Introduction
Lung cancer is the most common malignancy diagnosed
worldwide and represents the leading cause of cancer
associated-mortality. Among all types of lung cancer, non-small
cell lung cancer (NSCLC) is the most frequent, accounting for ~85%
of all lung cancer cases (1).
Although lung neoplasm pathogenesis is known to rise
constitutively, it is crucial to better understand the mechanisms
of cancer progression, including mutual interactions between
malignant and immune cells (2,3). Due to
immune regulatory properties, cancer cells regulate innate and
adaptive immune responses, abolishing antitumor activities and
supporting mechanisms promoting tumor progression (4,5). Among
tumor-infiltrating immune cells, the vast majority is represented
by myeloid cells, such as monocytes/macrophages, which are referred
to as tumor-associated macrophages (6,7). Due to
pleiotropic biological activities, myeloid cells play a central
role in the regulation of the tumor microenvironment by means of
secreted soluble factors, including proteins belonging to the tumor
necrosis factor (TNF) superfamily (8,9).
B-cell activating factor (BAFF) and a
proliferation-inducing ligand (APRIL) are relatively newly
discovered TNF superfamily members. Both ligands might act through
direct interaction with shared receptors, such as B-cell maturation
antigen (BCMA) and transmembrane activator and CML interactor
(TACI), while BAFF is also explicitly recognized by BAFF receptor
(BAFF-R). Both ligands serve a crucial role in B cell development,
maturation and immunoglobulin class switching (10). Furthermore, BAFF was shown to
stimulate anti-apoptotic signaling in tumor cells (8,11).
Previous studies demonstrated that BAFF could play a role in
neoplasm progression and aggressiveness (12–14). In
addition, it was reported that both BAFF and APRIL signaling might
increase tumor cell proliferation and enhance tumor cell viability
in response to chemotherapeutic drugs in hematopoietic malignancies
(8,9,15).
At present, to the best of our knowledge, the role
of BAFF and APRIL in solid tumors development and progression
remains unknown. However, the expression of their receptors was
mainly reported in tumor-infiltrating cells (16,17).
Interestingly, elevated blood levels of BAFF and APRIL are
associated with a higher stage of the disease and cancer
invasiveness, such as in breast cancer, chronic lymphocytic
leukemia and pancreatic cancer (12,18).
Furthermore, the elevated expression of BAFF and APRIL is
associated with increased cell migration, epithelial-mesenchymal
transistion and stemness, subsequently contributing to an
aggressive phenotype of breast cancer (12,19,20).
Similarly, APRIL overexpression was shown to be associated with
tumor progression and was therefore proposed as a potential
prognostic factor in rectal cancer, pancreatic adenocarcinoma and
various B-cell malignances (13). In
addition, in hepatocellular carcinoma cells, APRIL can play
pleiotropic biological activities. Subsequently, APRIL may support
tumor growth or reduce cell proliferation, depending on the
particular pathway activation (21).
Previous studies reported for the first time the importance of BAFF
and APRIL signaling in NSCLC (22,23).
However, to date, it remains unclear whether BAFF and APRIL may
serve a direct role in regulating lung cancer cell proliferation
and invasiveness (22,24). The present study aimed therefore to
evaluate the effects of BAFF and APRIL on NSCLC cell proliferation
and invasiveness.
Materials and methods
Cell lines
The human NSCLC cell lines A549 and H2030 were
purchased from the American Type Culture Collection. Cells were
cultured in Dulbecco's modified Eagles medium (PAN Biotech UK,
Ltd.) supplemented with 10% of heat-inactivated and filtered FBS
(PAN Biotech UK, Ltd.) and gentamycin (50 µg/ml; Gibco; Thermo
Fisher Scientific, Inc.) and placed at 37°C in a humidified
incubator containing 5% CO2. Cells were passaged when at
70–80% confluence. Cells from the second to fifth passage were used
for all experiments.
Reverse transcription quantitative
PCR
To assess the expression level of BAFF, APRIL,
BAFF-R, BCMA and TACI, cells were harvested, washed with PBS and
lysed in RLT buffer (Qiagen) supplemented with 1% β-mercaptoethanol
(Sigma-Aldrich; Merck KGaA) at room temperature. Total RNA was
extracted using the RNeasy Mini Kit (Qiagen) according to the
manufacturers' protocol and quantified using NanoDrop (NanoDrop
2000c/2000; Thermo Fisher Scientific, Inc.). Reverse transcription
was performed using the High-Capacity cDNA Reverse Transcription
Kit (Thermo Fisher Scientific, Inc.) according to manufacturers'
instructions. Commercially available TaqMan assays (Thermo Fisher
Scientific, Inc.; Table I) and
TaqMan Universal PCR Master Mix (Thermo Fisher Scientific, Inc.)
were used according to manufacturers' instructions. The
thermocyclig conditions were as follows: Polymerase activation at
95°C for 10 min followed by 40 cycles of denaturation at 95°C for
15 sec and annealing/extension at 60°C for 1 min. Samples were
analyzed in the StepOne Plus system (Thermo Fisher Scientific,
Inc.). Data were analyzed using StepOne Software v2.3 (Thermo
Fisher Scientific, Inc.). The relative expression levels were
normalized to endogenous control and were expressed as
2−ΔΔCq (25).
 | Table I.TaqMan assays used for reverse
transcription quantitative PCR. |
Table I.
TaqMan assays used for reverse
transcription quantitative PCR.
| Assay | Target species | Catalogue
number | Manufacturer |
|---|
| TNFSF13 (BAFF) | Human | Hs00198106_m1 | Thermo Fisher
Scientific, Inc. |
| TNFSF13
(APRIL) | Human | Hs00601664_g1 | Thermo Fisher
Scientific, Inc. |
| TNFRSF1
(BAFF-R) | Human | Hs00606874_g1 | Thermo Fisher
Scientific, Inc. |
| TNFRSF1 (TACI) | Human | Hs00963364_m1 | Thermo Fisher
Scientific, Inc. |
| TNFRSF1 (BCMA) | Human | Hs03045080_m1 | Thermo Fisher
Scientific, Inc. |
| GADPH | Human | Hs02786624_g1 | Thermo Fisher
Scientific, Inc. |
Western blotting
To evaluate the protein expression of BAFF, APRIL,
BAFF-R, BCMA and TACI, cells were harvested, washed in PBS and
lysed in RIPA buffer (Thermo Fisher Scientific, Inc.) supplemented
with complete protease inhibitor cocktail (Roche Diagnostics) for
15 min on ice. The cell debris were removed by centrifugation at
13,000 × g for 5 min at 4°C. The concentration of total protein was
assessed using Pierce™ BCA Protein Assay Kit (Thermo Fisher
Scientific, Inc.) according to manufacturers' instructions.
Proteins (20 µg) were mixed with Lammli sample buffer (1:4; Bio-Rad
Laboratories, Inc.) and heated at 95°C for 5 min. Proteins were
separated on 4–10% TGX gel (Bio-Rad Laboratories, Inc.) and
transferred onto nitrocellulose membranes (Bio-Rad Laboratories,
Inc.) using the TransBlot turbo system (Bio-Rad Laboratories,
Inc.). The membranes were blocked using 5% skimmed milk dissolved
in PBS and supplemented with 0.1% Tween-20 (Sigma-Aldrich; Merck
KGaA; T-PBS) for 1 h at room temperature. Membranes were incubated
with primary antibodies overnight at 4°C (Table II). Subsequently, membranes were
washed using 10X PBS (Corining) supplemented with 0,1% Tween-20 and
incubated with specific HRP-conjugated secondary antibodies
(Table II) for 1 h in room
temperature. Bands were detected using the SuperSignal West Femto
chemiluminescence substrate kit (Thermo Fisher Scientific, Inc.)
and visualized with ChemiDoc Touch System (Bio-Rad Laboratories,
Inc.). Relative expression levels were normalized to endogenous
control (β-actin) using ImageJ software v1.53 (National Institutes
of Health).
 | Table II.Description of antibodies used for
western blotting. |
Table II.
Description of antibodies used for
western blotting.
| Antibody | Target species | Host | Isotype | Clone | Dilution | Catalogue
number | Manufacturer |
|---|
| BAFF-R | Human, mouse and
rat | Mouse | IgG2b | H-1 | 1:150 | sc-365410 | Santa Cruz
Biotechnology, Inc. |
| TACI | Human, mouse and
rat | Mouse | IgG2b | C-9 | 1:200 | sc-365253 | Santa Cruz
Biotechnology, Inc. |
| BCMA | Human | Rat | IgG2a | 335004 | 1:100 | MAB193 | R&D Systems,
Inc. |
| β-actin
(Direct-Blot HRP) | Human, mouse and
rat | Mouse | IgG2b | 2F1-1 | 1:1000 | 643807 | BioLegend,
Inc. |
| Anti-mouse IgG
HRP-conjugated | Mouse | Goat | IgG | Polyclonal | 1:1000 | HAF007 | R&D Systems,
Inc. |
| Anti-rat IgG
HRP-conjugated | Rat | Goat | IgG | Polyclonal | 1:1000 | HAF005 | R&D Systems,
Inc. |
Ligand receptor interaction
blocking
To block the interactions of BAFF, APRIL and their
receptors in A549 and H2030 cell lines, blocking antibodies for
TACI (monoclonal mouse IgG1 clone #165609; R&D Systems, Inc.;
cat. no. MAB174) and BAFF-R (monoclonal mouse IgG2a, kappa clone
#8A7; Thermo Fisher Scientific, Inc.) were used separately or in
combination at 10 µg/ml for 24 h in standard cell culture
conditions (37°C, 5% CO2).
Cell proliferation assay
To assess the effects of BAFF and APRIL on lung
cancer cell proliferation, the cells were harvested and labeled
with carboxyfluorescein succinimidyl ester (CFSE; Sigma-Aldrich;
Merck KGaA). The cells were cultured for 72 h in 24 well plates
(37°C, 5% CO2) in the presence of recombinant human BAFF
or APRIL proteins at the concentrations of 50, 100 or 150 ng/ml or
without any stimulation (vehicle). The cells were acquired by using
TrypLE Select (Thermo Fisher Scientific, Inc.) at 24, 48 and 72 h.
Subsequently, cells were washed in PBS and stained with 7-AAD as
suggested by the manufacturer (0,25 µg per 1×106 cells;
Becton-Dickinson and Company), and incubated for 15 min at 4°C.
Cells were analyzed on FACSCalibur flow cytometry system
(Becton-Dickinson and Company). Data were analyzed using FlowJo
software version 10.6.1 (Tree Star, Inc.). To set the gates for the
analysis of proliferation (negative control), colcemid (50 ng/ml)
was used to arrest the mitosis in metastatasis stage. Cell
viability was assessed according to 7-AAD staining and viable cells
were considered as 7-AAD negative.
Invasion assay
To evaluate the influence of BAFF and APRIL on
non-small cell lung cancer invasiveness, a commercially available
invasion assay (Cell Biolabs, Inc.) was used according to the
manufacturers' instruction. Briefly, the membrane was rehydrated,
and cell culture media containing 10% FBS (vehicle) or 10% FBS with
BAFF (150 ng/ml) or 10% FBS with APRIL (150 ng/ml) was added to the
lower well of the invasion plate. Subsequently, cells in
single-cell suspension were placed in the insert. After 48 h
incubation at 37°C, the invasive cells were stained with cell stain
solution, lysed with extraction solution and the absorbance was
measured at 560 nm using a plate reader (Ledetect; Labexim
Products). The data were analyzed using MicroWin2000 software (OEM
version; Labexim Products).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6 software (GraphPad Software, Inc.). Data were compared
using Mann-Whitney U test or Kruskal-Wallis followed by Dunn's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference. The results from at least five independent
experiments are presented as the median ± interquartile range.
Results
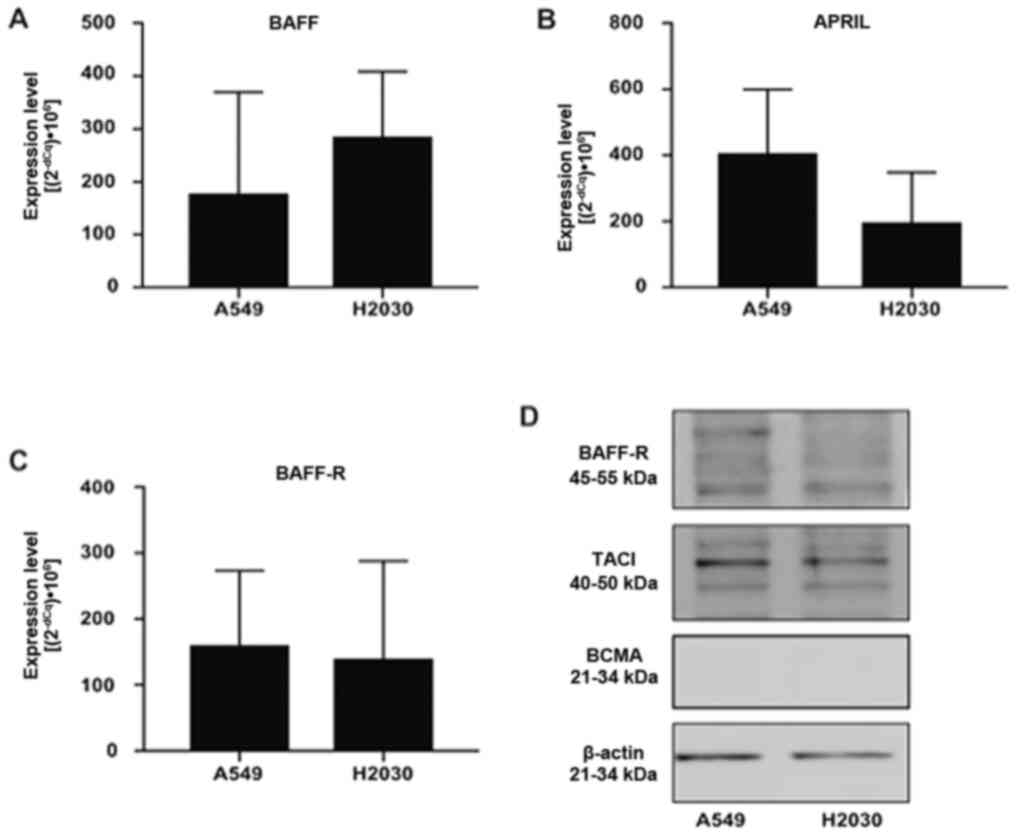
NSCLC cells express BAFF, APRIL and
their receptors
First, we aimed to investigate whether NSCLC cells
could express BAFF, APRIL and their receptors BCMA, TACI and BAFF-R
using RT-qPCR and western blotting (Fig.
1). The results demonstrated that A549 and H2030 cell lines
expressed BAFF, APRIL and BAFF-R (Fig.
1A-C). Conversely, the expression of TACI and BCMA was detected
in H2030 cells only, while in the A549 cell line, the expression of
both receptors was under the detection limit (data not shown).
Furthermore, no difference was found in the expression levels of
BAFF, APRIL and BAFF-R among the analyzed cell lines (Fig. 1A-C). In addition, results from
western blotting demonstrated that both cell lines expressed BAFF-R
and TACI at the protein level, while no expression of BCMA was
detected (Fig. 1D). These findings
suggested that NSCLC cells could respond directly to BAFF and APRIL
stimulation via BAFF-R and TACI signaling.
Effect of different BAFF and APRIL
doses on NSCLC cells viability
Having found that both cell lines possess the
potential to directly respond to BAFF and APRIL stimulation by the
interaction with TACI and BAFF-R, we subsequently aimed to
investigate the direct effects of both ligands on cancer cell
viability. As expected, the results demonstrated that stimulation
of H2030 and A549 cells with 50, 100 and 150 ng/ml BAFF (Fig. 2A and B) and APRIL (Fig. 2C and D) did not affect NSCLC cell
viability. These findings indicated that BAFF and APRIL were not
toxic for NSCLC cells and could be used in further functional
experiments.
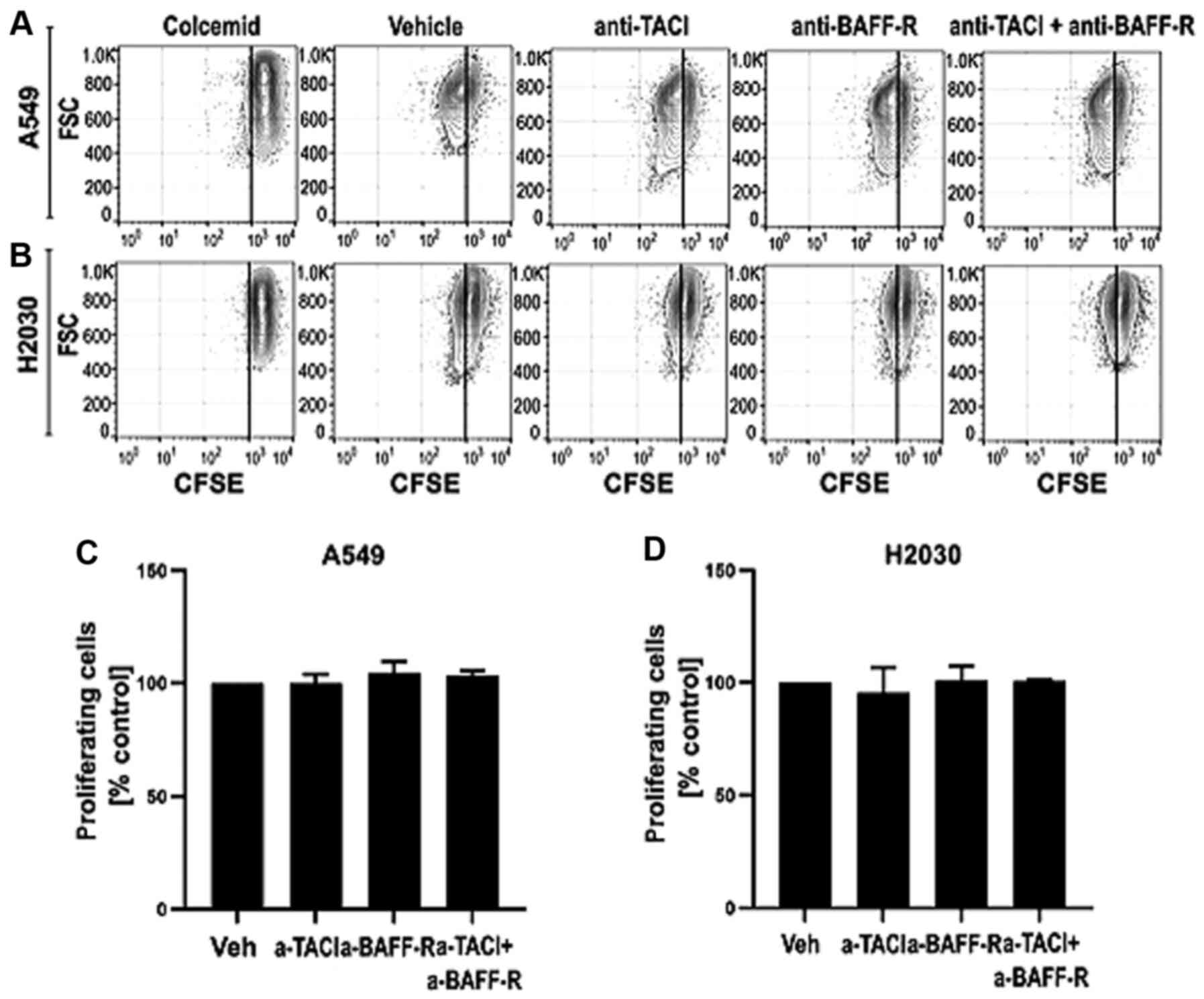
NSCLC-derived BAFF and APRIL do not
influence cell proliferation
Having found that both cell lines can produce the
analyzed ligands, we aimed to investigate whether A549 and H2030
cell-derived BAFF and APRIL may directly influence their
proliferation (Fig. 3). Functional
experiments were performed with receptor blocking using
functional-grade monoclonal antibodies. The results demonstrated
that blocking TACI and BAFF-R separately or in combination did not
influence the proliferation of A549 (Fig. 3C) and H2030 (Fig. 3D) cells. These results indicated that
endogenous BAFF and APRIL may not increase tumor growth directly by
increasing cancer cell proliferation.
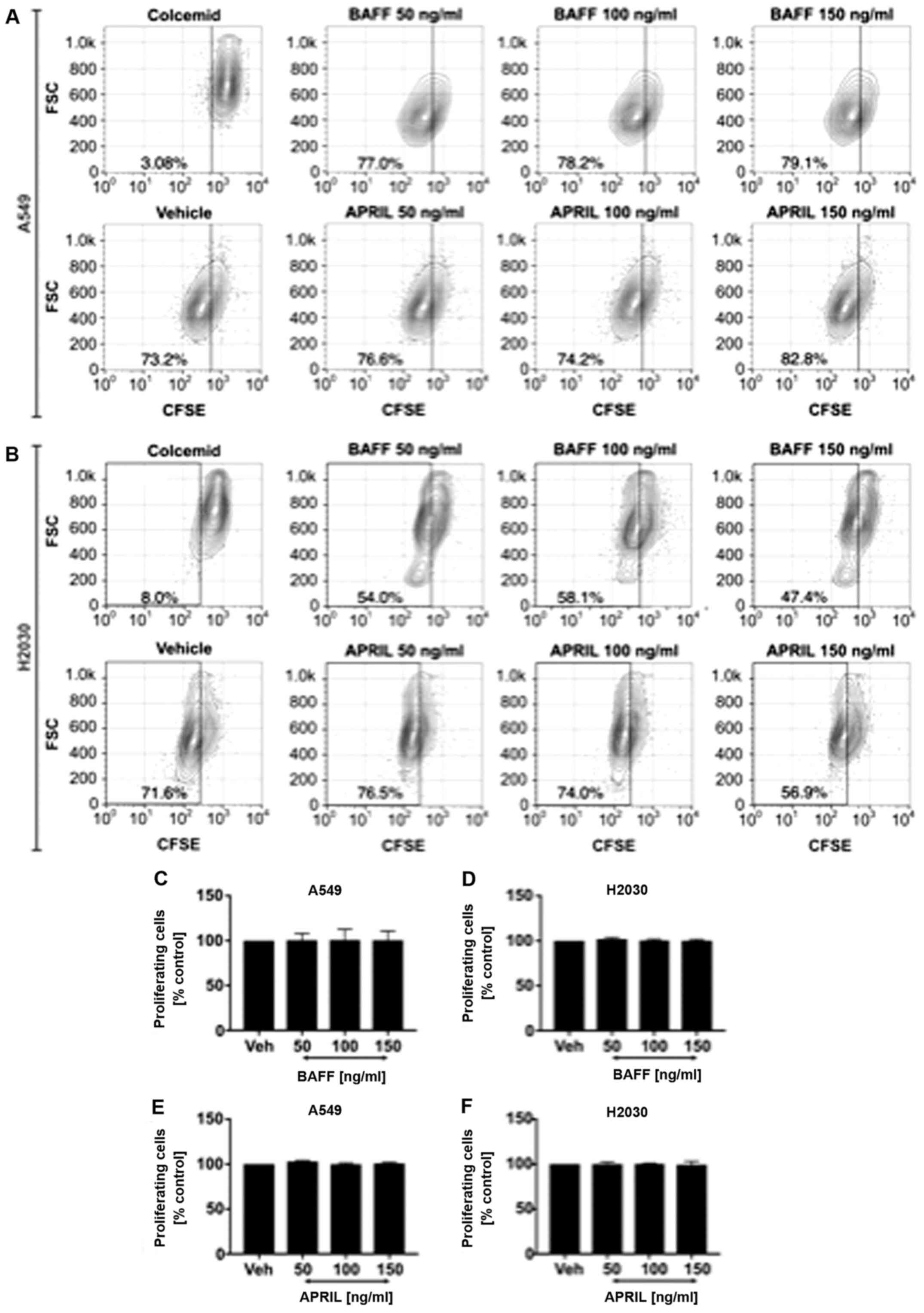
Exogenous BAFF and APRIL did not
influence NSCLC cell proliferation
Next, we wished to analyze whether exogenous BAFF
and APRIL could directly affect NSCLC cell proliferation. Cells
were stimulated with recombinant human BAFF and APRIL at 50, 100
and 150 ng/ml. The results demonstrated no effect of exogenous
stimulation with both ligands on A549 (Fig. 4A) and H2030 (Fig. 4B) cell proliferation, since no
differences in the frequency of proliferating cells was observed
(Fig. 4C-F). Furthermore, no effect
on cancer cell invasiveness in response to BAFF and APRIL
stimulation was observed compared with vehicle alone (Fig. 5).
Discussion
The present study demonstrated that NSCLC cells
possess the ability to respond directly to BAFF and APRIL
stimulation via interaction with TACI and BAFF-R, while BCMA was
undetectable at the protein level. Previous studies reported that
BAFF and APRIL can enhance the proliferation of normal and
malignant B cells (11,20). In addition, previous studies from our
group demonstrated that the expression of BCMA might serve as a
prognostic factor for treatment response in patients with acute
myeloid leukemia (8,9). In solid tumors, BAFF and APRIL
signaling is associated with cancer cell proliferation, such as in
breast cancer (12), esophageal
cancer (26), clear cell renal cell
carcinoma (27), adult male germ
cell tumor (28) and invasive
bladder carcinoma (29).
Furthermore, elevated local and systemic levels of BAFF and APRIL
were shown to be associated with a higher stage and disease
progression, such as in chronic graft versus host disease, systemic
lupus erythormatosus and breast cancer tumor size (12,13).
Surprisingly, the present study demonstrated that BAFF and APRIL
direct signaling did not serve an essential role in NSCLC
aggressiveness, as no effect of BAFF and APRIL on NSCLC cell
proliferation and invasiveness was reported. Conversely, previous
studies reported that APRIL signaling in NSCLC cells promote tumor
proliferation, migration and metastasis (23,30).
Consequently, both ligands may act as tumor supporters and
pro-metastatic factors in an indirect manner. In fact, high BAFF
and APRIL expression were shown in NSCLC tissues (22). It is therefore essential to determine
whether BAFF and APRIL signaling in tumor-infiltrating or stromal
cells, including tumor-infiltrating macrophages or tumor-associated
fibroblasts, may play a role in tumor progression and spread.
Macrophages (alternatively activated) and
fibroblasts are crucial components of tumor stroma. The progressive
effects of macrophages and fibroblasts on tumor growth are
associated with the release of growth factors and anti-inflammatory
mediators, their promotion of cancer invasiveness by releasing
matrix degradation enzymes, and their involvement in the
recruitment of suppressive cells, including T regulatory cells to
the tumor side (31,32). Both aforementioned cell subsets have
been shown to produce high levels of BAFF and APRIL; however, the
effects of both ligands on the activation and biological function
of tumor-infiltrating macrophages and tumor-associated fibroblasts
remain elusive (18,24,33–35).
BAFF via direct interaction with TACI may therefore induce monocyte
maturation towards macrophages and, thus, contribute to tumor
progression.
BAFF and APRIL signaling stimulate the expression of
anti-apoptotic proteins, such as Bcl-2, Bcl-XL and Bcl-2-related
protein A1 (36,37). Both ligands may therefore serve as
anti-apoptotic mediators and increase the survival of cancer cells
subjected to chemotherapy (11).
Interestingly, APRIL gene silencing was shown to increase the
apoptotic susceptibility of gastric cancer cells (38). However, the present study
demonstrated that BAFF and APRIL stimulation had no effects on the
viability of NSCLC cells in normal conditions. Further
investigation is therefore required to elucidate the impact of BAFF
and APRIL on cancer cell susceptibility to cytotoxic therapy.
In summary, the results from the present study
demonstrated that, despite the presence of TACI and BAFF-R in NSCLC
cells, both BAFF and APRIL did not exert direct effects on cancer
cell proliferation and invasiveness. Further studies are needed to
elucidate the mechanisms of previously reported ex vivo
associations between BAFF and APRIL and cancer progression
(12,13).
Acknowledgements
Not applicable.
Funding
This study was supported by the statutory founds of
Medical University of Bialystok, Poland (grant no.
N/ST/ZB/16/001/1113).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AE, MM and LB developed the research concept. AE and
LB designed the experiments. MW, MT, DL and KG performed the
experiments. MW, MT and JD performed statistical analysis and
designed figures. MW and MT drafted the mancuscript. AE, LB, JD and
MM revised the draft. AE, MM and BL confirmed the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gajewski TF, Schreiber H and Fu YX: Innate
and adaptive immune cells in the tumor microenvironment. Nat
Immunol. 14:1014–1022. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stankovic B, Bjørhovde HAK, Skarshaug R,
Aamodt H, Frafjord A, Müller E, Hammarström C, Beraki K, Bækkevold
ES, Woldbæk PR, et al: Immune Cell Composition in Human Non-small
Cell Lung Cancer. Front Immunol. 9:31012019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X, Wu S, Yang Y, Zhao M, Zhu G and Hou
Z: The prognostic landscape of tumor-infiltrating immune cell and
immunomodulators in lung cancer. Biomed Pharmacother. 95:55–61.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poh AR and Ernst M: Targeting macrophages
in cancer: From bench to bedside. Front Oncol. 8:492018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loyher PL, Hamon P, Laviron M,
Meghraoui-Kheddar A, Goncalves E, Deng Z, Torstensson S, Bercovici
N, Baudesson de Chanville C, Combadière B, et al: Macrophages of
distinct origins contribute to tumor development in the lung. J Exp
Med. 215:2536–2553. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bolkun L, Grubczak K, Schneider G, Zembko
P, Radzikowska U, Singh P, Kloczko J, Ratajczak MZ, Moniuszko M and
Eljaszewicz A: Involvement of BAFF and APRIL in Resistance to
Apoptosis of Acute Myeloid Leukemia. J Cancer. 7:1979–1983. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bolkun L, Lemancewicz D, Jablonska E,
Szumowska A, Bolkun-Skornicka U, Ratajczak-Wrona W, Dzieciol J and
Kloczko J: The impact of TNF superfamily molecules on overall
survival in acute myeloid leukaemia: Correlation with biological
and clinical features. Ann Hematol. 94:35–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meinl E, Thaler FS and Lichtenthaler SF:
Shedding of BAFF/APRIL receptors controls B cells. Trends Immunol.
39:673–676. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kern C, Cornuel JF, Billard C, Tang R,
Rouillard D, Stenou V, Defrance T, Ajchenbaum-Cymbalista F, Simonin
PY, Feldblum S, et al: Involvement of BAFF and APRIL in the
resistance to apoptosis of B-CLL through an autocrine pathway.
Blood. 103:679–688. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pelekanou V, Notas G, Athanasouli P,
Alexakis K, Kiagiadaki F, Peroulis N, Kalyvianaki K, Kampouri E,
Polioudaki H, Theodoropoulos P, et al: BCMA (TNFRSF17) induces
APRIL and BAFF mediated breast cancer cell stemness. Front Oncol.
8:3012018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moreaux J, Veyrune JL, De Vos J and Klein
B: APRIL is overexpressed in cancer: Link with tumor progression.
BMC Cancer. 9:832009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quinn J, Glassford J, Percy L, Munson P,
Marafioti T, Rodriguez-Justo M and Yong K: APRIL promotes
cell-cycle progression in primary multiple myeloma cells: Influence
of D-type cyclin group and translocation status. Blood.
117:890–901. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bolkun L, Lemancewicz D, Jablonska E,
Kulczynska A, Bolkun-Skornicka U, Kloczko J and Dzieciol J: BAFF
and APRIL as TNF superfamily molecules and angiogenesis parallel
progression of human multiple myeloma. Ann Hematol. 93:635–644.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang S, Li JY and Xu W: Role of
BAFF/BAFF-R axis in B-cell non-Hodgkin lymphoma. Crit Rev Oncol
Hematol. 91:113–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tai YT, Acharya C, An G, Moschetta M,
Zhong MY, Feng X, Cea M, Cagnetta A, Wen K, van Eenennaam H, et al:
APRIL and BCMA promote human multiple myeloma growth and
immunosuppression in the bone marrow microenvironment. Blood.
127:3225–3236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koizumi M, Hiasa Y, Kumagi T, Yamanishi H,
Azemoto N, Kobata T, Matsuura B, Abe M and Onji M: Increased B
cell-activating factor promotes tumor invasion and metastasis in
human pancreatic cancer. PLoS One. 8:e713672013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kampa M, Notas G, Stathopoulos EN, Tsapis
A and Castanas E: The TNFSF members APRIL and BAFF and their
receptors TACI, BCMA, and BAFFR in oncology, with a special focus
in breast cancer. Front Oncol. 10:8272020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Novak AJ, Grote DM, Stenson M, Ziesmer SC,
Witzig TE, Habermann TM, Harder B, Ristow KM, Bram RJ, Jelinek DF,
et al: Expression of BLyS and its receptors in B-cell non-Hodgkin
lymphoma: Correlation with disease activity and patient outcome.
Blood. 104:2247–2253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Notas G, Alexaki VI, Kampa M, Pelekanou V,
Charalampopoulos I, Sabour-Alaoui S, Pediaditakis I, Dessirier V,
Gravanis A, Stathopoulos EN, et al: APRIL binding to BCMA activates
a JNK2-FOXO3-GADD45 pathway and induces a G2/M cell growth arrest
in liver cells. J Immunol. 189:4748–4758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dou H, Yan Z, Zhang M and Xu X: APRIL,
BCMA and TACI proteins are abnormally expressed in non-small cell
lung cancer. Oncol Lett. 12:3351–3355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dou H, Yan Z, Zhang M and Xu X: APRIL
promotes non-small cell lung cancer growth and metastasis by
targeting ERK1/2 signaling. Oncotarget. 8:109289–109300. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qian Z, Qingshan C, Chun J, Huijun Z, Feng
L, Qiang W, Qiang X and Min Z: High expression of TNFSF13 in tumor
cells and fibroblasts is associated with poor prognosis in
non-small cell lung cancer. Am J Clin Pathol. 141:226–233. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hao Y, Triadafilopoulos G, Sahbaie P,
Young HS, Omary MB and Lowe AW: Gene expression profiling reveals
stromal genes expressed in common between Barrett's esophagus and
adenocarcinoma. Gastroenterology. 131:925–933. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee C, Park JW, Suh JH and Moon KC: High
expression of APRIL correlates with poor prognosis in clear cell
renal cell carcinoma. Pathol Res Pract. 211:824–828. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Korkola JE, Houldsworth J, Chadalavada RS,
Olshen AB, Dobrzynski D, Reuter VE, Bosl GJ and Chaganti RS:
Down-regulation of stem cell genes, including those in a 200-kb
gene cluster at 12p13.31, is associated with in vivo
differentiation of human male germ cell tumors. Cancer Res.
66:820–827. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sanchez-Carbayo M, Socci ND, Lozano J,
Saint F and Cordon-Cardo C: Defining molecular profiles of poor
outcome in patients with invasive bladder cancer using
oligonucleotide microarrays. J Clin Oncol. 24:778–789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun B, Wang H, Wang X, Huang H, Ding W,
Jing R, Shi G and Zhu L: A proliferation-inducing ligand: A new
biomarker for non-small cell lung cancer. Exp Lung Res. 35:486–500.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sahai E, Astsaturov I, Cukierman E,
DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR,
Hunter T, et al: A framework for advancing our understanding of
cancer-associated fibroblasts. Nat Rev Cancer. 20:174–186. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X and Song E: Turning foes to
friends: Targeting cancer-associated fibroblasts. Nat Rev Drug
Discov. 18:99–115. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J, He D, Chen Q, Guo X, Yang L, Lin
X, Li Y, Wu W, Yang Y, He J, et al: BAFF is involved in
macrophage-induced bortezomib resistance in myeloma. Cell Death
Dis. 8:e31612017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Manfroi B, McKee T, Mayol JF, Tabruyn S,
Moret S, Villiers C, Righini C, Dyer M, Callanan M, Schneider P, et
al: CXCL-8/IL8 produced by diffuse large B-cell lymphomas recruits
neutrophils expressing a proliferation-inducing ligand APRIL.
Cancer Res. 77:1097–1107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lowin T, Anssar TM, Bäuml M, Classen T,
Schneider M and Pongratz G: Positive and negative cooperativity of
TNF and Interferon-γ in regulating synovial fibroblast function and
B cell survival in fibroblast/B cell co-cultures. Sci Rep.
10:7802020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mackay F and Tangye SG: The role of the
BAFF/APRIL system in B cell homeostasis and lymphoid cancers. Curr
Opin Pharmacol. 4:347–354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He B, Chadburn A, Jou E, Schattner EJ,
Knowles DM and Cerutti A: Lymphoma B cells evade apoptosis through
the TNF family members BAFF/BLyS and APRIL. J Immunol.
172:3268–3279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ni SZ, Cao HY, Chen Z, Zhu Y and Xu ZK:
siRNA interference with a proliferation-inducing ligand gene in the
Sgr-7901 gastric carcinoma cell line. Asian Pac J Cancer Prev.
13:1511–1514. 2012. View Article : Google Scholar : PubMed/NCBI
|