Introduction
Human esophageal cancer is one of the most prevalent
cancer types worldwide. According to data published by the World
Health Organization, there were ~600,000 new confirmed cases of
esophageal cancer globally in 2020, accounting for 3.1% of all
malignant tumors, and thus, it was ranked as the eighth most
commonly occurring cancer. Esophageal cancer resulted in 540,000
deaths globally in 2020 according to data published by the
International Agency for Research on Cancer, accounting for 5.5% of
all deaths due to malignant tumors; thus, the mortality associated
with esophageal cancer was the sixth highest. Squamous cell
carcinoma is the most common type of esophageal cancer (1,2). There
are a number of treatments for esophageal cancer, including
endoscopic submucosal dissection for mucosal cancer, esophagectomy
for locally advanced cancer and neoadjuvant chemotherapy or
neoadjuvant chemoradiotherapy for terminal cancer (3).
Photodynamic therapy (PDT) has been considered to be
a favorable method for the treatment of various benign or malignant
diseases. There are three basic requirements for PDT: i)
Photosensitizers (PSs); ii) a laser of a specific wavelength; and
iii) oxygen (4). Oxygen molecules
(O2) receive the energy released from light-excited PSs,
and are converted into reactive oxygen species (ROS), which results
in the damage and death of neoplastic cells (5). A previous study has reported that PDT
can have curative effects that cause tumor tissue necrosis,
particularly in early stage tumors (6). PDT has been demonstrated to have a
number of advantages during the treatment of human esophageal
cancer, such as lower toxicity levels in healthy tissues (7). Furthermore, the tumor specificity is
high, and the therapeutic effect of inducing cellular necrosis in
skin, digestive tract, lung, vaginal and other organ tumors is
marked (8). The effects of PDT are
dependent on the application of PSs. PSs are specifically absorbed
by tumor tissues (9). A particular
wavelength of light can activate PSs and initiate the activation
processes that destroy tumor tissues (10).
Hematoporphyrin derivative (HpD) has been widely
applied in PDT as a first-generation PS (5). HpD (trade name, Hiporfin) has strong
photosensitivity and exhibits optimum absorption at a wavelength of
620–635 nm (11).
It is known that apoptosis is regulated and
controlled via two main pathways, namely the extrinsic and
intrinsic pathways. As two necessary pathways of cell apoptosis,
both of them eventually lead to the induction of Caspase activation
and the occurrence of apoptosis. Bcl-2 is regarded as an
anti-apoptotic gene that serves a role in the intrinsic pathway
(12). However, BAX serves a key
role in the promotion of apoptosis (13). The ratio of the expression products
of pro-apoptotic genes to anti-apoptotic genes decides whether the
cell undergoes apoptosis (14). As
a common result of different pathways, the Caspase protein family
is activated during the apoptotic process (15). Caspase-9 initiates apoptosis, and
Caspase-3 executes cell structure disassembly (16).
PI3K/Akt is a crucial signaling pathway. It is
related to biological behaviors such as; cell proliferation,
autophagy, cell cycle progression and apoptosis (17). Phosphorylated (p-)Akt can regulate
NF-κB expression, cell proliferation and migration (18). A previous study reported that the
blocking of the PI3K/Akt signaling pathway was associated with the
promotion of apoptosis and suppression of tumor growth (19).
To the best of our knowledge, the connection between
HpD-PDT and the PI3K/Akt signaling pathway has not been thoroughly
clarified. The present study aimed to investigate the effects of
HpD-PDT on the biological behavior of human esophageal squamous
cell carcinoma cells and determine whether the PI3K/Akt signaling
pathway is involved in the regulatory molecular mechanism of
HpD-PDT based on cellular experiments.
Materials and methods
Cell culture and reagents
The KYSE-150 human esophageal squamous cell
carcinoma cell line was purchased from Leibniz Institute
DSMZ-German Collection of Microorganisms and Cell Cultures GmbH and
short tandem repeat identification was performed. The cells were
incubated in RPMI-1640 medium (Nanjing BioChannel Biotechnology
Co., Ltd.). FBS (Nanjing BioChannel Biotechnology Co., Ltd.), 100
U/ml penicillin and 0.1 mg/ml streptomycin (Nanjing BioChannel
Biotechnology Co., Ltd.) were added to the medium and the final FBS
concentration was 10%. Cells were cultured in an incubator (Thermo
Fisher Scientific, Inc.) containing 5% CO2 at a
temperature of 37°C. All experiments involved cells in the
exponential phase of growth.
The Cell Counting Kit-8 (CCK-8) was obtained from
APeXBIO Technology LLC. LY294002 (cat. no. HY-10108) and 740Y-P
(cat. no. HY-P0175) were obtained from MedChemExpress. The
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) kit was
purchased from Beyotime Institute of Biotechnology.
PDT protocol
HpD was purchased from Chongqing Mele
Biopharmaceutical Co., Ltd. A 635-nm wavelength laser was borrowed
from Guoyihuake Company. After cells became attached, the cells
were divided into the following groups: Control, light, HpD and
HpD-PDT. The media in the control and Light groups were replaced
with fresh RPMI-1640 medium. At the same time, the media in the HpD
and HpD-PDT groups were replaced with medium containing 2 mg/l HpD.
After incubating cells for 4 h at 37°C, PBS was used to wash away
the residual medium. Next, fresh medium was added into all the
wells. The light and HpD-PDT groups were irradiated with a 635-nm
laser light at the same energy density (5 J/cm2). The
energy density was calculated using the following formula: Light
energy density (J/cm2)=power density (W/cm2)
× time (sec). All procedures were performed in the darkroom and
plates were covered with foil.
Detection of intracellular HpD
HpD exhibits red fluorescence when exposed to a
390–450-nm light source, rendering it well-suited for early
clinical tumor fluorescence diagnosis (20). Therefore, by utilizing a
fluorescence microscope within the appropriate wavelength range, it
was possible to directly visualize the cellular uptake of HpD.
Cells (5×105 cells/ml) were seeded into 6-well plates
and incubated with 2 mg/l HpD for different durations (1, 2, 4 and
8 h) at 37°C. The intracellular uptake of HpD was assessed by
fluorescence microscopy (Nikon ECLIPSE Ti; Nikon Corporation).
Cell viability
KYSE-150 cells (1×105 cells/ml) were
added into 96-well plates and incubated in RPMI-1640 medium. The
old media were substituted with fresh media containing HpD at
various concentrations, ranging from 0 to 8 mg/l, the next day.
After incubating the cells for 4 h at 37°C in the dark, PBS was
used to wash away HpD that had not been absorbed by cells. The
plates were irradiated under 635-nm light with different energy
levels (0, 2.5, 5 and 7.5 J/cm2). Subsequently, cells
were cultured in a dark room. After 24 h, the culture medium was
substituted with 100 µl fresh medium containing CCK-8 reagent.
After incubating cells for 2 h in the dark, the optical densities
(ODs) were measured at 450 nm using a microplate reader (Thermo
Fisher Scientific, Inc.). The following formula was used to
estimate cell viability: Cell viability (%)=[(mean OD of experiment
group-mean OD of the blank group)/(mean OD of control group-mean OD
of blank group)] ×100. The wells of the control group only
contained fresh RPMI-1640 medium and CCK-8 solution.
ROS measurement
The DCFH-DA kit was used to measure ROS levels.
KYSE-150 cells were seeded in 6-well plates (1×105
cells/ml) and incubated for 12 h in the cell culture incubator at a
temperature of 37°C. The culture medium for the HpD and HpD-PDT
groups was replaced with medium containing 2 mg/l HpD. The control
and Light groups were maintained in serum-free medium. After
incubating all cells in the dark for 4 h in a 37°C incubator, the
HpD-PDT group was subjected to light exposure (5 J/cm2).
The medium was completely replaced with medium containing DCFH-DA
diluted to a concentration of 10 µM. After 30 min, KYSE-150 cells
were rinsed with PBS at least three times. All cells were
trypsinized and collected. A flow cytometer (Cytoflex S; Beckman
Coulter, Inc.) was used to detect the fluorescence intensity level.
The data was analyzed using CytExpert (version 2.4; Beckman
Coulter) and GraphPad Prism software (version 8.3.0;
Dotmatics).
Wound healing assay
KYSE-150 cells were cultured in 6-well plates. When
the cells were almost 100% confluent, the HpD and HpD-PDT groups
were incubated with RPMI-1640 medium containing HpD (1 mg/l) for 4
h. Several sterile 10-µl pipet tips were used to create scratches.
Residual floating cells were washed away carefully with PBS. The
cell culture medium was substituted with serum-free medium. The
light and HpD-PDT groups were subjected to the irradiation process
immediately (2.5 J/cm2). Cells in the control and HpD
groups continued to be incubated in the dark at 37°C in the
incubator. All images were captured immediately or 12 h later under
a light microscope. ImageJ was used to convert the original images
to grayscale images and to measure the area of the scratches. The
scratch migration rate was calculated using the following formula:
Migration rate=(Scratch area at 0 h-scratch area at 12 h)/scratch
area at 0 h.
Hoechst 33342 staining
Cells were cultured overnight in 12-well plates
(5×105 cells/ml). After subjecting them to different
treatments (control, HpD, light and HpD-PDT), cells were
continuously cultured for 24 h. Subsequently, KYSE-150 cells were
incubated for 30 min in a dark room with Hoechst 33342 staining
solution (Beyotime Institute of Biotechnology) at room temperature.
Pre-cooled PBS was used to wash off the residual solution at least
three times. A Thunder Imager fluorescence microscope (Leica
Microsystems GmbH) was used to capture images.
Cell apoptosis
The cell apoptosis rate was measured using an
Annexin V-FITC/PI Kit (Vazyme Biotech Co., Ltd.). KYSE-150 cells
were grown in 6-well plates (2×105 cells per well).
After subjecting the cells to different treatments (control, HpD,
light and HpD-PDT), the HpD and HpD-PDT group were incubated with
fresh medium containing 2 mg/l HpD for 4 h. Subsequently, the Light
group and HpD-PDT group received laser irradiation (5
J/cm2). Cells were incubated for 24 h in a 5%
CO2 incubator at 37°C. After cells were trypsinized,
washed and centrifuged at 300 × g for 5 min at 4°C to remove the
supernatant, the cell precipitate in each tube was resuspended
using 100 µl binding buffer containing an appropriate volume of
FITC and PI. Subsequently, cells were placed in the dark for 20
min. Finally, 400 µl binding buffer was added to the cell
suspension. FITC-positive cells were detected using a flow
cytometer (Cytoflex S; Beckman Coulter). The cell apoptosis rate
was calculated by determining the proportion of early apoptotic
cells (FITC-positive/PI-negative) and late apoptotic cells
(FITC-positive/PI-positive) among all cells. The data were analyzed
using CytExpert (version 2.4; Beckman Coulter) and GraphPad Prism
software (version 8.3.0; Dotmatics).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
After cell grouping (control, HpD, light and
HpD-PDT), the HpD group and HpD-PDT group were incubated with fresh
medium containing 2 mg/l HpD for 4 h. The Light group and HpD-PDT
group received laser irradiation (5 J/cm2). All groups
of cells were incubated in the dark at 37°C in a cell culture
incubator for 24 h. RNAiso Plus (Takara Bio, Inc.) was used to
extract RNA at 24 h post-HpD-PDT. Subsequently, 75% ethanol was
used to purify the extracted RNA. An ultraviolet spectrophotometer
was used to measure the concentration of RNA. Reverse transcription
was performed to synthesize cDNA. A HiScript II Q RT SuperMix for
qPCR kit (Vazyme Biotech Co., Ltd.) was used for reverse
transcription. This process was conducted under the following
reaction conditions: 50°C for 15 min and 85°C for 5 sec. SYBR Green
Mix (Vazyme Biotech Co., Ltd.) was used to perform qPCR using
StepOne Plus (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The program was set as follows: i) Holding stage, 95°C for 30 sec;
ii) cycling stage (40 cycles), 95°C for 10 sec and 60°C for 30 sec;
and iii) melting curve stage, 95°C for 15 sec, 60°C for 1 min and
95°C for 15 sec. The 2−ΔΔCq method was used (21). GAPDH was chosen as the reference
gene. TsingKe Biological Technology synthesized the primers. All
primer sequences are listed in Table
I.
 | Table I.Primer sequences for target
genes. |
Table I.
Primer sequences for target
genes.
| Gene name | Primer sequences
(5′-3′) |
|---|
| GAPDH | F:
CACCATCTTCCAGGAGCGAG |
|
| R:
GATGGCATGGACTGTGGTCA |
| Bcl-2 | F:
TTCTTTGAGTTCGGTGGGGTC |
|
| R:
TGCATATTTGTTTGGGGCAGG |
| BAX | F:
TCCACCAAGAAGCTGAGCGAG |
|
| R:
GTCCAGCCCATGATGGTTCT |
| Caspase 3 | F:
CATGGAAGCGAATCAATGGACT |
|
| R:
CTGTACCAGACCGAGATGTCA |
| Caspase 7 | F:
AGTGACAGGTATGGGCGTTC |
|
| R:
CGGCATTTGTATGGTCCTCTT' |
| PTEN | F:
TTTGAAGACCATAACCCACCAC |
|
| R:
ATTACACCAGTTCGTCCCTTTC |
Western blotting
RIPA lysis buffer (Beyotime Institute of
Biotechnology) containing PMSF and phosphatase inhibitors purchased
from Bimake.com was used to lyse KYSE-150 cells. A BCA
kit (Beyotime Institute of Biotechnology) was used to quantify
protein concentrations. SDS-PAGE was performed to isolate proteins
with 12.5% gels. The mass of protein loaded per lane was 30 µg.
Target proteins were transferred to PVDF membranes (Roche
Diagnostics GmbH). Bovine serum albumin powder (Biosharp) was
dissolved in tris-buffered saline with 0.1% Tween 20 (TBST) to
block membranes and membranes were agitated for 1.5 h at room
temperature. The concentration of bovine serum albumin solution was
5%. The membranes were incubated with primary antibodies overnight
at 4°C. TBST was used to wash the membranes three times the next
day. Next, the membranes were incubated with the secondary antibody
for 2 h at 4°C. A NcmECL Ultra kit (New Cell and Molecular Biotech
Co., Ltd.) was used to identify the target protein bands. All
images were processed using ImageJ (version 1.53 C; National
Institutes of Health). Phospho-PI3K (rabbit anti-human; cat. no.
4228; dilution, 1:1,000), mTOR (rabbit anti-human; cat. no. 2983;
dilution, 1:2,000) and phospho-mTOR (Ser2448) (rabbit anti-human;
cat. no. 5536; dilution, 1:1,000) primary antibodies were provided
by Cell Signaling Technology, Inc. The following antibodies were
supplied by Proteintech Group, Inc.: Bcl-2 (mouse anti-human; cat.
no. 60178-1-Ig; dilution, 1:2,000), BAX (mouse anti-human; cat. no.
60267-1-Ig; dilution, 1:5,000), Caspase-3 (mouse anti-human; cat.
no. 66470-2-Ig; dilution, 1:1,000), LC3 (rabbit anti-human; cat.
no. 14600-1-AP; dilution, 1:2,000), p62 (mouse anti-human; cat. no.
18420-1-AP; dilution, 1:5,000), GAPDH (mouse anti-human; cat. no.
60004-1-Ig; dilution, 1:50,000), β-actin (mouse anti-human; cat.
no. 66009-1-Ig; dilution, 1:20,000), PI3Kinase p85 (mouse
anti-human; cat. no. 60225-1-Ig; dilution, 1:5,000), AKT (mouse
anti-human; cat. no. 60203-2-Ig; dilution, 1:5,000), phospho-AKT
Ser473 (mouse anti-human; cat. no. 66444-1-Ig; dilution, 1:5,000),
HRP-conjugated Affinipure Goat Anti-Rabbit (cat. no. SA00001-2;
dilution, 1:2,000), HRP-conjugated Affinipure Goat Anti-Mouse (cat.
no. SA00001-1; dilution, 1:2,000), E-cadherin (mouse anti-human;
cat. no. 20874-1-AP; dilution, 1:20,000) and N-cadherin (mouse
anti-human; 22018-1-AP; dilution, 1:2,000).
Combined experiments
For the combined experiments, cells
(1×105cells/ml) were added into 96- and 6-well plates.
In the experimental group, cells were incubated in serum-free
medium containing 740Y-P or LY294002 at a range of concentrations
(10, 20, 30 and 40 µM) at 37°C, whereas the control group was
treated with RPMI 1640 medium. After 12 h, experimental group cells
were incubated in serum-free medium containing 2 mg/l HpD. After 4
h, the cells were irradiated with the laser in the PDT-alone group
and combined group.
Statistical analysis
All experiments were independently repeated three
times. Data analysis and mapping were performed using GraphPad
Prism software (version 8.3.0; Dotmatics). Data are presented as
the mean ± SD and were analyzed by one-way ANOVA, with Tukey's
method was used for post-hoc analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
HpD-PDT suppresses KYSE-150 cell
viability
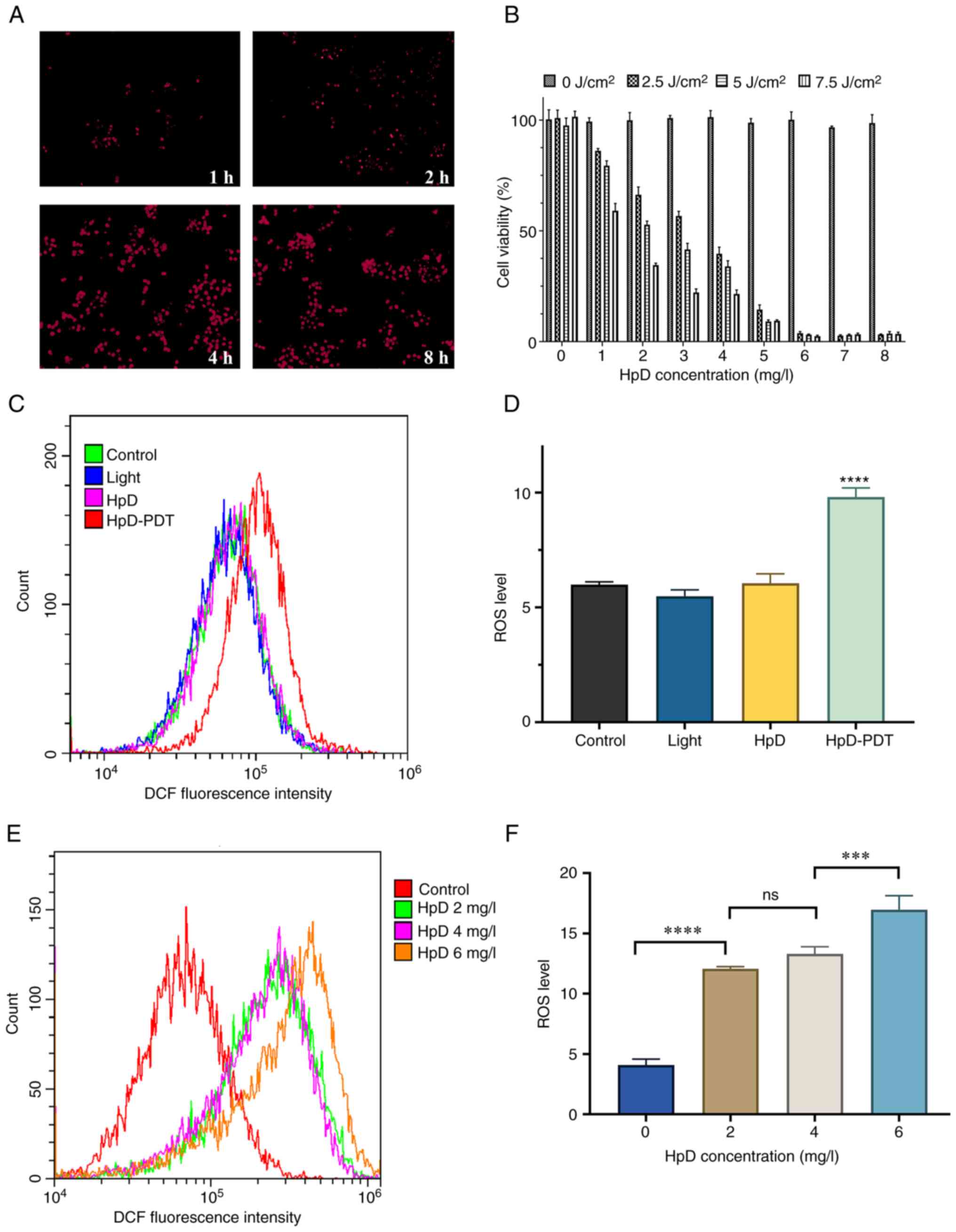
The cellular uptake of HpD increased over time.
After a 4-h incubation, cells had absorbed a substantial amount of
HpD. After an 8-h incubation, the cellular uptake of HpD was
similar to that after 4 h of incubation (Fig. 1A). Cells were incubated with HpD in
the dark for 4 h in the subsequent experiments, allowing the PS
sufficient time to enter the cells, while minimizing changes in the
microenvironment of the cell culture medium (22). To investigate the most appropriate
laser energy density and concentration of HpD, KYSE-150 cells were
subjected to HpD and energy at different concentration gradients.
When cells were in a completely dark environment (0
J/cm2), increasing concentrations of HpD had no
cytotoxicity. The cell viability after HpD-PDT treatment was
decreased in a dose-dependent manner. The efficacy was dependent on
the combination of the HpD concentration and energy density
(Fig. 1B). Cell viability plateaued
when the HpD concentration was increased to 6 mg/l. When the energy
density was 5 J/cm2 and the HpD concentration was 2
mg/l, the mean survival rate of KYSE-150 cells was 52.77%. To avoid
interference from excessively high or low HpD concentrations and
light intensities on the experimental results, parameters with
moderate light intensity and HpD concentration were chose. Finally,
a concentration of 5 J/cm2 in combination with 2 mg/l
HpD was selected for all subsequent experiments except for the
wound healing assay.
ROS levels are significantly increased
after HpD-PDT
The intracellular ROS levels were quantified after
HpD-PDT. Analysis based on flow cytometry demonstrated that the
relative fluorescence intensities of the control, light, HpD and
HpD-PDT groups were 5.99±0.13, 5.49±0.27, 6.05±0.41 and 9.81±0.39,
respectively. The ROS level of the control group was notably lower
than that of the HpD-PDT group (Fig. 1C
and D). However, the differences in ROS levels among the
control, light and HpD groups were not statistically significant
(P=0.1099). The present study also evaluated the production of ROS
following PDT treatment (light energy density, 5 J/cm2)
at various concentrations of HpD. There was no significant
difference in ROS production between the concentrations of 2 and 4
mg/l. When the HpD concentration was increased to 6 mg/l, there was
a significant increase in ROS production compared with the 4 mg/l
group (Fig. 1E and F).
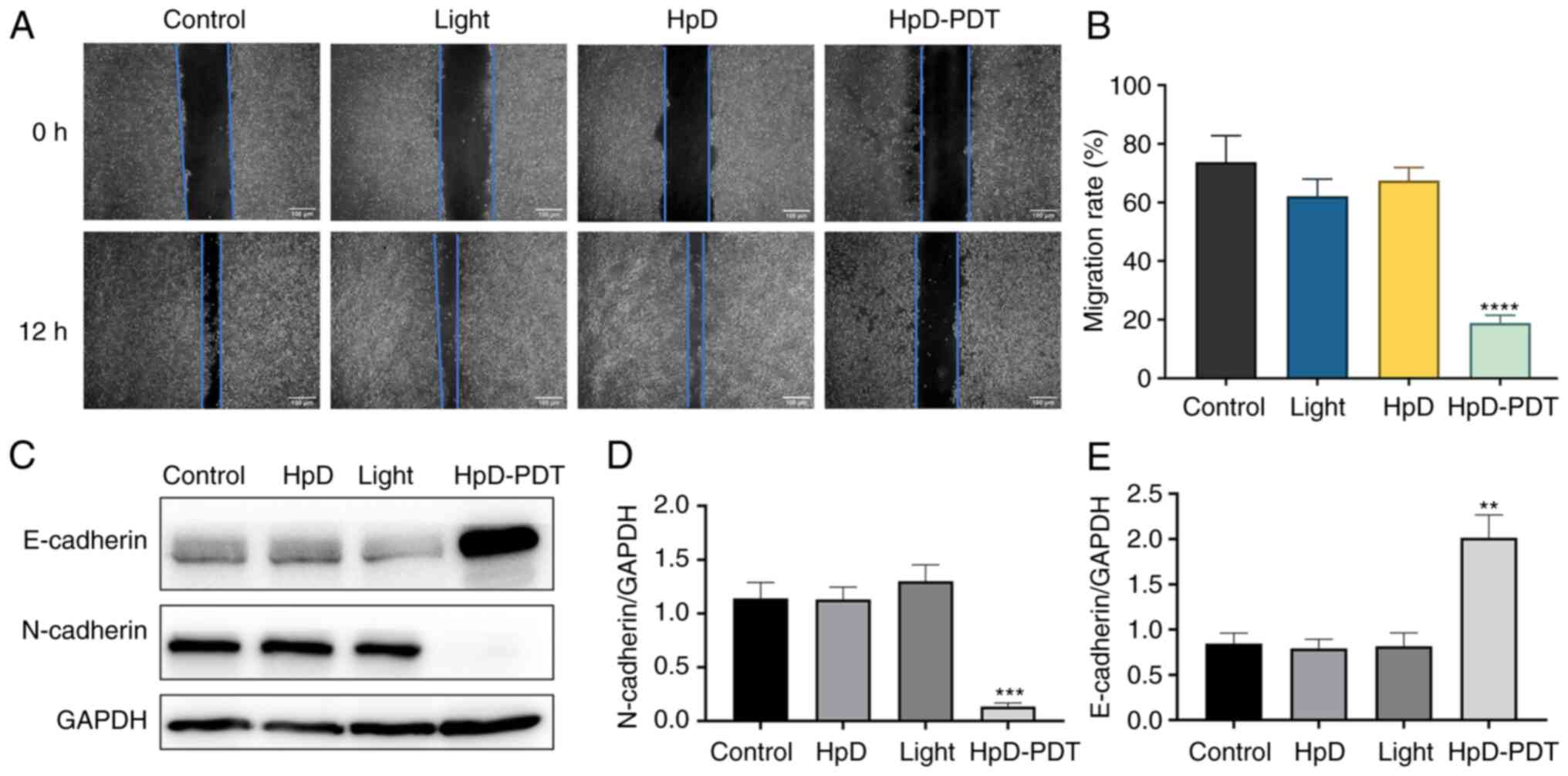
HpD-PDT suppresses KYSE-150 cell
migration
Based on the results of the cell viability assay,
low-dose light (2.5 J/cm2) in combination with 1 mg/l
HpD was selected for the wound healing assay, to avoid the effects
of cell death on cell migration. After 12 h, the scratch healing
rates of the control, light, HpD and HpD-PDT groups were
73.77±0.09, 62.08±0.06, 67.45±0.04 and 18.89±0.03%, respectively.
These results suggested that HpD-PDT significantly suppressed the
migration of KYSE-150 cells (Fig. 2A
and B). It is well-known that epithelial-mesenchymal transition
(EMT) serves a key role in cell migration. N-cadherin is a
mesenchymal marker, while E-cadherin is an epithelial marker. A
marker event of EMT is the downregulation of E-cadherin and the
increased expression of N-cadherin, also known as the ‘cadherin
switch’. It results in lost epithelial cell integrity, decreased
epithelial intercellular adhesion and enhanced cell migration
(23,24). To understand the underlying
mechanism of PDT in the inhibition of cell migration, western
blotting was performed to assess E-cadherin and N-cadherin
expression. E-cadherin protein expression was upregulated after
HpD-PDT treatment. By contrast, N-cadherin expression was decreased
significantly compared with that of the control group. These
results indicated that HpD-PDT inhibited the EMT process, which in
turn inhibited cell migration (Fig.
2C-E).
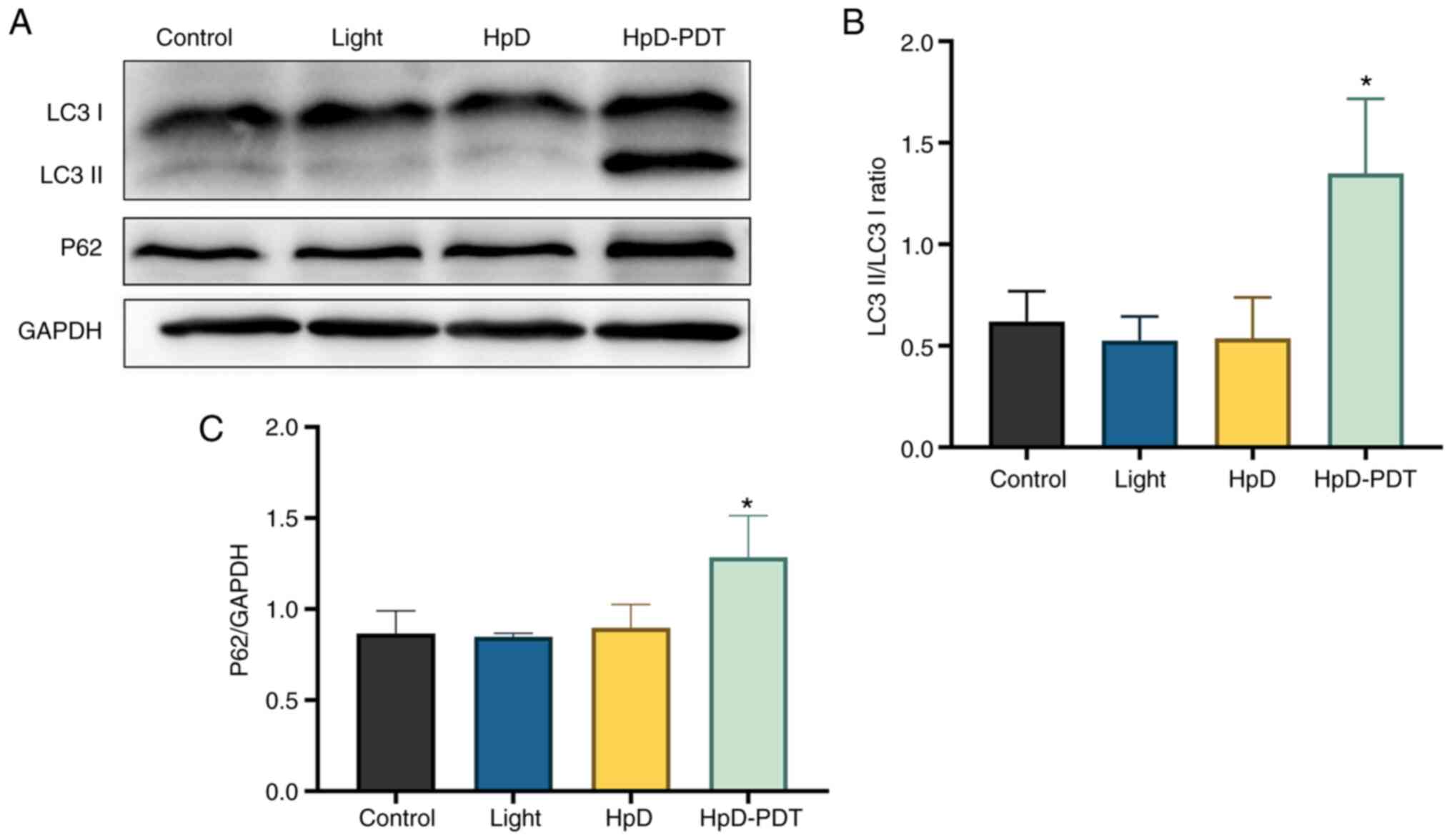
HpD-PDT induces cell autophagy
To understand the relationship between cell
autophagy and HpD-PDT treatment, the expression levels of
autophagy-associated proteins were assessed. LC3 has been studied
most often as a distinctive autophagy marker. LC3 I can be
transformed to LC3 II during autophagy, indicating the level of
autophagy activity. p62 is considered a vital protein during
autophagy (25). p62 can interact
with LC3 on the isolation membrane through the LC3-interacting
area. During the process of autophagosome formation in cells, p62
is incorporated into the autophagosome and is subsequently degraded
(26). Autophagy activation is
usually accompanied by p62 degradation (27). LC3 II protein levels were increased
at 24 h after irradiation in the HpD-PDT group, causing the ratio
of LC3 II to LC3 I to be significantly increased compared with
those in the control (Fig. 3A and
B). However, the protein expression levels of p62 were also
significantly upregulated after HpD-PDT treatment compared with
those in the control (Fig. 3C). We
hypothesized that although HpD-PDT could induce autophagy, the
increased p62 protein levels indicated a decrease in autophagic
flux.
HpD-PDT-induced apoptosis in KYSE-150
cells
To visualize apoptotic cells, a Hoechst 33342
staining assay was performed. The nuclei of apoptotic cells
exhibited bright blue fluorescence. Only a few apoptotic cells
could be found among the control, light and HpD groups. By
contrast, numerous apoptotic cells were observed in the HpD-PDT
group (Fig. 4A). Subsequently, cell
apoptosis levels were examined via flow cytometry. Annexin
V-FITC/PI staining was performed at 24 h after PDT. Both early and
late apoptotic cells manifested as FITC-positive cells. The
percentages of apoptotic cells were determined (Fig. 4B and C). In the HpD-PDT group, the
percentage of apoptotic cells was markedly higher than that in
other groups. To further investigate the relationship between
apoptosis and HpD-PDT treatment, RT-qPCR was used to evaluate the
expression of specific genes related to apoptosis. The results of
RT-qPCR (Fig. 4D) demonstrated that
Bcl-2 expression was significantly downregulated after PDT compared
with that in the control; thus, the ratio of BAX to Bcl-2 was
significantly increased. Additionally, HpD-PDT significantly
upregulated Caspase-3 gene expression compared with the control
(Fig. 4D). The protein expression
levels of cleaved Caspase-3, which is vital for converting normal
cells into apoptotic cells (28),
were significantly increased following HpD-PDT compared with the
control (Fig. 5A). The expression
levels of Bcl-2 were also notably decreased in HpD-PDT-treated
cells compared with control. The expression levels of BAX did not
show significant intergroup differences. These results indicated
that HpD-PDT induced apoptosis in KYSE-150 cells.
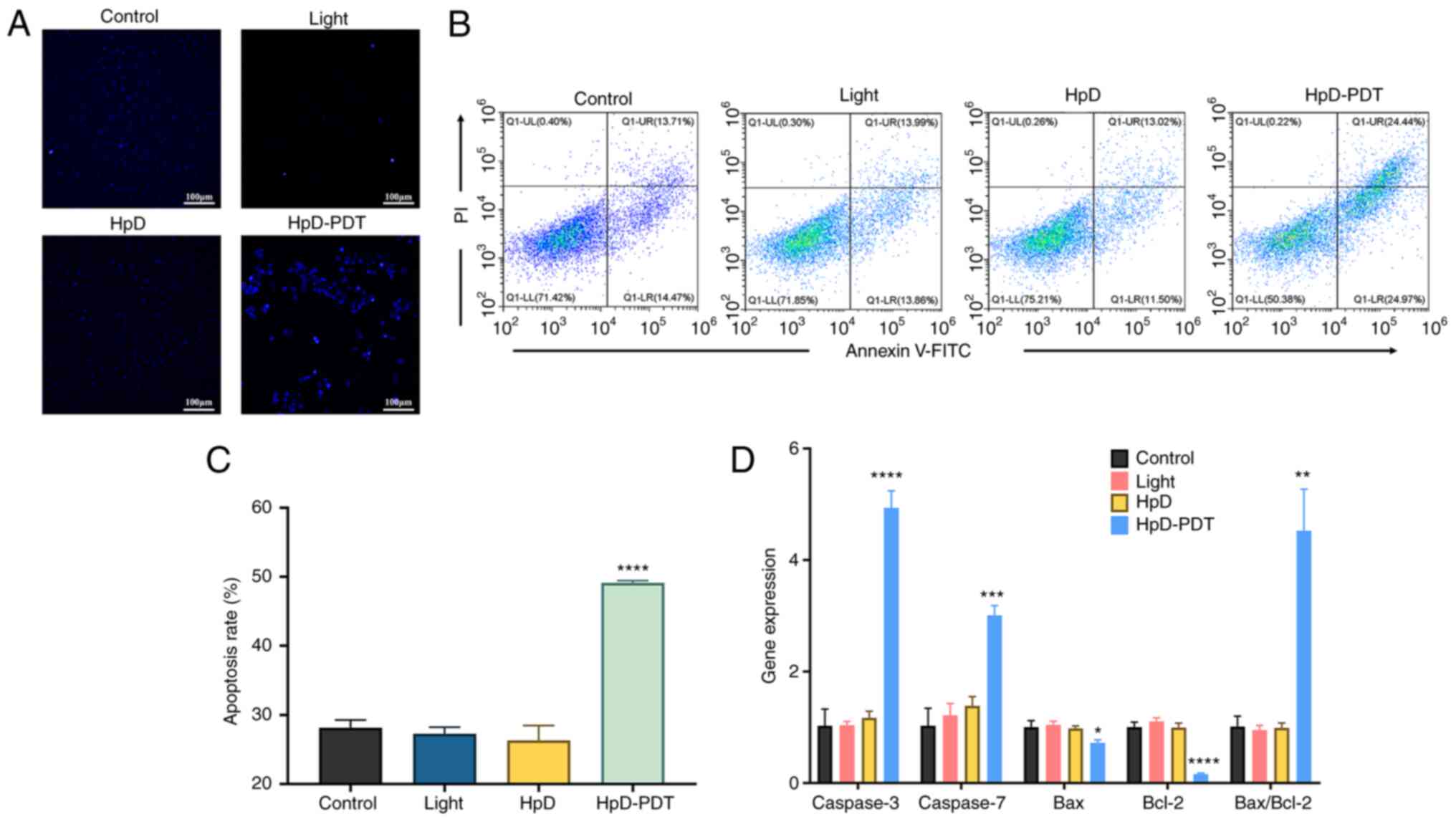 | Figure 4.HpD-PDT induces apoptosis in KYSE-150
cells. (A) Cells were stained with Hoechst 33342 and images were
captured using a fluorescence microscope (scale bar, 100 µm). (B)
Cell apoptosis rates were determined using flow cytometry with an
annexin V-FITC/PI kit. (C) The apoptosis rates of the control,
light, HpD and HpD-PDT groups were 28.15±1.12, 27.28±0.95,
26.31±2.16 and 49.13±0.32%, respectively. The percentage of
apoptotic cells was significantly increased after HpD-PDT treatment
(mean ± SD; n=3; ****P<0.001 vs. control group). (D) Expression
levels of apoptosis-related genes were detected by reverse
transcription-quantitative PCR 24 h after laser irradiation (mean ±
SD; n=3; *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001
vs. control group). HpD, ematoporphyrin derivative; PDT,
photodynamic therapy. |
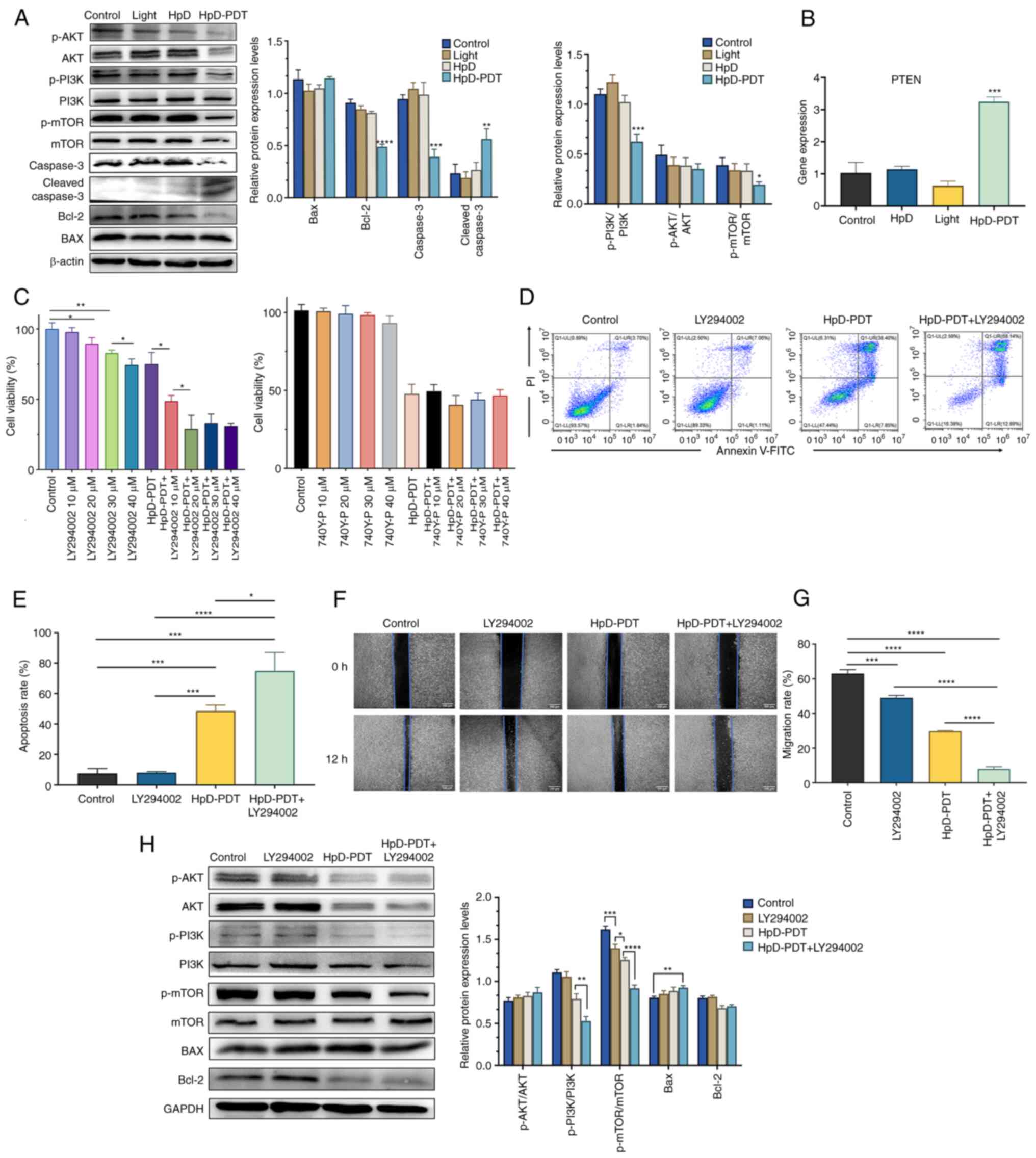 | Figure 5.Role of the PI3K/AKT/mTOR signaling
pathway in HpD-PDT treatment. (A) Phosphorylation levels of PI3K,
AKT and mTOR were detected by western blotting. The expression
levels of apoptosis-related proteins were also detected (mean ± SD;
n=3; *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 vs.
control group). (B) PTEN expression was measured by reverse
transcription-quantitative PCR 24 h after laser irradiation (mean ±
SD; n=3; ***P<0.001 vs. control group). (C) Effect of combined
treatment on cell viability was measured using a Cell Counting
Kit-8 assay 24 h after laser irradiation (*P<0.05 and
**P<0.01). (D) Flow cytometry analysis of apoptosis in KYSE-150
cells was performed based on Annexin V/PI staining. (E) Cell
apoptosis was higher in the combination group than in the HpD-PDT
alone group (mean ± SD; n=3; LY294002 + HpD-PDT group vs. HpD-PDT
group; *P<0.05, ***P<0.001 and ****P<0.0001). (F)
Representative images of cellular migration after different
treatments (scale bar, 100 µm). The original images were converted
to grayscale images using ImageJ. (G) Combination of HpD-PDT with
LY294002 could enhance the efficiency of inhibition of cell
migration compared with that observed for HpD-PDT alone (mean ± SD;
n=3; ***P<0.001; ****P<0.0001). (H) Phosphorylation levels of
PI3K, AKT and mTOR in the LY294002 + HpD-PDT group were detected by
western blotting. The protein expression levels of BAX and Bcl-2
were also detected (mean ± SD; n=3; *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001). HpD, ematoporphyrin derivative;
p-, phosphorylated; PDT, photodynamic therapy. |
HpD-PDT reduces phosphorylation levels
in the PI3K/Akt/mTOR signaling pathway
KYSE-150 cells were collected at 24 h after HpD-PDT.
Western blotting was subsequently performed to determine changes in
the PI3K/Akt/mTOR signaling pathway after HpD-PDT treatment. The
results demonstrated that the ratios of p-PI3K/PI3K and p-mTOR/mTOR
were significantly decreased after HpD-PDT (Fig. 5A). The PI3K signaling pathway can be
negatively regulated by PTEN (29).
By decreasing the phosphatidylinositol-3, 4, 5-phosphate levels
inside the cell, PTEN can serve a physiological role to inhibit the
activation of downstream proteins of the PI3K signaling pathway
(30). Therefore, PTEN expression
could be used to indirectly detect the activity of the PI3K
signaling pathway. PTEN expression examined by qPCR was
significantly increased after HpD-PDT compared with the control
(Fig. 5B). These findings
demonstrated that HpD-PDT inhibited the activation of the PI3K
signaling pathway. After pre-treating the cells with the PI3K
activator 740Y-P for 12 h, cell viability was assessed using a
CCK-8 assay. As the concentration of 740Y-P increased, it did not
enhance cell viability following HpD-PDT treatment (Fig. 5C). An artificial inhibitor of PI3K
(LY294002) was used to determine the relationship between the
PI3K/Akt/mTOR signaling pathway and HpD-PDT. LY294002, a well-known
PI3K signaling pathway inhibitor, could simultaneously inhibit the
activity of PI3Kα, PI3Kδ and PI3Kβ (31). The CCK-8 assay was used to verify
whether LY294002 exerted notable levels of cytotoxicity. Cells were
incubated with LY294002 for 12 h before irradiation in the LY294002
only group (LY294002 group) and combination group (LY294002 +
HpD-PDT group). The results of the CCK-8 assay demonstrated no
significant difference at a 10 µM LY294002 concentration in the
LY294002 group. However, the cell viability gradually decreased as
the LY294002 concentration increased. If the LY294002 concentration
reached 40 µM, the degree of the inhibition effect was almost
similar to that observed with HpD-PDT. In addition, cell viability
was significantly decreased in the LY294002 10 µM + HpD-PDT group
compared with the HpD-PDT alone group (Fig. 5C). When the LY294002 concentration
increased to 20 µM in the combination group, cell viability was
further reduced. No statistical differences were observed among the
LY294002 20 µM + HpD-PDT, LY294002 30 µM + HpD-PDT and LY294002 40
µM + HpD-PDT groups. Continuing to increase the concentration of
LY294002 did not result in a better enhancement of PDT efficacy.
Finally, 20 µM LY294002 was selected for the subsequent experiment.
Flow cytometry was performed again to assess the apoptosis rate. In
the combination group, the apoptosis rate was markedly higher than
that in the LY294002 only and HpD-PDT only groups (Fig. 5D and E). The present findings
suggested that the combination of HpD-PDT with LY294002 enhanced
the pro-apoptotic effects of HpD-PDT. To further explore their
combinatorial effects on KYSE-150 cell migration, a wound healing
assay was performed. In the combined treatment group, the migration
of cells was significantly suppressed compared with that observed
in the HpD-PDT alone or LY294002 alone groups (Fig. 5F and G). Furthermore, protein
expression levels in the combined treatment group and the
HpD-PDT-alone group were assessed using western blotting. The
ratios of p-PI3K/PI3K and p-mTOR/mTOR in the LY294002 + HpD-PDT
group were significantly decreased compared with those in the
HpD-PDT alone group. By contrast, the ratio of p-AKT/AKT did not
exhibit significant inter-group differences. The expression levels
of Bcl-2 were also markedly decreased in both the HpD-PDT alone
group and the LY294002 + HpD-PDT group. However, there were no
significant differences between the two groups (Fig. 5H).
Discussion
PDT has been regarded as an accepted non-invasive PS
application-based therapy that can be activated by light (10). Compared with other treatments, such
as surgery, radiotherapy, chemotherapy or a combination of these
therapies used for esophageal squamous cell carcinoma, PDT has
certain advantages, including high tumor specificity, minimal side
effects and relatively minor tumor resistance (32). Certain scholars have proposed that
the tumor microenvironment could potentially lead to PDT
resistance. Intervening in the tumor microenvironment may reduce
this resistance (33,34). PDT has been recommended and applied
for the treatment of malignant tumor (35). Nevertheless, to the best of our
knowledge, its underlying mechanism has not been studied
thoroughly.
A positive association was observed between ROS
production and the HpD concentration in PDT. With the increase in
HpD concentration, there was a simultaneous increase in ROS
production, while the maximum inhibition of cell viability was
achieved. At an HpD concentration of 6 mg/l, both the inhibition of
cell viability and ROS production reached significantly high levels
compared with the control. This implied that increasing ROS
production was crucial for the efficacy of HpD-PDT.
The present study revealed that HpD-PDT had the
ability to reduce KYSE-150 cell viability, in a HpD concentration
and laser energy density-dependent manner, and HpD-PDT could
inhibit the migration of esophageal squamous cells by suppressing
the EMT process. Tumor cells exhibiting a strong ability to invade
usually exhibit an increased degree of malignancy (36). The extracellular matrix (ECM) is
critical for processes such as migration and invasion (18). It has been reported that MMPs could
affect ECM degradation, which could affect the metastasis and
invasion of tumor cells (37).
MMP-2 and MMP-9, two representative proteins, are closely related
to tumor metastasis (38). The
PI3K/Akt signaling pathway is also closely linked with the
regulation of MMP expression (39).
p-Akt could trigger the regulation of MMP-2 and MMP-9 (40,41).
Further research is required to determine whether PDT can
indirectly regulate the MMP protein family and influence cell
invasion through the PI3K/AKT signaling pathway.
Autophagy has been considered a mechanism by which
tumor cells respond to stress and starvation during changes in the
external environment (42). This
cellular self-digestion could remove damaged proteins or impaired
organelles to keep the cells alive; however, it could also cause
autophagic death of tumor cells (27). Therefore, autophagy has a dual
regulatory ability because it can lead to cell survival or death
based on different external stimulus conditions (43). A number of tumor therapies induce
autophagic death by adjusting cell signaling pathways, and can
indirectly induce autophagy by exerting cytotoxic effects (42). However, to the best of our
knowledge, the efficacy of autophagy induction during the cancer
treatment process remains unclear. The PI3K/AKT/mTOR signaling
pathway has been studied extensively to investigate and determine
its role in autophagy regulation (44–46).
The PI3K pathway has been demonstrated to act as a crucial
mechanism for cellular autophagy that helps deal with changes in
ROS levels. The downstream protein Akt participates in this process
by activating the mTOR complex (mTORC)1 (44). Furthermore, Akt could inhibit
autophagy-related gene expression. Under low ROS level conditions,
mTORC1 and mTORC2 could suppress cell autophagy (44). Under high ROS level conditions,
mTORC2 could accelerate cellular aging via the promotion of
autophagy (44). As expected, LC3
II expression was markedly increased after HpD-PDT, which in turn
resulted in the ratio of LC3 II to LC3 I increasing significantly,
indicating the occurrence of autophagy. p62/sequestosome 1
expression increased after HpD-PDT treatment. p62 is involved in
the autophagy process as a substrate. It is degraded when the
autophagosome is formed. Alternatively, it can be accumulated due
to impairments in autophagic flux (42). We hypothesized that HpD-PDT may
block the autophagic flux, causing p62 accumulation and ultimately
resulting in cell death (47).
Briefly, HpD-PDT initiated autophagy in KYSE-150 cells, followed by
the blockade of autophagic flux.
Apoptosis is considered to be a programmed cell
death process. This process is related to the activation of the
cysteine protease family (48). As
aforementioned, apoptosis is regulated and controlled via two main
pathways. Both pathways eventually lead to Caspase activation.
However, the final apoptotic process is initiated with the cleavage
of Caspase-3, which causes DNA to be broken into fragments and the
cytoskeleton to be degraded. Finally, those fragments are swallowed
by phagocytic cells (49). The
present study revealed that HpD-PDT triggered Caspase-dependent
apoptosis in KYSE-150 cells. The levels of cleaved Caspase-3 were
significantly increased following HpD-PDT. In addition, the protein
expression levels of Bcl-2, which is known as an anti-apoptotic
gene, were downregulated after HpD-PDT treatment. However, the
protein expression levels of BAX did not exhibit significant
intergroup differences. These results indicated that the balance
between pro-apoptotic and anti-apoptotic effects was broken.
Additionally, the increase in the ROS levels may be the factor
driving these changes.
The present study demonstrated that the ratios of
p-PI3K/PI3K and p-mTOR/mTOR were significantly decreased following
HpD-PDT. It is evident that the PI3K/AKT/mTOR signaling pathway
plays a significant role in the process through which PDT exerts
the aforementioned therapeutic effects.
CCK-8 assays were performed using cells pre-treated
with 740Y-P, and it was observed that 740Y-P was unable to
counteract the inhibitory effect of HpD-PDT on cell viability. We
hypothesized that PDT may encompass certain mechanisms for tumor
cell eradication, with the modulation of the PI3K/AKT/mTOR
signaling pathway representing merely one facet of the multifaceted
interplay. In situations where the PI3K/AKT/mTOR signaling pathway
cannot be inhibited, PDT has the potential to induce cell death via
alternative mechanisms, such as pyroptosis and ferroptosis
(50–54).
LY294002, a broad-spectrum PI3K inhibitor that could
inhibit PI3Kα, PI3Kδ and PI3Kβ (31), was used in subsequent experiments.
The results demonstrated that the combination of HpD-PDT and
LY294002 showed a synergistic effect on the promotion of apoptosis,
inhibition of cell viability and suppression of migration. The
inhibitor of PI3K could enhance the curative effects of HpD-PDT.
These findings could provide a novel clinical combination therapy
that could be used for esophageal cancer treatment in the
future.
The present study revealed that the ratios of
p-PI3K/PI3K and p-mTOR/mTOR in the LY294002 + HpD-PDT group were
significantly decreased compared with those in the HpD-PDT alone
group (Fig. 5H). The LY294002 and
control groups only exhibited statistical differences with regard
to the ratio of p-mTOR/mTOR (Fig.
5H). The inconsistent protein expression levels suggested that
the regulatory mechanism of HpD-PDT may not be limited solely to
the PI3K/AKT/mTOR signaling pathway but may involve the regulation
of multiple mechanisms.
In clinical practice, it has been observed that a
significant proportion of patients with esophageal cancer
undergoing first PDT treatment do not achieve optimal photodynamic
results. A repeat endoscopy is necessary for these patients to
receive the secondary PDT 24 h later (55,56).
This phenomenon may be attributed to several factors, including
large tumor volume, limited light penetration depth, variable
responses to PSs and inter-patient variability. However, increasing
light intensity, while more efficient in targeting the tumor, also
carries the risk of inducing esophageal damage and substantial
scarring, leading to esophageal stenosis, a condition particularly
prevalent among patients with early esophageal cancer (50,55).
Consequently, the pursuit of high-intensity light exposure in PDT
comes with inherent risks.
Our primary objective was to enhance patient
responsiveness to PDT, while minimizing its adverse effects. The
potential of PI3K inhibitors to augment PDT efficacy offers the
prospect of reducing intravenous PS dosages and light exposure
intensities, thereby mitigating side effects and improving
therapeutic outcomes.
The present experiments were exclusively conducted
using the KYSE-150 poorly differentiated esophageal cancer cell
line. To improve the generalizability of the present findings, a
diverse array of cell lines should be used for validation.
Furthermore, it is essential to establish animal tumor models to
provide empirical validation for the present conclusions through
in vivo experiments.
In conclusion, HpD-PDT could reduce esophageal
cancer cell viability, induce apoptosis and inhibit migration by
downregulating the PI3K/AKT/mTOR signaling pathway. The combination
of HpD-PDT and an inhibitor of PI3K (LY294002) could enhance the
therapeutic efficacy compared with that observed for HpD-PDT alone.
Further investigations on combination therapy are required to
achieve improved clinical outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW, JN and XL conceived the study and conducted data
analysis. XW and JN wrote the original draft, which was
subsequently revised by LY and XL. XW and LY conducted the data
interpretation. XW, JN, LY and XL confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morgan E, Soerjomataram I, Rumgay H,
Coleman HG, Thrift AP, Vignat J, Laversanne M, Ferlay J and Arnold
M: The global landscape of esophageal squamous cell carcinoma and
esophageal adenocarcinoma incidence and mortality in 2020 and
projections to 2040: New estimates from GLOBOCAN 2020.
Gastroenterology. 163:649–658.e2. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kato H and Nakajima M: Treatments for
esophageal cancer: A review. Gen Thorac Cardiovasc Surg.
61:330–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Correia JH, Rodrigues JA, Pimenta S, Dong
T and Yang Z: Photodynamic Therapy review: Principles,
Photosensitizers, applications, and future directions.
Pharmaceutics. 13:13322021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dobson J, de Queiroz GF and Golding JP:
Photodynamic therapy and diagnosis: Principles and comparative
aspects. Vet J. 233:8–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, et al: Photodynamic therapy of cancer: An update. CA Cancer J
Clin. 61:250–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zahra M, Chota A, Abrahamse H and George
BP: Efficacy of green synthesized nanoparticles in photodynamic
therapy: A therapeutic approach. Int J Mol Sci. 24:109312023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Cai L, He J, Li X, Li L, Chen X
and Lan P: Influence and mechanism of 5-aminolevulinic
acid-photodynamic therapy on the metastasis of esophageal
carcinoma. Photodiagnosis Photodyn Ther. 20:78–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lan M, Zhao S, Liu W, Lee CS, Zhang W and
Wang P: Photosensitizers for photodynamic therapy. Adv Healthc
Mater. 8:e19001322019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwiatkowski S, Knap B, Przystupski D,
Saczko J, Kędzierska E, Knap-Czop K, Kotlińska J, Michel O,
Kotowski K and Kulbacka J: Photodynamic therapy-mechanisms,
photosensitizers and combinations. Biomed Pharmacother.
106:1098–1107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Gemert JC, Berenbaum MC and Gijsbers
GH: Wavelength and light-dose dependence in tumour phototherapy
with haematoporphyrin derivative. Br J Cancer. 52:43–49. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kashyap D, Garg VK and Goel N: Intrinsic
and extrinsic pathways of apoptosis: Role in cancer development and
prognosis. Adv Protein Chem Struct Biol. 125:73–120. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carpenter R and Brady MF: BAX Gene.
StatPearls [Internet] Treasure Island (FL): StatPearls Publishing;
2023
|
|
14
|
Cheung TH, Chung TK, Lo KW, Yu MY,
Krajewski S, Reed JC and Wong YF: Apotosis-related proteins in
cervical intraepithelial neoplasia and squamous cell carcinoma of
the cervix. Gynecol Oncol. 86:14–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Korsmeyer SJ: BCL-2 gene family and the
regulation of programmed cell death. Cancer Res. 59 (7
Suppl):1693s–1700s. 1999.PubMed/NCBI
|
|
16
|
Thornberry NA and Lazebnik Y: Caspases:
Enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumar D, Haldar S, Gorain M, Kumar S,
Mulani FA, Yadav AS, Miele L, Thulasiram HV and Kundu GC:
Epoxyazadiradione suppresses breast tumor growth through
mitochondrial depolarization and caspase-dependent apoptosis by
targeting PI3K/Akt pathway. BMC Cancer. 18:522018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang L, Lin H, Chen Q, Yu L and Bai D:
MPPa-PDT suppresses breast tumor migration/invasion by inhibiting
Akt-NF-κB-dependent MMP-9 expression via ROS. BMC Cancer.
19:11592019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar S, Patil HS, Sharma P, Kumar D,
Dasari S, Puranik VG, Thulasiram HV and Kundu GC: Andrographolide
inhibits osteopontin expression and breast tumor growth through
down regulation of PI3 kinase/Akt signaling pathway. Curr Mol Med.
12:952–966. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liutkeviciute-Navickiene J, Mordas A,
Rutkovskiene L and Bloznelyte-Plesniene L: Skin and mucosal
fluorescence diagnosis with different light sources. Eur J
Dermatol. 19:135–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)). Method. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gupta S, Dwarakanath BS, Muralidhar K and
Jain V: Cellular uptake, localization and photodynamic effects of
haematoporphyrin derivative in human glioma and squamous carcinoma
cell lines. J Photochem Photobiol B. 69:107–120. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pal M, Bhattacharya S, Kalyan G and Hazra
S: Cadherin profiling for therapeutic interventions in Epithelial
Mesenchymal Transition (EMT) and tumorigenesis. Exp Cell Res.
368:137–146. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Loh CY, Chai JY, Tang TF, Wong WF, Sethi
G, Shanmugam MK, Chong PP and Looi CY: The E-Cadherin and
N-Cadherin Switch in Epithelial-to-Mesenchymal Transition:
Signaling, therapeutic implications, and challenges. Cells.
8:11182019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Glick D, Barth S and Macleod KF:
Autophagy: Cellular and molecular mechanisms. J Pathol. 221:3–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie J, Wang S, Li Z, Ao C, Wang J, Wang L,
Peng X and Zeng K: 5-aminolevulinic acid photodynamic therapy
reduces HPV viral load via autophagy and apoptosis by modulating
Ras/Raf/MEK/ERK and PI3K/AKT pathways in HeLa cells. J Photochem
Photobiol B. 194:46–55. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Crowley LC and Waterhouse NJ: Detecting
cleaved caspase-3 in apoptotic cells by flow cytometry. Cold Spring
Harb Protoc. 2016:2016. View Article : Google Scholar
|
|
29
|
Li A, Qiu M, Zhou H, Wang T and Guo W:
PTEN, insulin resistance and cancer. Curr Pharm Des. 23:3667–3676.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen CY, Chen J, He L and Stiles BL: PTEN:
Tumor suppressor and metabolic regulator. Front Endocrinol
(Lausanne). 9:3382018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chaussade C, Rewcastle GW, Kendall JD,
Denny WA, Cho K, Grønning LM, Chong ML, Anagnostou SH, Jackson SP,
Daniele N and Shepherd PR: Evidence for functional redundancy of
class IA PI3K isoforms in insulin signalling. Biochem J.
404:449–458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Didamson OC and Abrahamse H: Targeted
photodynamic diagnosis and therapy for esophageal cancer: Potential
role of functionalized nanomedicine. Pharmaceutics. 13:19432021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gallagher-Colombo SM, Finlay JC and Busch
T: Tumor Microenvironment as a Determinant of Photodynamic Therapy
Resistance. Resistance to Photodynamic Therapy in Cancer.
Resistance to Targeted Anti-Cancer Therapeutics. Rapozzi V and Jori
G: 5. Springer; Cham: pp. 65–97. 2015, View Article : Google Scholar
|
|
34
|
Rapozzi V and Jori G: Resistance to
Photodynamic Therapy in Cancer. Resistance to Targeted Anti-Cancer
Therapeutics. 5. 1st edition. Springer; Cham: pp. p2482015
|
|
35
|
Rodrigues JA and Correia JH: Photodynamic
therapy for colorectal cancer: An update and a look to the future.
Int J Mol Sci. 24:122042023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tahtamouni L, Ahram M, Koblinski J and
Rolfo C: Molecular regulation of cancer cell migration, invasion,
and metastasis. Anal Cell Pathol (Amst).
2019:13565082019.PubMed/NCBI
|
|
37
|
Mao W, Sun Y, Zhang H, Cao L, Wang J and
He P: A combined modality of carboplatin and photodynamic therapy
suppresses epithelial-mesenchymal transition and matrix
metalloproteinase-2 (MMP-2)/MMP-9 expression in HEp-2 human
laryngeal cancer cells via ROS-mediated inhibition of MEK/ERK
signalling pathway. Lasers Med Sci. 31:1697–1705. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Josefsen LB and Boyle RW: Unique
diagnostic and therapeutic roles of porphyrins and phthalocyanines
in photodynamic therapy, imaging and theranostics. Theranostics.
2:916–966. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu L, Ye Y and Zhu X: MMP-9 secreted by
tumor associated macrophages promoted gastric cancer metastasis
through a PI3K/AKT/Snail pathway. Biomed Pharmacother.
117:1090962019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jung JS, Jung K, Kim DH and Kim HS:
Selective inhibition of MMP-9 gene expression by mangiferin in
PMA-stimulated human astroglioma cells: Involvement of PI3K/Akt and
MAPK signaling pathways. Pharmacol Res. 66:95–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hwang YP, Yun HJ, Choi JH, Han EH, Kim HG,
Song GY, Kwon KI, Jeong TC and Jeong HG: Suppression of EGF-induced
tumor cell migration and matrix metalloproteinase-9 expression by
capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK,
p38 MAPK, and AP-1 signaling. Mol Nutr Food Res. 55:594–605. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mathew R and White E: Autophagy in
tumorigenesis and energy metabolism: Friend by day, foe by night.
Curr Opin Genet Dev. 21:113–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He C, Xia J, Gao Y, Chen Z and Wan X:
Chlorin A-mediated photodynamic therapy induced apoptosis in human
cholangiocarcinoma cells via impaired autophagy flux. Am J Transl
Res. 12:5080–5094. 2020.PubMed/NCBI
|
|
44
|
Kma L and Baruah TJ: The interplay of ROS
and the PI3K/Akt pathway in autophagy regulation. Biotechnol Appl
Biochem. 69:248–264. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu Z, Han X, Ou D, Liu T, Li Z, Jiang G,
Liu J and Zhang J: Targeting PI3K/AKT/mTOR-mediated autophagy for
tumor therapy. Appl Microbiol Biotechnol. 104:575–587. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yan J, Dou X, Zhou J, Xiong Y, Mo L, Li L
and Lei Y: Tubeimoside-I sensitizes colorectal cancer cells to
chemotherapy by inducing ROS-mediated impaired autophagolysosomes
accumulation. J Exp Clin Cancer Res. 38:3532019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang H, Ewetse MP, Ma C, Pu W, Xu B, He P,
Wang Y, Zhu J and Chen H: The ‘Light Knife’ for gastric cancer:
Photodynamic therapy. Pharmaceutics. 15:1012022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen D, Wang B, Zhao Z, Zhang G, Wang P,
Zhang L, Liu X, Zhang H, Zeng Q and Wang X: Modified
5-aminolevulinic acid photodynamic therapy induces cutaneous
squamous cell carcinoma cell pyroptosis via the JNK signaling
pathway. Biochim Biophys Acta Mol Cell Res. 1871:1196032023.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li L, Song D, Qi L, Jiang M, Wu Y, Gan J,
Cao K, Li Y, Bai Y and Zheng T: Photodynamic therapy induces human
esophageal carcinoma cell pyroptosis by targeting the
PKM2/caspase-8/caspase-3/GSDME axis. Cancer Lett. 520:143–159.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pan WL, Tan Y, Meng W, Huang NH, Zhao YB,
Yu ZQ, Huang Z, Zhang WH, Sun B and Chen JX:
Microenvironment-driven sequential ferroptosis, photodynamic
therapy, and chemotherapy for targeted breast cancer therapy by a
cancer-cell-membrane-coated nanoscale metal-organic framework.
Biomaterials. 283:1214492022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang ZJ, Huang YP, Li XX, Liu ZT, Liu K,
Deng XF, Xiong L, Zou H and Wen Y: A Novel Ferroptosis-Related
4-Gene prognostic signature for cholangiocarcinoma and photodynamic
therapy. Front Oncol. 11:7474452021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bartusik-Aebisher D, Osuchowski M,
Adamczyk M, Stopa J, Cieślar G, Kawczyk-Krupka A and Aebisher D:
Advancements in photodynamic therapy of esophageal cancer. Front
Oncol. 12:10245762022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yamashita H, Kadota T, Minamide T,
Sunakawa H, Sato D, Takashima K, Nakajo K, Murano T, Shinmura K,
Yoda Y, et al: Efficacy and safety of second photodynamic therapy
for local failure after salvage photodynamic therapy for esophageal
cancer. Dig Endosc. 34:488–496. 2022. View Article : Google Scholar : PubMed/NCBI
|