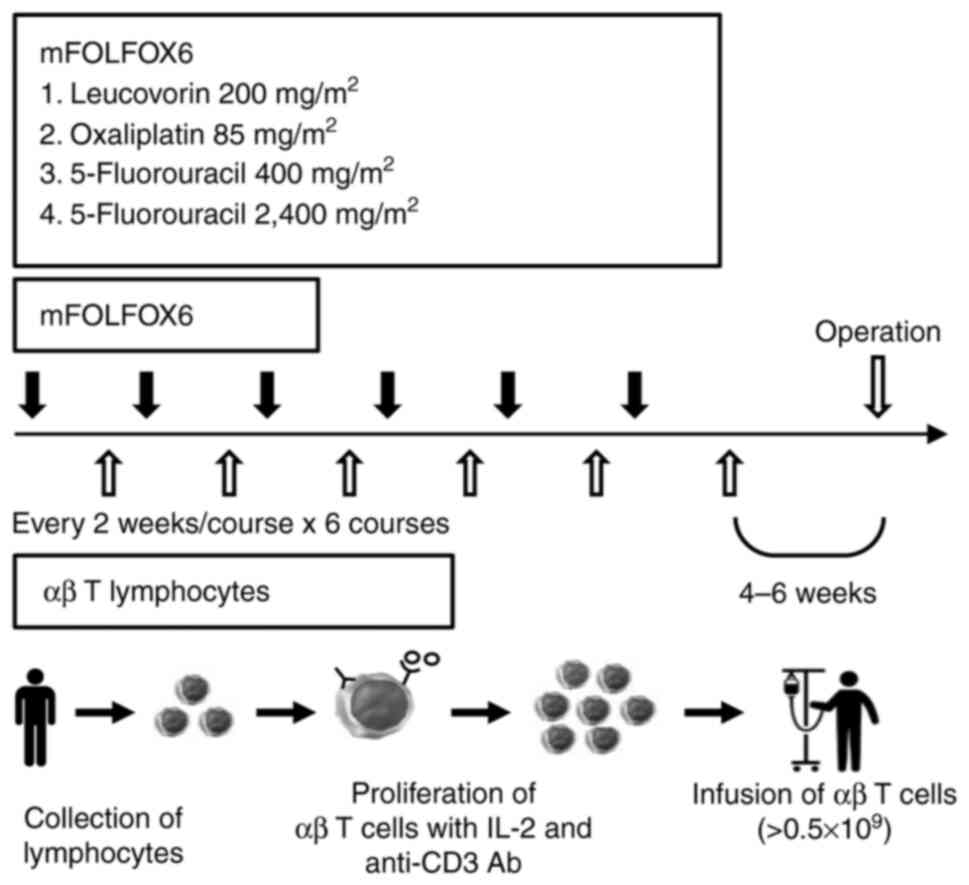
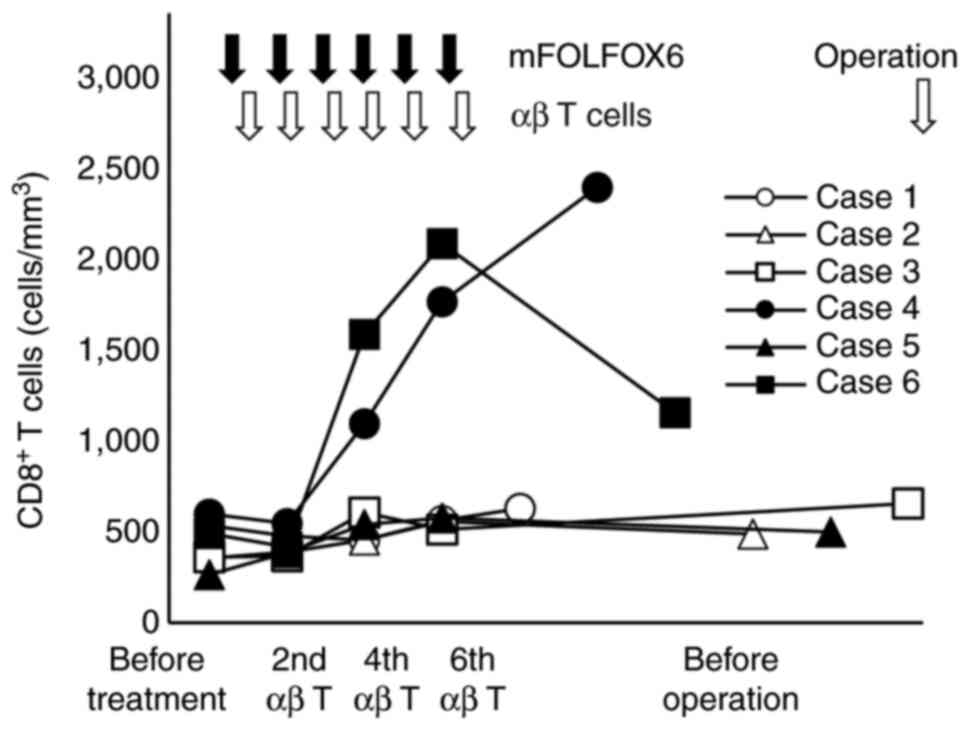
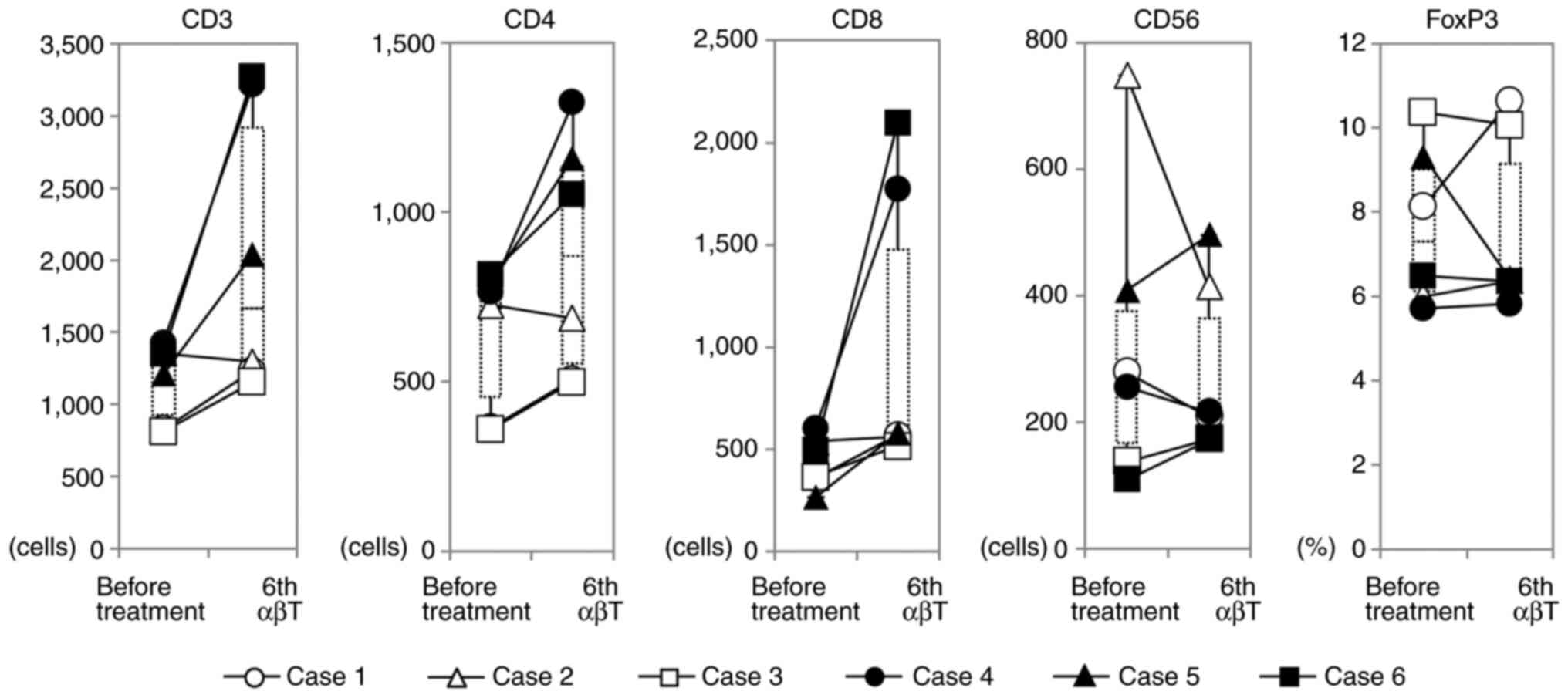
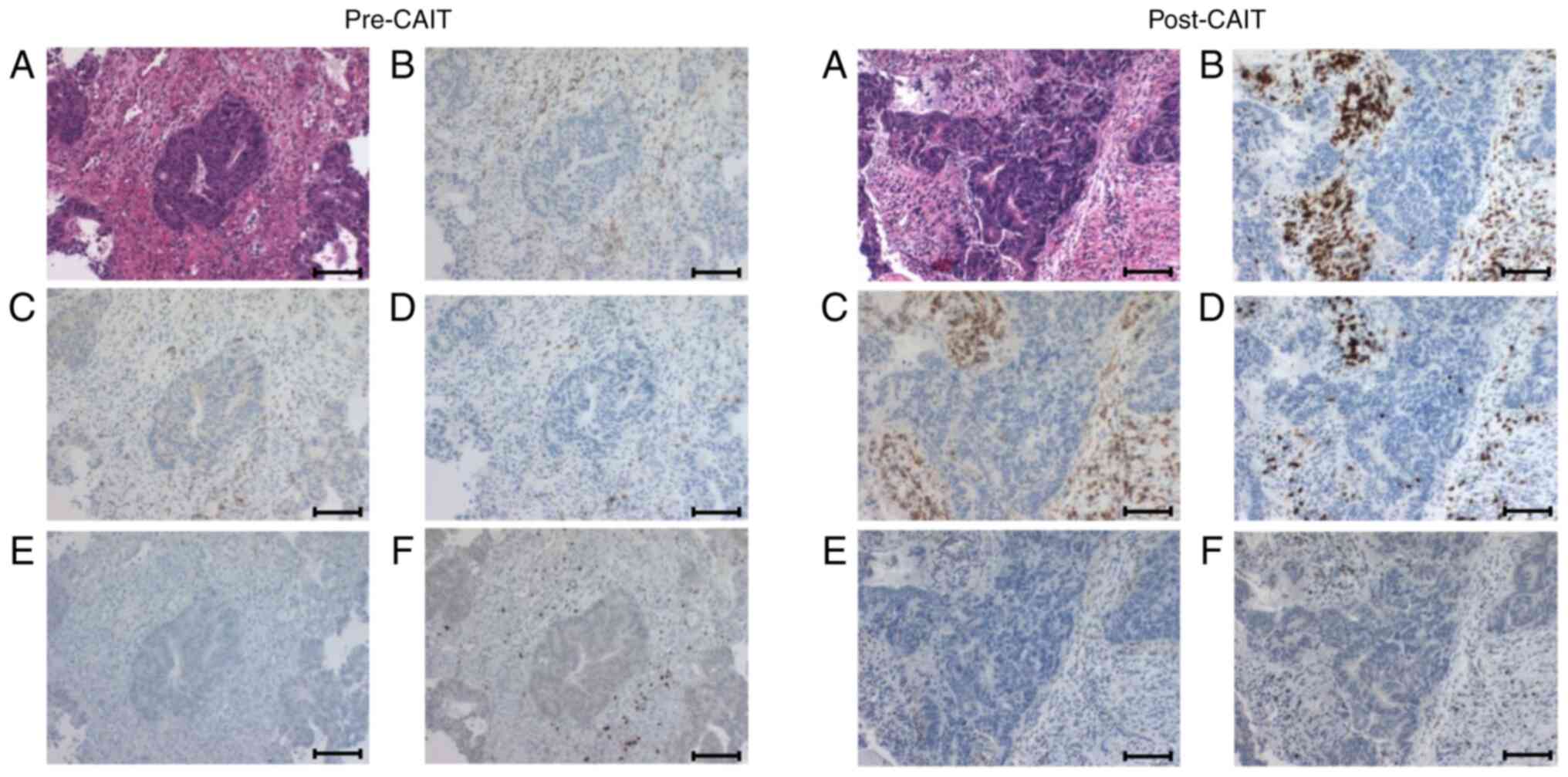
|
1
|
Benson AB, Venook AP, Al Hawary MM,
Cederquist L, Chen Y, Ciombor KK, Cohen S, Cooper HS, Deming D,
Engstrom PF, et al: Rectal cancer, version 2.2018, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
16:874–901. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peeters KCMJ, Marijnen CAM, Nagtegaal ID,
Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius
B, Leer JW, et al: The TME trial after a median follow-up of 6
years: Increased local control but no survival benefit in
irradiated patients with resectable rectal carcinoma. Ann Surg.
246:693–701. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkesson J, Birgisson H, Pahlman L,
Cedermark B, Glimelius B and Gunnarsson U: Swedish rectal cancer
trial: Long lasting benefits from radiotherapy on survival and
local recurrence rate. J Clin Oncol. 23:5644–5650. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sauer R, Liersch T, Merkel S, Fietkau R,
Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann
H, et al: Preoperative versus postoperative chemoradiotherapy for
locally advanced rectal cancer: Results of the German
CAO/ARO/AIO-94 randomized phase III trial after a median follow-up
of 11 years. J Clin Oncol. 30:1926–1933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tomida A, Uehara K, Hiramatsu K, Maeda A,
Sakamoto E, Okada Y, Kurumiya Y, Nakayama G, Nakamura M, Aiba T, et
al: Neoadjuvant CAPOX and bevacizumab alone for locally advanced
rectal cancer: Long-term results from the N-SOG 03 trial. Int J
Clin Oncol. 24:403–410. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Benson AB, Venook AP, Al-Hawary MM, Arain
MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D,
Garrido-Laguna I, et al: NCCN guidelines insights: Rectal cancer,
version 6.2020. J Natl Compr Canc Netw. 18:806–815. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takayama T, Sekine T, Makuuchi M, Yamasaki
S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi
Y and Kakizoe T: Adoptive immunotherapy to lower postsurgical
recurrence rates of hepatocellular carcinoma: A randomised trial.
Lancet. 356:802–807. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwai K, Soejima K, Kudoh S, Umezato Y,
Kaneko T, Yoshimori K, Tokuda H, Yamaguchi T, Mizoo A, Setoguchi Y,
et al: Extended survival observed in adoptive activated T
lymphocyte immunotherapy for advanced lung cancer: Results of a
multicenter historical cohort study. Cancer Immunol Immunother.
61:1781–1790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abbasi S, Totmaj MA, Abbasi M, Hajazimian
S, Goleij P, Behroozi J, Shademan B, Isazadeh A and Baradaran B:
Chimeric antigen receptor T (CAR-T) cells: Novel cell therapy for
hematological malignancies. Cancer Med. 12:7844–7858. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mulé JJ, Shu S, Schwarz SL and Rosenberg
SA: Adoptive immunotherapy of established pulmonary metastases with
LAK cells and recombinant interleukin-2. Science. 225:1487–1489.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshida Y, Naito M, Yamada T, Aisu N,
Daibo K, Mera T, Tanaka T, Naito K, Yasumoto K, Kamigaki T, et al:
Adoptive chemoimmunotherapy using activated αβ T cells for stage IV
colorectal cancer. Anticancer Res. 36:3741–3746. 2016.PubMed/NCBI
|
|
13
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumours. 8th edition.
John Wiley & Sons; Hoboken: 2017
|
|
14
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Cancer Institute, . Common
Terminology Criteria for Adverse Events v4.0. August
24–2021https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Japanese Society for Cancer of the Colon
Rectum, . Japanese classification of colorectal, appendiceal, and
anal carcinoma: The 3-d, english edition [Secondary Publication]. J
Anus Rectum Colon. 3:175–195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamigaki T, Ibe H, Okada S, Matsuda E,
Tanaka M, Oguma E, Kinoshita Y, Ogasawara S, Ono A, Makita K, et
al: Improvement of impaired immunological status of patients with
various types of advanced cancers by autologous immune cell
therapy. Anticancer Res. 35:4535–4543. 2015.PubMed/NCBI
|
|
19
|
Ishii F, Yoshida Y, Yamauchi Y, Aisu N,
Kojima D, Mera T, Kato D, Tanaka T, Naito K, Yasumoto K, et al:
Hepatectomy for liver metastases of colorectal cancer after
adoptive chemoimmunotherapy using activated αβ T-cells. Anticancer
Res. 37:3933–3939. 2017.PubMed/NCBI
|
|
20
|
Uehara K, Hiramatsu K, Maeda A, Sakamoto
E, Inoue M, Kobayashi S, Tojima Y, Yoshioka Y, Nakayama G, Yatsuya
H, et al: Neoadjuvant oxaliplatin and capecitabine and bevacizumab
without radiotherapy for poor-risk rectal cancer: N-SOG 03 phase II
trial. Jpn J Clin Oncol. 43:964–971. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kamiya T, Uehara K, Nakayama G, Ishigure
K, Kobayashi S, Hiramatsu K, Nakayama H, Yamashita K, Sakamoto E,
Tojima Y, et al: Early results of multicenter phase II trial of
perioperative oxaliplatin and capecitabine without radiotherapy for
high-risk rectal cancer: CORONA I study. Eur J Surg Oncol.
42:829–835. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hasegawa H, Okabayashi K, Tsuruta M, Koike
J, Funahashi K, Yokomizo H, Yoshimatsu H, Kan H, Yamada T, Ishida
H, et al: Updated survival results of FACT trial: Multicenter phase
II trial of neoadjuvant chemotherapy with mFOLFOX6 for stage II/III
rectal cancer with a T3/T4 tumor. Ann Oncol. 28:v171–v172. 2017.
View Article : Google Scholar
|
|
23
|
André T, Boni C, Navarro M, Tabernero J,
Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F
and de Gramont A: Improved overall survival with oxaliplatin,
fluorouracil, and leucovorin as adjuvant treatment in stage II or
III colon cancer in the MOSAIC trial. J Clin Oncol. 27:3109–3116.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kotaka M, Yoshino T, Oba K, Shinozaki K,
Touyama T, Manaka D, Matsui T, Ishigure K, Hasegawa J, Inoue K, et
al: Initial safety report on the tolerability of modified FOLFOX6
as adjuvant therapy in patients with curatively resected stage II
or III colon cancer (JFMC41-1001-C2: JOIN trial). Cancer Chemother
Pharmacol. 76:75–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin H, Wang L, Zhong X, Zhang X, Shao L
and Wu J: Meta-analysis of neoadjuvant chemotherapy versus
neoadjuvant chemoradiotherapy for locally advanced rectal cancer.
World J Surg Oncol. 19:1412021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Adams S, Gray RJ, Demaria S, Goldstein L,
Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et
al: Prognostic value of tumor-infiltrating lymphocytes in
triple-negative breast cancers from two phase III randomized
adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin
Oncol. 32:2959–2966. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyashita M, Sasano H, Tamaki K, Hirakawa
H, Takahashi Y, Nakagawa S, Watanabe G, Tada H, Suzuki A, Ohuchi N
and Ishida T: Prognostic significance of tumor-infiltrating CD8+
and FOXP3+ lymphocytes in residual tumors and alterations in these
parameters after neoadjuvant chemotherapy in triple-negative breast
cancer: A retrospective multicenter study. Breast Cancer Res.
17:1242015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Diederichsen AC, Hjelmborg Jv, Christensen
PB, Zeuthen J and Fenger C: Prognostic value of the CD4+/CD8+ ratio
of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR
expression on tumour cells. Cancer Immunol Immunother. 52:423–428.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mosely SI, Prime JE, Sainson RC, Koopmann
JO, Wang DY, Greenawalt DM, Ahdesmaki MJ, Leyland R, Mullins S,
Pacelli L, et al: Rational selection of syngeneic preclinical tumor
models for immunotherapeutic drug discovery. Cancer Immunol Res.
5:29–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Q and Ding ZY: The ways of isolating
neoantigen-specific T cells. Front Oncol. 10:13472020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simoni Y, Becht E, Fehlings M, Loh CY, Koo
SL, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, et al:
Bystander CD8+ T cells are abundant and phenotypically distinct in
human tumour infiltrates. Nature. 557:575–579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bando H, Tsukada Y, Inamori K, Togashi Y,
Koyama S, Kotani D, Fukuoka S, Yuki S, Komatsu Y, Homma S, et al:
Preoperative chemoradiotherapy plus nivolumab before surgery in
patients with microsatellite stable and microsatellite
instability-high locally advanced rectal cancer. Clin Cancer Res.
28:1136–1146. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takimoto R, Kamigaki T, Gotoda T,
Takahashi T, Okada S, Ibe H, Oguma E and Goto S: Esophageal cancer
responsive to the combination of immune cell therapy and low-dose
nivolumab: Two case reports. J Med Case Rep. 15:1912021. View Article : Google Scholar : PubMed/NCBI
|