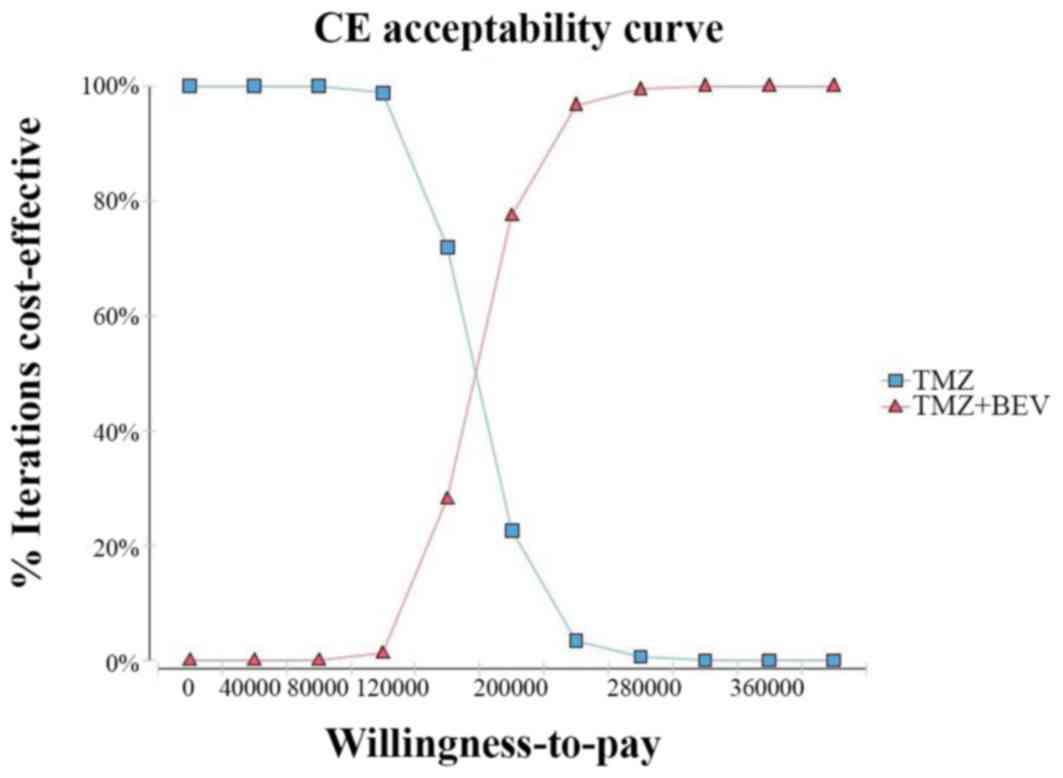
The acceptability curve revealed that the acceptable
proportion of BEV and TMZ co-administration being a cost-effective
treatment for this patient population in China, compared with TMZ
monotherapy, was zero, unless the WTP increased to $80,000/QALY
(Fig. 4). Additionally,
probabilistic sensitivity analysis (1,000 iterations) demonstrated
that the ICER was consistently greater than $26,508.00/QALY
(Fig. 5).
Discussion
According to the Markov model used in the present
study, the total costs were estimated at $50,190.52 for the BEV+TMZ
arm and $19,295.53 for the TMZ arm (a difference of $30,894.99).
The BEV+TMZ arm exhibited a higher number of QALYs gained (0.71)
compared with the TMZ arm (0.53). The ICER, or the cost per QALY
gained, was $171,638.83 in the base case analysis, which was higher
than the accepted WTP threshold ($26,508.00) in China (15). Thus, the addition of BEV to TMZ
treatment cannot be considered a cost-effective option for
unresected glioblastoma, from a Chinese societal perspective.
BEV, a monoclonal antibody, was first approved by
the US Food and Drug Administration for the treatment of recurrent
glioblastoma in 2009 (16). A
systematic review examining the treatment of newly diagnosed
glioblastoma showed that additional BEV significantly increased the
median PFS time (10.6–13.6 months vs. 6.2–7.6 months) (HR, 0.74;
95% CI, 0.62–0.88; P=0.0009) (17).
However, several pharmacoeconomic studies have concluded that
combining BEV with other antineoplastic drugs is not cost-effective
(16–18). In Canada, the ICER associated with
BEV treatment was $607,966/QALY for high-grade glioma, which far
exceeded the WTP threshold of $100,000/QALY, and did not fall below
$450,000/QALY in one-way sensitivity analysis (18). The estimated ICER of chemotherapy
plus BEV was $295,164/QALY for cervical cancer, based on a phase
III randomized trial (19).
Moreover, in a USA study, BEV and chemotherapy increased the mean
QALYs by 0.13 for patients with advanced non-small cell lung
cancer, compared with chemotherapy only. However, the ICER was ~5.6
times that of the accepted WTP threshold in the USA (20).
In accordance with the World Health Organization
Guide to Generalized Cost-Effectiveness Analysis (15), the present study also determined that
BEV/TMZ combination therapy was not a cost-effective treatment
option for patients with unresected glioblastoma in China. In
consideration of its efficacy and high cost, BEV was included in
the National Health Insurance Directory (2017) following
negotiations between the government and pharmaceutical companies.
Prices of BEV haver remained the same. Although the price reduction
did not reduce the ICER below the WTP threshold, a further price
reduction, social assistance and/or medical insurance may
contribute to making BEV more affordable for patients.
A cost reduction in BEV treatment would evidently
improve cost-effectiveness. One-way sensitivity analysis
demonstrated that the ICER increased from $153,324.46/QALY to
$197,603.80/QALY in response to a change in BEV treatment costs
from $3,312.18/month to $4,968.27/month. Although the cost of BEV
was previously reduced by 64.17%, the ICER remained markedly higher
than the WTP threshold. The high cost of targeted therapies present
a financial barrier to multiple treatments in the Chinese
healthcare system. However, reflecting on the promising treatment
improvements shown, provinces with a high GDP should consider the
inclusion of BEV treatment in the local supplement list.
In the present study, a Markov decision tree model
was constructed to simulate the glioblastoma disease process.
However, there were some limitations: i) The cost-effectiveness
analysis model was based on a phase II trial; ii) the patients were
not Chinese; and iii) the treatment costs were determined using
prices from Sichuan, which are subject to variation throughout the
rest of China.
In summary, the results of the present study
indicated that a combination of BEV and TMZ as a first-line
treatment option for unresected glioblastoma, is not cost-effective
from a societal perspective in China, and that the ICER is
significantly higher than the WTP threshold ($26,508.00) in China.
However, an appropriate price reduction and social assistance
should be considered to ensure BEV is more affordable for this
patient population.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
TX conceived and designed the experiments. ZYC and
MZ performed the experiments. ZYC and MZ performed data analysis.
ZYC and FYT provided the reagents, materials and analysis tools.
ZYC wrote the manuscript. FYT revised the work critically for
important intellectual content. All authors have read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Therese A, Dolece k, Jennifer M, Prop p,
Nancy E, Strou p and Carol Kruchko: CBTRUS statistical report:
Primary brain and central nervous system tumors diagnosed in the
united states in 2005–2009. Neuro Oncol. 14 (Suppl):v1–v49. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aldape KD, Okcu MF, Bondy ML and Wrensch
M: Molecular epidemiology of glioblastoma. Cancer J. 9:99–106.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balana C, De Las Penas R, Sepúlveda JM,
Gil-Gil MJ, Luque R, Gallego O, Carrato C, Sanz C, Reynes G,
Herrero A, et al: Bevacizumab and temozolomide versus temozolomide
alone as neoadjuvant treatment in unresected glioblastoma: The
GENOM 009 randomized phase II trial. J Neurooncol. 127:569–579.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomized phase III study: 5-Year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Purmonen T, Martikainen JA, Soini EJ,
Kataja V, Vuorinen RL and Kellokumpu-Lehtinen PL: Economic
evaluation of sunitinib malate in second-line treatment of
metastatic renal cell carcinoma in Finland. Clin Ther. 30:382–392.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miller DK and Homan SM: Determining
transition probabilities: Confusion and suggestions. Med Decis
Making. 14:52–58. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu B, Ye M, Chen H and Shen JF: Costs of
trastuzumab in combination with chemotherapy for HER2-positive
advanced gastric or gastroesophageal junction cancer: An economic
evaluation in the Chinese context. Clin Ther. 34:468–479. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
http://www.sc.gov.cn/10462/12771/2018/1/31/10444117.shtmlMarch
15–2018
|
|
10
|
http://www.boc.cn/sourcedb/whpj/Jan 31–2018
|
|
11
|
Garside R, Pitt M, Anderson R, Rogers G,
Dyer M, Mealing S, Somerville M, Price A and Stein K: The
effectiveness and cost-effectiveness of carmustine implants and
temozolomide for the treatment of newly diagnosed high-grade
glioma: A systematic review and economic evaluation. Health Technol
Assess. 11:iii–iv, ix-221. 2007. View
Article : Google Scholar
|
|
12
|
https://book.douban.com/subject/26922525/Jan
31–2018
|
|
13
|
Wu B, Miao Y, Bai Y, Ye M, Xu Y, Chen H,
Shen J and Qiu Y: Subgroup economic analysis for glioblastoma in a
health resource-limited setting. PLoS One. 7:e345882012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
http://www.stats.gov.cn/english/PressRelease/201801/t20180118_1574943.htmlJan
31–2018
|
|
15
|
Murray CJ, Evans DB, Acharya A and
Baltussen RM: Development of WHO guidelines on generalized
cost-effectiveness analysis. Health Econ. 9:235–251. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cohen MH, Shen YL, Keegan P and Pazdur R:
FDA drug approval summary: Bevacizumab (Avastin) as treatment of
recurrent glioblastoma multiforme. Oncologist. 14:1131–1138. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu P, He YS, Huang Q, Ding T, Cen YC, Zhao
HY and Wei X: Bevacizumab treatment for newly diagnosed
glioblastoma: Systematic review and meta-analysis of clinical
trials. Mol Clin Oncol. 4:833–838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kovic B1 and Xie F: Economic evaluation of
bevacizumab for the first-line treatment of newly diagnosed
glioblastoma multiforme. J Clin Oncol. 33:2296–2302. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Minion LE, Bai J, Monk BJ, Robin Keller L,
Ramez EN, Forde GK, Chan JK and Tewari KS: A Markov model to
evaluate cost-effectiveness of antiangiogenesis therapy using
bevacizumab in advanced cervical cancer. Gynecol Oncol.
137:490–496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goulart B and Ramsey S: A trial-based
assessment of the cost-utility of bevacizumab and chemotherapy
versus chemotherapy alone for advanced non-small cell lung cancer.
Value Health. 14:836–845. 2011. View Article : Google Scholar : PubMed/NCBI
|