Introduction
Breast cancer is the most common cancer type and a
leading cause of cancer-related death in women worldwide. Breast
cancer is clinically classified into four subtypes that are
characterized by the expression of hormone receptors: Estrogen
receptor (ER)-positive, progesterone receptor (PR)-positive, human
epidermal receptor 2 (HER2)-positive, and triple-negative for ER,
PR and HER2 (1,2). Breast cancer is treated by various
methods, including surgery, chemotherapy, radiotherapy and
endocrine therapy (3,4). For early-stage breast cancer, there is
a good chance of patients achieving cancer-free status following
multimodal therapy (3). By
contrast, advanced breast cancer is typically regarded as
incurable, with patients surviving for only 2–4 years (5). Thus, there is an urgent need to
develop novel therapies and drugs that can prolong survival and
improve the quality of life of patients with advanced breast
cancer.
Deubiquitinases are components of the proteasome
system that remove conjugated ubiquitin molecules from target
proteins, and thus regulate their stability and bioactivity
(6). Deubiquitinases are involved
in several biological processes related to cancer development,
including proliferation, cell cycle progression, apoptosis and
metastasis (7). Increasing evidence
has shown that deubiquitinases contribute to breast cancer growth,
metastasis, immunosuppression, chemosensitivity and
radiosensitivity (8). OTU
domain-containing 7B (OTUD7B), a deubiquitinase is a member of the
A20 subgroup of the ovarian tumor protein superfamily, which is
often dysregulated in human cancer and may serve as a valuable
prognostic predictor (9–14). In ERα-positive breast cancer, OTUD7B
deubiquitylates ERα, leading to its stabilization; through this
mechanism, OTUD7B promotes the proliferation of breast cancer cells
(15). In ERα-negative breast
cancer, silencing OTUD7B markedly inhibits the migration and
invasion of cancer cells in vitro, and significantly impairs
lung metastasis in vivo (16). Furthermore, high OTUD7B expression
is associated with poor paclitaxel response in patients with
triple-negative breast cancer (17).
In the present study, co-immunoprecipitation (co-IP)
assay was used to investigate whether OTUD7B interacts with
forkhead box protein M1 (FOXM1) and reduces FOXM1 ubiquitination to
stabilize the FOXM1 protein. FOXM1 is a proliferation-specific
transcription factor that is involved in the initiation,
progression, metastasis and drug resistance of breast cancer
(18). Furthermore, the present
study investigated whether OTUD7B inhibits the proliferation and
stemness of breast cancer cells by regulating the protein stability
of FOXM1.
Materials and methods
Cell culture and tumor tissues
MCF-10A, MDA-MB-436, MDA-MB-231, MDA-MB-468,
MDA-MB-453 and MCF-7 cells were obtained from Xiamen Immocell
Biotechnology Co., Ltd., and were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) containing
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.).
Cells were cultured at 37°C and 5% CO2 in an
incubator.
A total of 10 pairs of breast cancer tissue samples
and adjacent normal tissue samples (1 cm away from the edge of the
tumor and free of tumor cells) were obtained from Sanming First
Hospital Affiliated to Fujian Medical University (Sanming, China).
The age range of the 10 female patients was 43–62 years (mean age,
53±6.5 years). All of the patients did not receive any other
treatment, such as chemotherapy, target therapy or other
preoperative treatment, except for surgical operation.
Plasmid construction
Plasmid encoding His-ubiquitin (His-Ub) was obtained
from Xiamen Anti-HeLa Biological Technology Trade Co., Ltd. The
plasmids encoding hemagglutinin (HA)-OTUD7B and Flag-FOXM1 were
constructed by subcloning OTUD7B and FOXM1 cDNA into pCDNA3.1-HA
and pCDNA3.1-Flag vectors (Xiamen Anti-HeLa Biological Technology
Trade Co., Ltd.), respectively. FOXM1 cDNA was also inserted into
plv-EF1a-MCS-c-Flag-bsd vector (Xiamen Anti-HeLa Biological
Technology Trade Co., Ltd), and the resulting plasmid was named
plv-Flag-FOXM1, which was used to produce stable OTUD7B-knockdown
and FOXM1-overexpression cells. Plasmids encoding a short hairpin
(sh)RNA targeting OTUD7B (shOTUD7B) and a negative control shRNA
(shNC) were constructed using the pLKO.1 vector (Xiamen Anti-HeLa
Biological Technology Trade Co., Ltd). The sequences for plasmid
construction are listed in Table
I.
 | Table I.Sequences for plasmid construction
used in the present study. |
Table I.
Sequences for plasmid construction
used in the present study.
| Plasmid name | Sequence,
5′-3′ |
|---|
| shOTUD7B-1 | F:
CCGGCAGATTCTGTGGCTAACAAACCTCGAGGTTTGTTAGCCACAGAATCTGTTTTT |
|
| R:
AATTAAAAACAGATTCTGTGGCTAACAAACCTCGAGGTTTGTTAGCCACAGAATCTG |
| shOTUD7B-2 | F:
CCGGTTGAAGAGTTTCACGTCTTTGCTCGAGCAAAGACGTGAAACTCTTCAATTTTT |
|
| R:
AATTAAAAATTGAAGAGTTTCACGTCTTTGCTCGAGCAAAGACGTGAAACTCTTCAA |
| shOTUD7B-3 | F:
CCGGTGGAAATGCTCACGGTTTATACTCGAGTATAAACCGTGAGCATTTCCATTTTT |
|
| R:
AATTAAAAATGGAAATGCTCACGGTTTATACTCGAGTATAAACCGTGAGCATTTCCA |
| shNC | F:
CCGGTTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGAATTTTT |
|
| R:
AATTAAAAATTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGAA |
| Flag-FOXM1 | F:
GTACCGAGCTCGGATCCGCCACCATGAAAACTAGCCCCCGTCG |
|
| R:
TCTTTGTAGTCCTCGAGCTGTAGCTCAGGAATAAACTG |
| HA-OTUD7B | F:
CTAGAGAATTCGGATCCATGACCCTGGACATGGATGC |
|
| R:
GCTTCCATGGCTCGAGTCAGAACCTGTGCACCAGGAG |
| Plv-Flag-FOXM1 | F:
CTAGAGAATTCGGATCCATGAAAACTAGCCCCCGTCG |
|
| R:
CCATGGCTCGAGCCCGGGCTGTAGCTCAGGAATAAACTG |
To determine the knockdown or overexpression
efficiency of plasmids in different breast cancer cells,
MDA-MB-468, MDA-MB-453 and MCF7 cells (2×106 cells/well)
were cultured in six-well plates and 5 µg shNC, shOTUD7B, pCDNA3.1
vector, HA-OTUD7B or Flag-FOXM1 were transfected into cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
After 24 h, cells were collected for western blotting. The results
of which showed that plasmids encoding Flag-FOXM1 or HA-OTUD7B
markedly increased the expression of FOXM1 or OTUD7B in cells,
whereas shOTUD7B markedly decreased the expression of OTUD7B in
cells. Notably, shOTUD7B-1 had the most significant effect and was
thus used in subsequent experiments (Fig. S1).
Construction of stable
OTUD7B-knockdown cell lines and OTUD7B-knockdown and
FOXM1-overexpressing cell lines
Stable OTUD7B-knockdown MDA-MB-468, MDA-MB-453 and
MCF7 cells; and stable OTUD7B-knockdown and FOXM1-overexpressing
MDA-MB-468, MDA-MB-453 and MCF7 cells were constructed as follows:
9 µg shNC, shOTUD7B or plv-Flag-FOXM1, 3 µg pMD2G (Xiamen Anti-HeLa
Biological Technology Trade Co., Ltd) and 6 µg psPAX2 (Xiamen
Anti-HeLa Biological Technology Trade Co., Ltd) were co-transfected
into 293T cells (Xiamen Immocell Biotechnology Co., Ltd.) in a
10-cm dish using Lipofectamine 2000. After 48 h cultivation at
37°C, the supernatant containing the lentivirus encoding shOTUD7B
or FOXM1 was collected. The lentivirus was enriched and the titer
was determined. To construct the stable OTUD7B-knockdown cell
lines, the lentivirus encoding shOTUD7B was transduced into
MDA-MB-468, MDA-MB-453 and MCF7 cells at a multiplicity of
infection of 10 in the presence of 8 µg/ml polybrene. After 48 h,
the medium was replaced with fresh medium containing 1 µg/ml
puromycin (Gibco; Thermo Fisher Scientific, Inc.). At 10 days
post-infection, cells were collected for gene expression analysis.
To construct the stable OTUD7B-knockdown and FOXM1-overexpressing
cell lines, the lentivirus encoding FOXM1 was transduced into
stable OTUD7B-knockdown MDA-MB-468, MDA-MB-453 and MCF7 cells at a
multiplicity of infection of 10 in the presence of 8 µg/ml
polybrene. After 48 h, the medium was replaced with fresh medium
containing 5 µg/ml blasticidin (Gibco; Thermo Fisher Scientific,
Inc.). At 10 days post-infection, cells were collected for gene
expression analysis.
Cell proliferation assay
MDA-MB-468, MDA-MB-453 and MCF7 cells; stable
OTUD7B-knockdown MDA-MB-468, MDA-MB-453 and MCF7 cells; stable
OTUD7B-knockdown and FOXM1-overexpressing MDA-MB-468, MDA-MB-453
and MCF7 cells were seeded into 96-well plates (1×104
cells/well) and cultured overnight. After 0, 24, 48 or 72 h, 10 µl
Cell Counting Kit 8 (CCK8) solution (Beyotime Institute of
Biotechnology) was added and cells were incubated for 4 h at 37°C.
The optical density was determined using an absorbance reader
(SpectraMax®; Molecular Devices, LLC) at 450 nm.
Colony formation assay
Clusters of cells containing >50 cells were
considered colonies. The same cells used in the cell proliferation
assay were seeded in 6-well plates at a density of 1×103
cells/well. After 14 days of culture, cell colonies were fixed with
4% paraformaldehyde overnight at 4°C. The fixed colonies were then
stained with 0.1% crystal violet for 10 min at 26°C. A light
microscope was used to count the colony numbers.
Sphere formation assay
The same cells used in the cell proliferation assay
were seeded in 6-well plates (1×104 cells/well)
containing serum-free DMEM supplemented with 2% B27 NeuroMix (Absin
Bioscience, Inc.), 20 ng/ml epithelial growth factor
(Sigma-Aldrich; Merck KGaA), 20 ng/ml basic fibroblast growth
factor (Sigma-Aldrich; Merck KGaA) and 4 µg/ml insulin. After 7
days of culture at 37°C, the sphere diameters were measured under a
light microscope.
Flow cytometric analysis
The same cells used in the cell proliferation assay
were plated in 6-well plates (2×106 cells/well). After
24 h, the cells were harvested and incubated with FITC-conjugated
antibodies for CD44 (cat. no. 12211-MM02-F; 1:200; Sino Biological,
Inc.), CD24 (cat. no. 655154; 1:200; BD Biosciences) or EpCAM (cat.
no. 10694-MM06-F; 1:200; Sino Biological, Inc.) at 26°C for 30 min.
The fluorescence intensity was determined using a NovoCyte flow
cytometer (ACEA Biosciences; Agilent Technologies, Inc.) and
NovoExpress software (1.4.1; ACEA Biosciences; Agilent
Technologies, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from MCF-10A, MDA-MB-436,
MDA-MB-231, MDA-MB-468, MDA-MB-453 and MCF-7 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. RNA was reverse
transcribed to cDNA using a reverse transcriptase cDNA synthesis
kit (cat. no. RR036Q; Takara Bio, Inc.) according to the
manufacturer's protocol. qPCR was conducted on the obtained cDNA
with a Roche Lightcycler 480 system (Roche Diagnostics) and TB
Green® premix (cat. no. RR820Q; Takara Biotechnology
Co., Ltd.). The qPCR program included preheating (95°C for 30 sec),
40 cycles of heating (95°C for 5 sec), cooling and extension (60°C
for 30 sec), and dissolution curve procedures (95°C for 5 sec, 60°C
for 60 sec and 95°C for 1 sec). The relative expression levels of
mRNA were calculated using the 2−ΔΔCq method (19). The qPCR primers are listed in
Table II with 18S used as the
reference gene.
 | Table II.Reverse transcription-quantitative
PCR primers used in the present study. |
Table II.
Reverse transcription-quantitative
PCR primers used in the present study.
| Gene | Sequence,
5′-3′ |
|---|
| 18S | F:
AGGCGCGCAAATTACCCAATCC |
|
| R:
GCCCTCCAATTGTTCCTCGTTAAG |
| OTUD7B | F:
AGTTGAGAAGGAAGCGTTGA |
|
| R:
TATACCAGCCCTGACTCTTTATTC |
Western blotting
MCF-10A, MDA-MB-436, MDA-MB-231, MDA-MB-468,
MDA-MB-453 and MCF-7 cells; stable OTUD7B-knockdown MDA-MB-468,
MDA-MB-453 and MCF7 cells; stable OTUD7B-knockdown and
FOXM1-overexpressing MDA-MB-468, MDA-MB-453 and MCF7 cells were
lysed in RIPA buffer containing protease and phosphatase inhibitors
(Beyotime Institute of Biotechnology). Protein quantification was
performed using the bicinchoninic acid method. A total of 20 µg
protein/lane was separated by SDS-PAGE on 10% gels and transferred
to polyvinylidene difluoride membranes (Bio-Rad Laboratories,
Inc.). After blocking with 5% skimmed milk at 25°C for 1 h, the
membranes were incubated with primary antibodies against OTUD7B
(cat. no. 16605-1-AP; 1:1,000), Nanog (cat. no. 14295-1-AP;
1:1,000), SOX2 (cat. no. 11064-1-AP; 1:1,000), Flag (cat. no.
20543-1-AP; 1:20,000), HA (cat. no. 51064-2-AP; 1:5,000), His (cat.
no. 10001-0-AP; 1:1,000), β-actin (cat. no. 20536-1-AP; 1:1,000),
β-tubulin (cat. no. 10094-1-AP; 1:2,000), FOXM1 (cat. no.
13147-1-AP; 1:2,000) or GAPDH (cat. no. 10494-1-AP; 1:5,000) (all
from Proteintech Group, Inc.) at 25°C for 2 h, followed by
incubation with horseradish peroxidase-conjugated goat anti-rabbit
IgG (cat. no. SA00001-2; 1:2,000; Proteintech Group, Inc.) at 25°C
for 1 h. An enhanced chemiluminescence kit (Thermo Fisher
Scientific, Inc.) was used to visualize the membranes. ImageJ v1.48
(National Institutes of Health) was used for densitometry. β-actin,
β-tubulin and GAPDH were used as internal reference proteins.
Co-IP
MDA-MB-468 cells were seeded in 6-well plates
(2×106 cells/well) overnight at 37°C and then
transfected with 1.6 µg pCDNA3.1 vector, pCDNA3.1-HA-OTUD7B,
pCDNA3.1-Flag-FOXM1 or His-Ub per well with Lipofectamine 2000
following the manufacturer's instructions for 24 h at 37°C.
Subsequently, cells were harvested and lysed in immunoprecipitation
buffer (150 mmol/l NaCl; 50 mmol/l Tris-HCl, Ph 7.4; 40 mmol/l
β-glycerophosphate; 1 mmol/l Na4OV3; 10
mmol/l NaF; 2 mmol/l EDTA) supplemented with 1 mmol/l PMSF and
protease inhibitor on ice for 30 min. The cells in
immunoprecipitation buffer were centrifuged at 4°C at 14,000 × g
for 15 min, and the supernatant was immediately transferred to a
new centrifuge tube to obtain the cell lysate. protein A/G agarose
beads (50 µl; Beyotime Institute of Biotechnology) were incubated
with anti-HA (cat. no. 66006-2-Ig; 1 µg) or anti-Flag (cat. no.
66008-4-Ig; 1 µg) (both from Proteintech Group, Inc.) at 4°C
overnight, followed by incubation with 500 µl cell lysates at 4°C
for 6 h. The mixture was centrifuged at 14,000 × g at 4°C for 15
min and the supernatant was discarded. The agarose
bead-antigen-antibody complex was then cleaned three times with
immunoprecipitation buffer. After the supernatant was discarded,
the precipitation was added to 60 µl 2X loading buffer, heated at
100°C for 5 min, and then subjected to western blotting.
Detection of the effect of OTUD7B on
FOXM1 degradation
MDA-MB-468 cells were seeded in 6-well plates
(2×106 cells/well) overnight at 37°C and then
transfected with 1.6 µg pCDNA3.1 vector, pCDNA3.1-HA-OTUD7B or
pCDNA3.1-Flag-FOXM1 with Lipofectamine 2000 for 24 h at 37°C,
according to the manufacturer's instructions. Subsequently, the
cells were cultured in medium containing 50 µg/ml cyclohexane
(MilliporeSigma) for 0, 2, 4, 8 and 12 h at 37°C. Subsequently, the
cells were collected for western blotting. Subsequently, ImageJ
v1.48 was used to calculate FOXM1 protein levels at different time
points.
Statistical analysis
The experiments were independently repeated three
times. Data are presented as the mean ± standard deviation.
Unpaired Student's t-test was used for comparisons between unpaired
groups, paired Student's t-test was used for comparisons between
paired groups, and one-way ANOVA followed by Tukey's post-hoc test
was used for comparisons among multiple groups. Prior to
statistical analysis, the normality of data was verified by the
Shapiro-Wilk test. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using GraphPad Prism v5.0 (Dotmatics).
Results
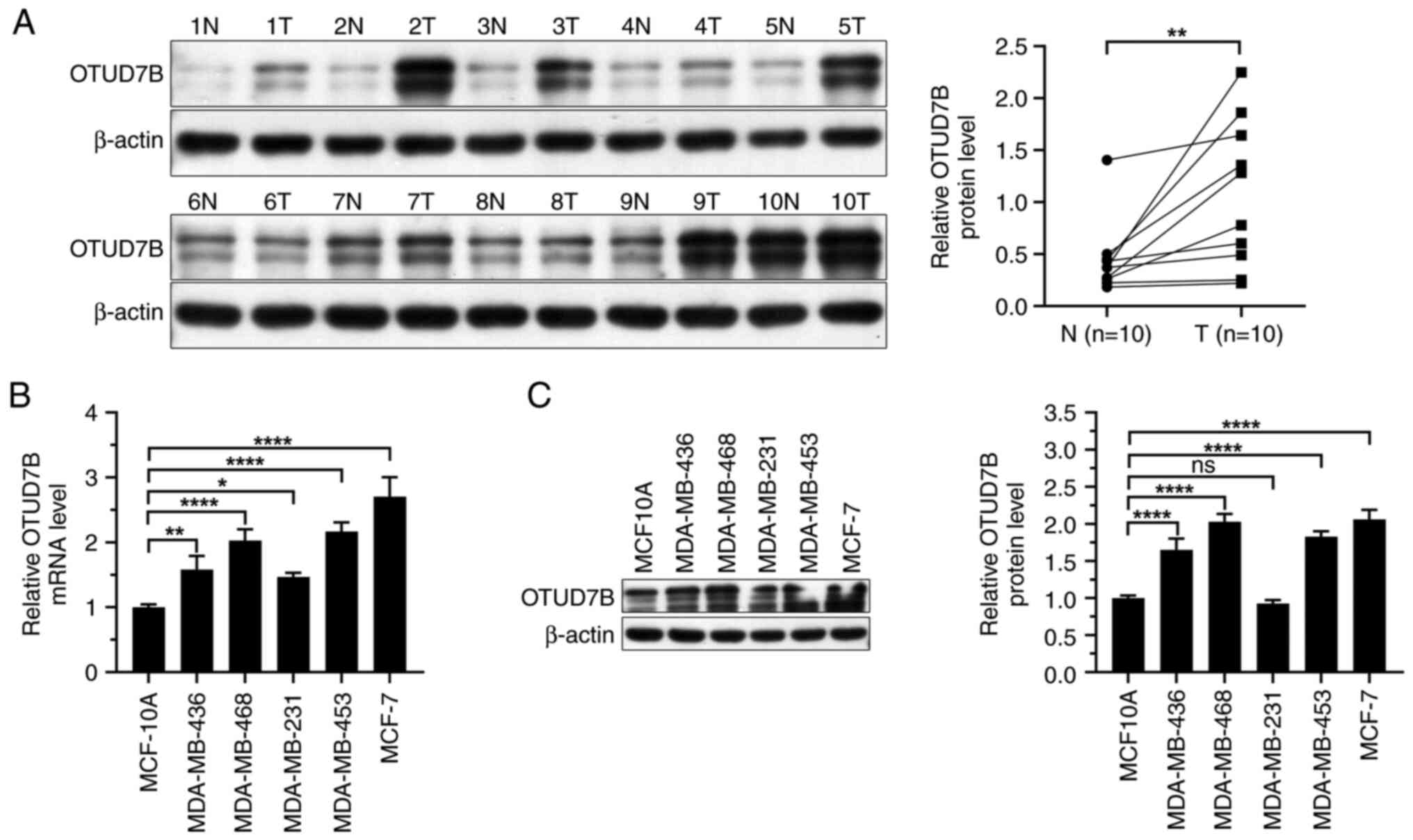
OTUD7B is upregulated in breast cancer
tissues and cells
To clarify the role of OTUD7B in breast
carcinogenesis, first the expression levels of OTUD7B in breast
cancer tissues and cells were examined. Western blotting showed
that the expression levels of OTUD7B were higher in breast cancer
tissues than those in adjacent normal tissues (Fig. 1A). Next, the mRNA and protein
expression levels of OTUD7B were analyzed in several breast cancer
cell lines. OTUD7B mRNA and protein expression levels were
increased in breast cancer cell lines compared with those in
MCF-10A cells (Fig. 1B and C).
Among the breast cancer cell lines, MDA-MB-468, MDA-MB-453 and
MCF-7 cells showed relatively higher OTUD7B expression levels;
therefore, these three cell lines were selected for further
analysis.
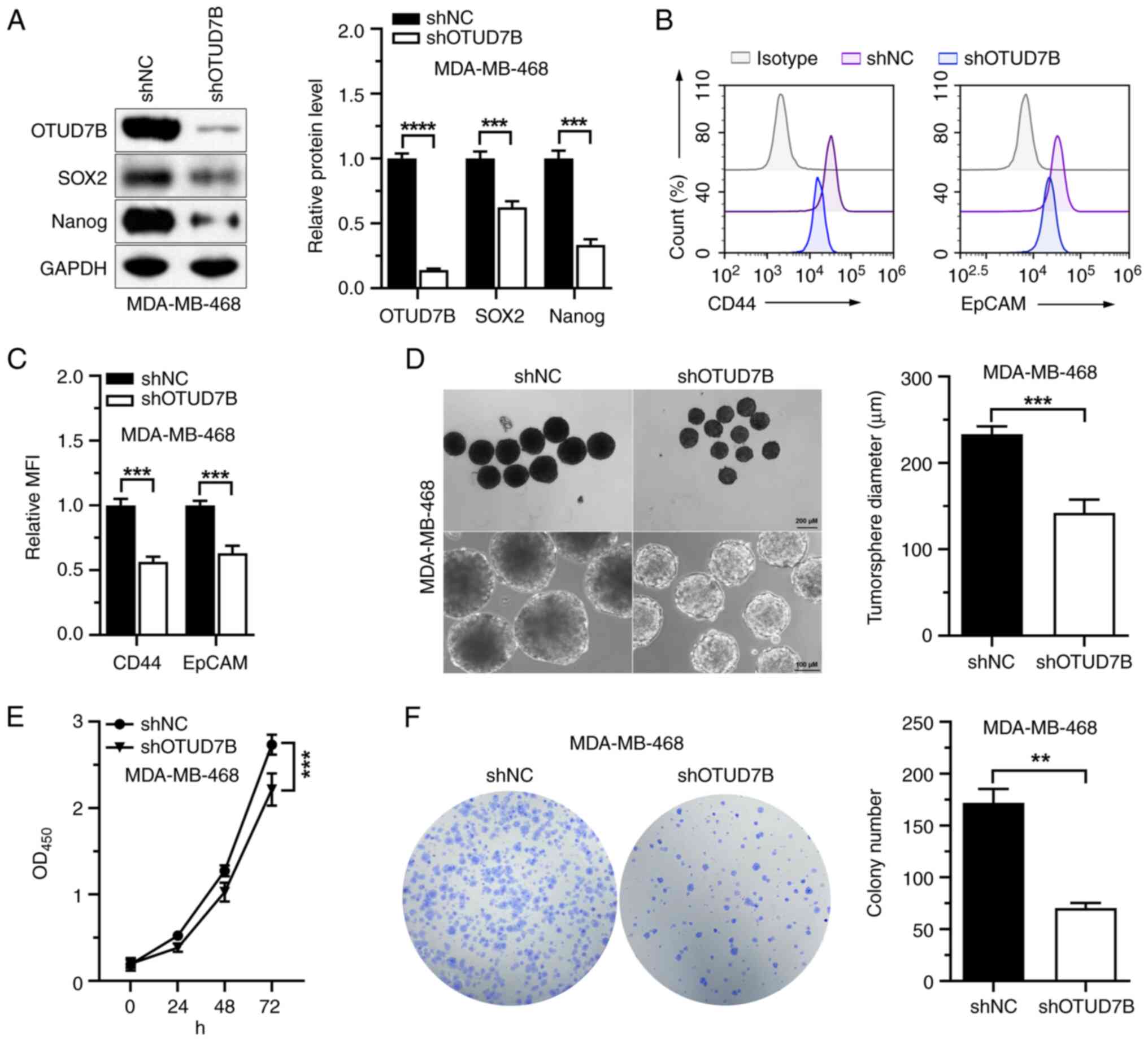
OTUD7B knockdown reduces the stemness
and proliferation of MDA-MB-468 cells
A previous study has shown that OTUD7B maintains the
stemness of neural progenitor cells by removing polyubiquitin
conjugates from SOX2 protein, increasing its stability (20). Thus, whether OTUD7B affects the
stemness of MDA-MB-468 cells was investigated. Western blotting
showed that shOTUD7B was successfully transfected into MDA-MB-468
cells and reduced the protein expression levels of OTUD7B in the
cells (Fig. 2A). The protein
expression levels of SOX2 and Nanog, two stemness-associated
proteins, were markedly suppressed by OTUD7B knockdown (Fig. 2A). Furthermore, the mean
fluorescence intensity of CD44 and EpCAM were also significantly
decreased in MDA-MB-468 cells with stable knockdown of OTUD7B
(Fig. 2B and C). Sphere formation
assays showed that the tumorsphere diameters of MDA-MB-468 cells
were significantly reduced by OTUD7B knockdown (Fig. 2D). The impact of OTUD7B on cell
proliferation was investigated and it was found that OTUD7B
knockdown inhibited the proliferation and colony formation
abilities of MDA-MB-468 cells (Fig. 2E
and F). These data indicated that OTUD7B knockdown may suppress
the stemness and proliferation of MDA-MB-468 cells.
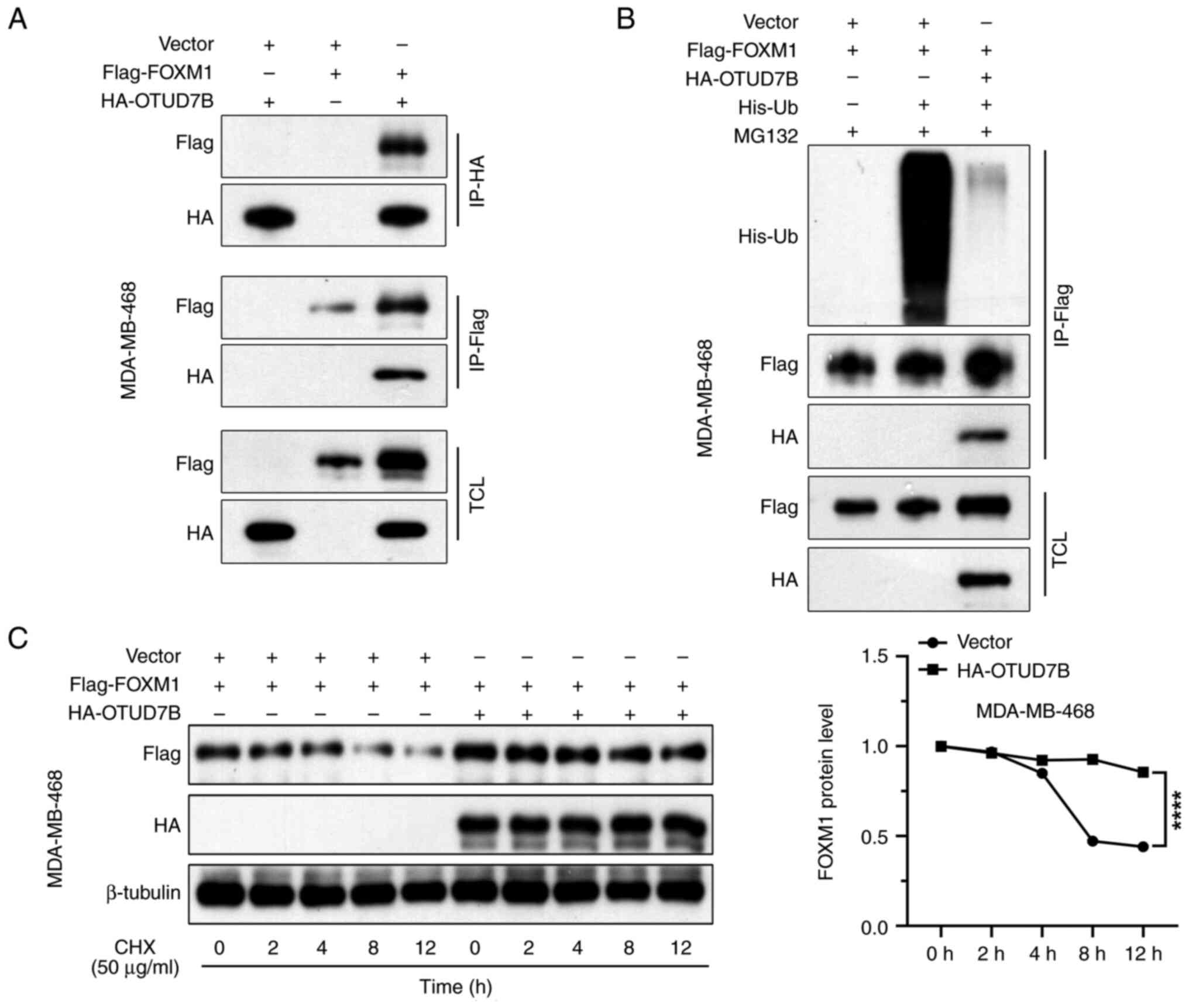
OTUD7B interacts with and stabilizes
FOXM1 via deubiquitination
FOXM1 is a transcriptional activator that is
involved in the stemness of several types of human cancer cells
(21), and its degradation rates
are accelerated in OTUD7b-depleted cells (22). Whether FOXM1 is involved in the
effects of OTUD7B on the stemness of breast cancer cells was
explored in the present study, as was whether OTUD7B interacts with
FOXM1 in breast cancer cells. HA-OTUD7B and Flag-FOXM1 plasmids
were co-transfected into breast cancer cells and the interaction
between OTUD7B and FOXM1 was detected by co-IP experiments.
FLAG-tagged FOXM1 was detected in the HA-OTUD7B immunoprecipitates
and vice versa (Fig. 3A).
OTUD7B is a deubiquitinase; therefore, whether
OTUD7B affects FOXM1 ubiquitination and stability was explored. As
shown in Fig. 3B, in the
immunoprecipitate pulled down by the Flag antibody, the His-Ub
level in the group with overexpression of HA-OTUD7B was markedly
lower than that in the group without overexpression of HA-OTUD7B,
indicating that OTUD7B reduced the ubiquitination level of
Flag-FOXM1. Protein biosynthesis was pharmacologically inhibited
using cycloheximide in MDA-MB-468 cells and FOXM1 protein levels
over 12 h with or without OTUD7B overexpression were evaluated. It
was found that Flag-FOXM1 protein levels consistently decreased
from 0 to 12 h after cycloheximide treatment and this decrease was
significantly alleviated by OTUD7B overexpression in MDA-MB-468
cells (Fig. 3C). These findings
suggested that OTUD7B stabilizes FOXM1 protein by reducing its
polyubiquitination.
FOXM1 overexpression rescues the
OTUD7B knockdown-induced inhibition of stemness and proliferation
in breast cancer cells
To examine the role of FOXM1 in the OTUD7B-mediated
effects on the proliferation and stemness of breast cancer cells,
the effects of FOXM1 overexpression on the expression of stemness
markers were analyzed. It was revealed that the protein expression
levels of SOX2, Nanog, CD44, CD24 and EpCAM were increased by FOXM1
overexpression in OTUD7B-knockdown breast cancer cells (Figs. 4 and 5). The reduced tumorsphere diameter of
OTUD7B-knockdown breast cancer cells was also reversed by FOXM1
overexpression (Fig. 6).
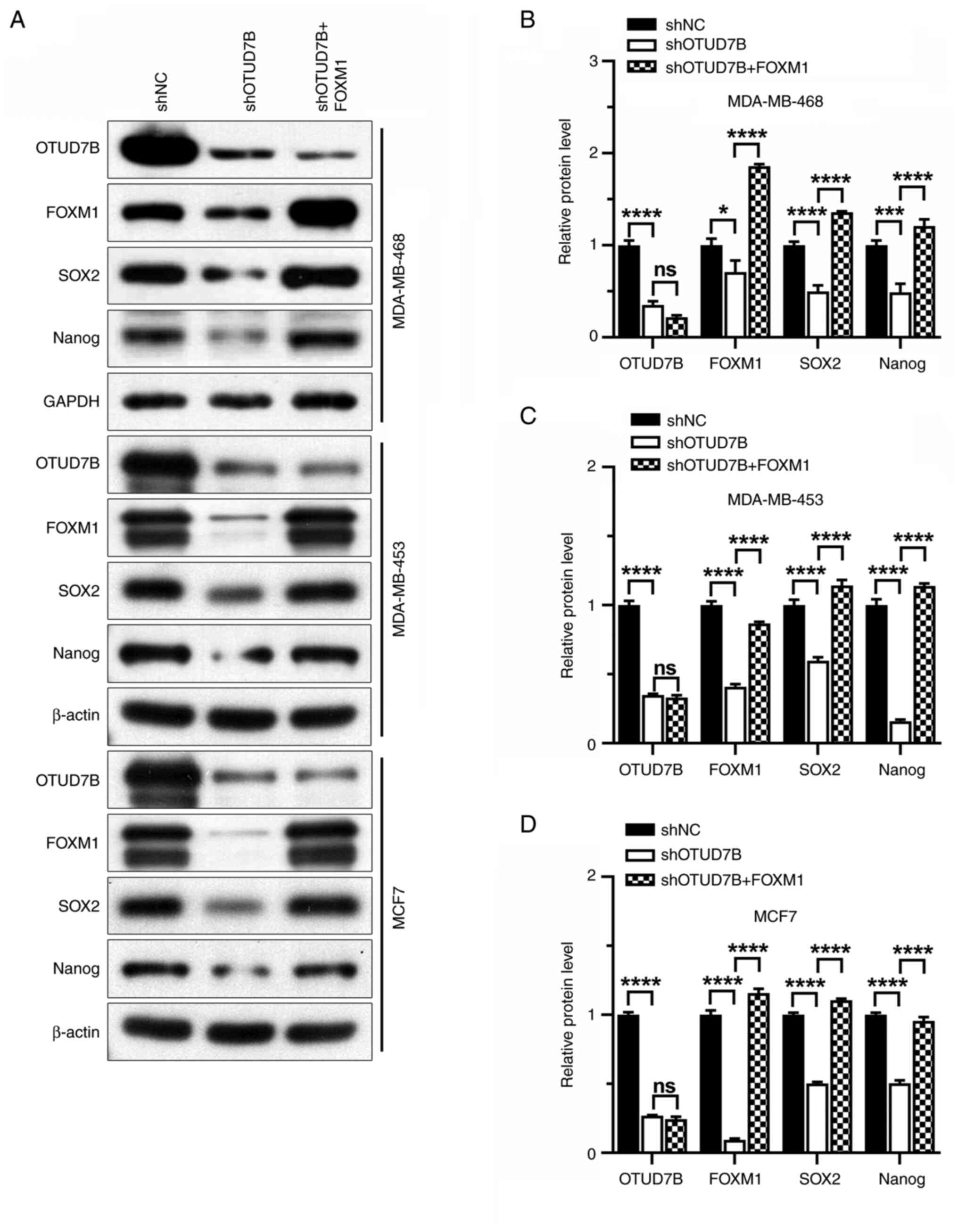 | Figure 4.Overexpression of FOXM1 increases the
protein expression levels of SOX2 and Nanog in OTUD7B-knockdown
breast cancer cells. (A) Western blotting of OTUD7B, FOXM1, SOX2
and Nanog proteins in MDA-MB-468, MDA-MB-453 and MCF7 cells.
Statistical analysis of relative protein expression levels of
OTUD7B, FOXM1, SOX2 and Nanog in (B) MDA-MB-468, (C) MDA-MB-453 and
(D) MCF7 cells. *P<0.05, ***P<0.001 and ****P<0.0001.
OTUD7B, OTU domain-containing 7B; FOXM1, forkhead box protein M1;
NC, negative control; sh, short hairpin ns, not significant. |
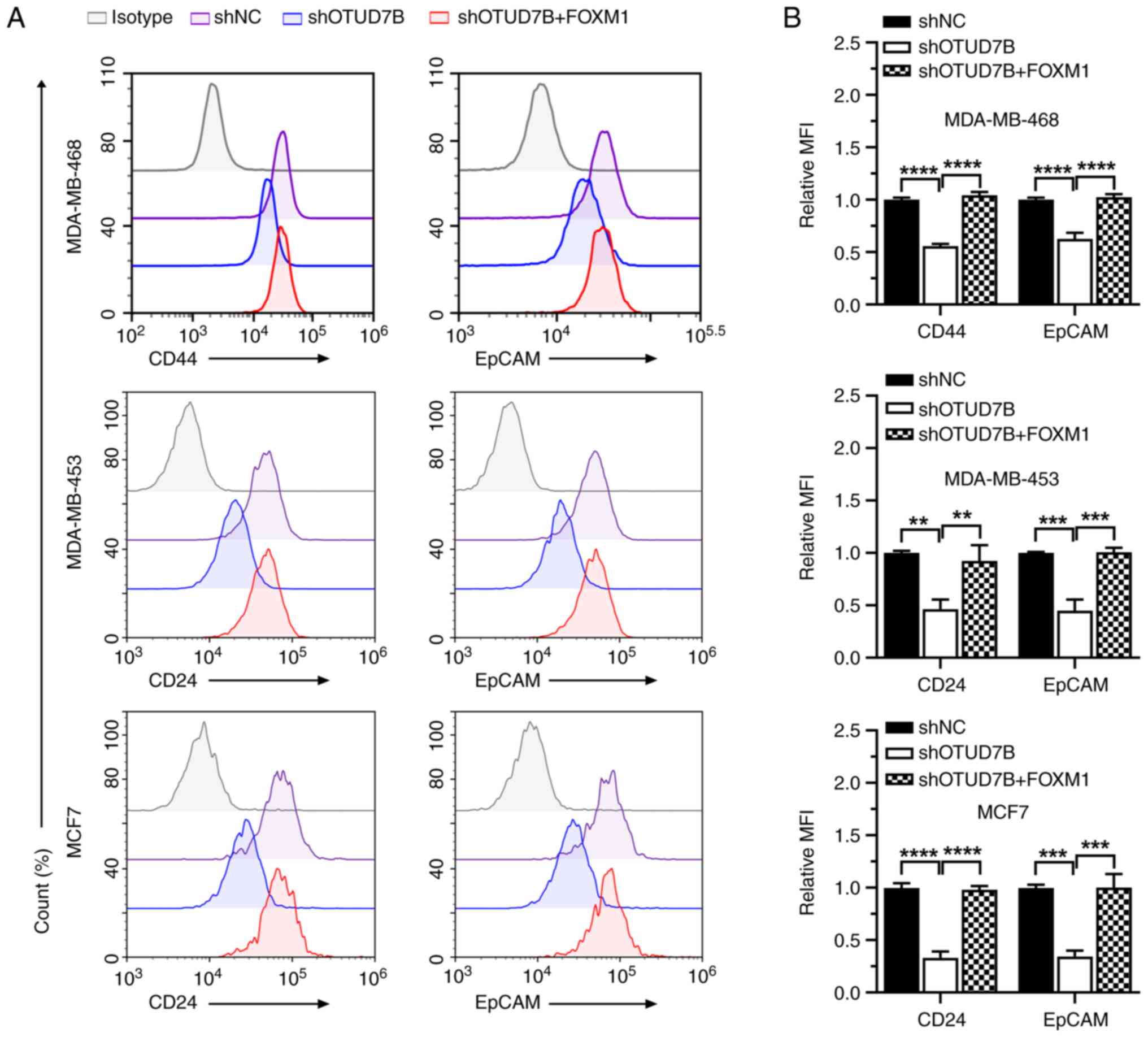 | Figure 5.Overexpression of FOXM1 increases the
expression levels of stemness markers in OTUD7B-knockdown breast
cancer cells. (A) Flow cytometric analysis of the expression levels
of CD44, CD24 and EpCAM in MDA-MB-468, MDA-MB-453 and MCF7 cells.
(B) Statistical analysis of the MFI of CD44, CD24 and EpCAM in
MDA-MB-468, MDA-MB-453 and MCF7 cells. **P<0.01, ***P<0.001
and ****P<0.0001. OTUD7B, OTU domain-containing 7B; FOXM1,
forkhead box protein M1; NC, negative control; sh, short hairpin;
MFI, mean fluorescence intensity. |
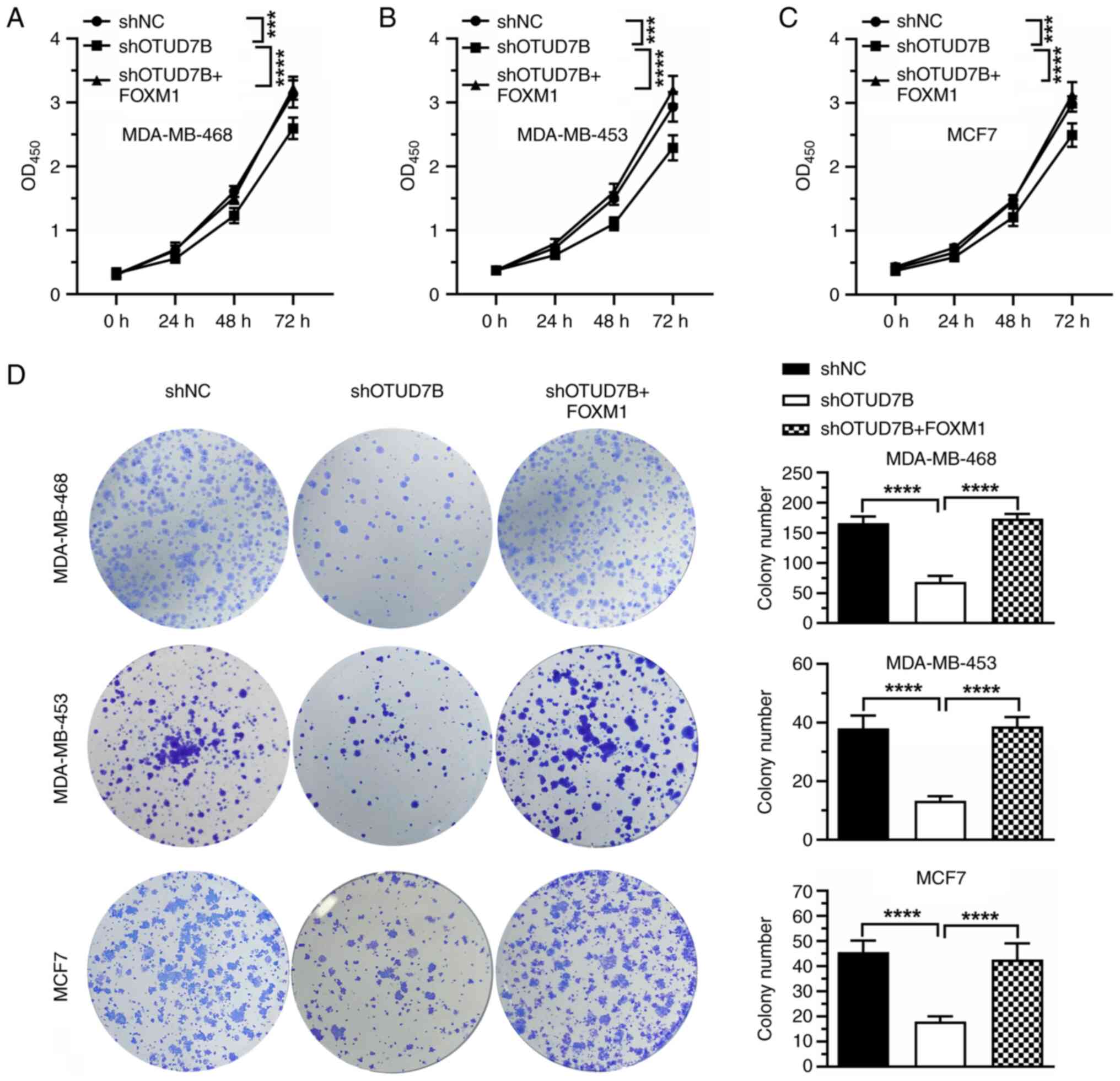
To determine the role of FOXM1 in OTUD7B
knockdown-mediated cell proliferation inhibition, FOXM1 was
overexpressed in OTUD7B-knockdown breast cancer cells and the
proliferation of the cells was analyzed by CCK8 assay. As shown in
Fig. 7A-C, OTUD7B knockdown
inhibited the proliferation of MDA-MB-468, MDA-MB-453 and MCF-7
cells, whereas the overexpression of FOXM1 blocked the inhibitory
effect induced by OTUD7B knockdown. Similar results were observed
in colony formation assays, which showed that overexpression of
FOXM1 alleviated the inhibitory effect of OTUD7B silencing on the
colony formation ability of MDA-MB-468, MDA-MB-453 and MCF-7 cells
(Fig. 7D).
Discussion
In the present study, it was revealed that OTUD7B
interacts with and deubiquitinates FOXM1 in breast cancer cells,
leading to the stabilization of FOXM1. Furthermore, knocking down
OTUD7B inhibited the proliferation and stemness of breast cancer
cells by enhancing FOXM1 degradation.
OTUD7B was first identified as a negative regulator
of NF-κB that inhibits the polyubiquitination of
receptor-interacting protein 1 signal adapter proteins (23–25).
OTUD7B dysregulation has been found to participate in the
development of numerous types of cancer, but studies on the role of
OTUD7B in oncogenesis have yielded conflicting results. In breast,
pancreatic and endometrial cancer, OTUD7B may play an oncogenic
role by facilitating the proliferation, progression and metastasis
of tumor cells (9,13,26).
However, in hepatocellular carcinoma (HCC), OTUD7B may function as
a tumor suppressor, which is evidenced by the fact that OTUD7B
knockdown accelerates the migration and invasion of HCC cells in
vitro and promotes metastasis in vivo. Furthermore,
reduced OTUD7B expression is associated with poor prognosis in
patients with HCC (10,12). The opposite results have been shown
in lung cancer. Zhang et al (11) reported that OTUD7B expression was
negatively correlated with tumor size, lymph node metastasis and
tumor-node-metastasis stage in patients with non-small cell lung
cancer. However, patients with high OTUD7B expression had favorable
prognoses. Additionally, Lin et al (27) showed that OTUD7B augmented
EGF-induced Akt signal transduction, promoting lung cancer cell
proliferation and migration. These results indicated that the
functions of OTUD7B in cancer may be cancer dependent.
In ERα-positive breast cancer, OTUD7B directly
interacts with and stabilizes ERα and knocking down OTUD7B inhibits
cell proliferation by promoting ERα degradation (15). In ERα-negative breast cancer,
silencing OTUD7B inhibits cell migration and invasion in
vitro and impairs lung metastasis of cancer cells in
vivo by increasing lysine-specific demethylase 1 (LSD1)-Ub and
targeting LSD1 for p62-mediated proteolysis (16). In the present study, it was
demonstrated that knocking down OTUD7B inhibited the proliferation
and stemness of breast cancer cells by enhancing FOXM1 degradation,
which indicates that OTUD7B may function as an oncoprotein in
breast cancer.
Cancer stem cells are characterized by increased
self-renewal and differentiation capacity, leading to metastasis,
drug resistance and cancer relapse, which ultimately result in
treatment failure (28,29). As a proliferation-specific
transcription factor, FOXM1 has been implicated in the initiation,
progression, metastasis and drug resistance of breast cancer
(22). In agreement with the
findings of the present study, several recent studies have also
reported that FOXM1 facilitates the stemness of both ER-positive
and ER-negative breast cancer cells (30–32).
Due to the important roles played by deubiquitinases
in each stage of breast cancer progression, much effort has been
put into developing inhibitors for breast cancer treatment, which
has led to promising results. A number of small molecule inhibitors
of different deubiquitinases including ZRANB1, CSN5, UCHL3, USP14,
USP1 and USP2 have been developed, and are beginning to be used to
interfere with proliferation, apoptosis, metastasis and DNA repair,
and to enhance sensitivity to immunotherapy and chemotherapy in
breast cancer (33–38). In the present study, the oncogenic
role played by OTUD7B in breast cancer was demonstrated and the
results suggested that OTUD7B is a possible target for drug design
in this malignancy. MDA-MB-468 and MDA-MB-453 cells are
triple-negative breast cancer cells (39,40)
and MCF-7 cells are double-positive breast cancer cells (41). The results of the present study
showed that OTUD7B and FOXM1 may regulate the proliferation and
stemness of these three cell lines and suggested that drugs
targeting OTUD7B or FOXM1 may have the potential to treat
triple-negative breast cancer and double-positive breast
cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was funded by the Natural Science Foundation of
Fujian (grant nos. 2020J011265 and 2021J011385), the Sanming
Science and Technology Project (grant no. 2019-S-1) and the Fujian
Provincial Health Technology Project (grant no. 2021CXA046).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ and WC were involved in conceptualization of the
present study. XF performed the statistical analysis. HW and SH
performed the experiments. JX obtained the informed consent from
the patients and collected the cancer tissues. DZ wrote the
original draft. HW, SH, XF, WC and JX revised the manuscript. DZ
supervised the study and was involved in project administration. HW
and DZ acquired the funding. HW, SH and DZ confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript and have agreed to be accountable for all aspects of the
work.
Ethics approval and consent to
participate
The study procedures were approved by The Ethics
Committees of Sanming First Hospital Affiliated to Fujian Medical
University [Sanming, China, approval no. 2020(12)] and written
informed consent was obtained from all of the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OTUD7B
|
OTU domain-containing 7B
|
|
FOXM1
|
forkhead box protein M1
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
HER2
|
human epidermal receptor 2
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
HCC
|
hepatocellular carcinoma
|
|
Co-IP
|
co-immunoprecipitation
|
|
CHX
|
cycloheximide
|
|
MFI
|
mean fluorescence intensity
|
|
ns
|
not significant
|
References
|
1
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harbeck N, Penault-Llorca F, Cortes J,
Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J and Cardoso F:
Breast cancer. Nat Rev Dis Primers. 5:662019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barzaman K, Karami J, Zarei Z,
Hosseinzadeh A, Kazemi MH, Moradi-Kalbolandi S, Safari E and
Farahmand L: Breast cancer: Biology, biomarkers, and treatments.
Int Immunopharmacol. 84:1065352020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mevissen TET and Komander D: Mechanisms of
deubiquitinase specificity and regulation. Annu Rev Biochem.
86:159–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lai KP, Chen J and Tse WKF: Role of
deubiquitinases in human cancers: Potential targeted therapy. Int J
Mol Sci. 21:25482020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li S, Zhang H and Wei X: Roles and
Mechanisms of Deubiquitinases (DUBs) in breast cancer progression
and targeted drug discovery. Life (Basel). 11:9652021.PubMed/NCBI
|
|
9
|
Pareja F, Ferraro DA, Rubin C,
Cohen-Dvashi H, Zhang F, Aulmann S, Ben-Chetrit N, Pines G, Navon
R, Crosetto N, et al: Deubiquitination of EGFR by Cezanne-1
contributes to cancer progression. Oncogene. 31:4599–4608. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang JH, Wei W, Guo ZX, Shi M and Guo RP:
Decreased Cezanne expression is associated with the progression and
poor prognosis in hepatocellular carcinoma. J Transl Med.
13:412015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang B, Wang H, Yang L, Zhang Y, Wang P,
Huang G, Zheng J, Ren H and Qin S: OTUD7B and NIK expression in
non-small cell lung cancer: Association with clinicopathological
features and prognostic implications. Pathol Res Pract.
212:893–898. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang JH, Zhong XP, Zhang YF, Wu XL, Li SH,
Jian PE, Ling YH, Shi M, Chen MS, Wei W, et al: Cezanne predicts
progression and adjuvant TACE response in hepatocellular carcinoma.
Cell Death Dis. 8:e30432017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lei S, He Z, Chen T, Guo X, Zeng Z, Shen Y
and Jiang J: Long noncoding RNA 00976 promotes pancreatic cancer
progression through OTUD7B by sponging miR-137 involving EGFR/MAPK
pathway. J Exp Clin Cancer Res. 38:4702019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu S, Liu Y, Gui A, Xia Z, Liu W, Gu JJ,
Zuo J, Yang L and Zhang Q: Deubiquitinase OTUD7B is a potential
prognostic biomarker in diffuse large B-cell lymphoma. J Cancer.
13:998–1004. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang J, Wu Z, Tian Z, Chen W and Wu G:
OTUD7B stabilizes estrogen receptor α and promotes breast cancer
cell proliferation. Cell Death Dis. 12:5342021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gong Z, Li A, Ding J, Li Q, Zhang L, Li Y,
Meng Z, Chen F, Huang J, Zhou D, et al: OTUD7B Deubiquitinates LSD1
to Govern Its Binding Partner Specificity, Homeostasis, and Breast
Cancer Metastasis. Adv Sci (Weinh). 8:e20045042021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chiu HW, Lin HY, Tseng IJ and Lin YF:
OTUD7B upregulation predicts a poor response to paclitaxel in
patients with triple-negative breast cancer. Oncotarget. 9:553–565.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen H, Yu Y, Yang M, Huang H, Ma S, Hu J,
Xi Z, Guo H, Yao G, Yang L, et al: YTHDF1 promotes breast cancer
progression by facilitating FOXM1 translation in an m6A-dependent
manner. Cell Biosci. 12:192022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cui CP, Zhang Y, Wang C, Yuan F, Li H, Yao
Y, Chen Y, Li C, Wei W, Liu CH, et al: Dynamic ubiquitylation of
Sox2 regulates proteostasis and governs neural progenitor cell
differentiation. Nat Commun. 9:46482018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sher G, Masoodi T, Patil K, Akhtar S,
Kuttikrishnan S, Ahmad A and Uddin S: Dysregulated FOXM1 signaling
in the regulation of cancer stem cells. Semin Cancer Biol.
86:107–121. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bonacci T, Suzuki A, Grant GD, Stanley N,
Cook JG, Brown NG and Emanuele MJ: Cezanne/OTUD7B is a cell
cycle-regulated deubiquitinase that antagonizes the degradation of
APC/C substrates. EMBO J. 37:e987012018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Evans PC, Taylor ER, Coadwell J, Heyninck
K, Beyaert R and Kilshaw PJ: Isolation and characterization of two
novel A20-like proteins. Biochem J. 357:617–623. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Evans PC, Smith TS, Lai MJ, Williams MG,
Burke DF, Heyninck K, Kreike MM, Beyaert R, Blundell TL and Kilshaw
PJ: A novel type of deubiquitinating enzyme. J Biol Chem.
278:23180–23186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Enesa K, Zakkar M, Chaudhury H, Luong le
A, Rawlinson L, Mason JC, Haskard DO, Dean JL and Evans PC:
NF-kappaB suppression by the deubiquitinating enzyme Cezanne: A
novel negative feedback loop in pro-inflammatory signaling. J Biol
Chem. 283:7036–7045. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu QZ, Liu HY, Zhao XY, Qiu LJ, Zhou TT,
Wang XY, Chen HP and Xiao ZQ: DJ-1 activates the noncanonical NF-κB
pathway via interaction with Cezanne to inhibit the apoptosis and
promote the proliferation of Ishikawa cells. Mol Biol Rep.
48:6075–6083. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin DD, Shen Y, Qiao S, Liu WW, Zheng L,
Wang YN, Cui N, Wang YF, Zhao S and Shi JH: Upregulation of OTUD7B
(Cezanne) promotes tumor progression via AKT/VEGF pathway in lung
squamous carcinoma and adenocarcinoma. Front Oncol. 9:8622019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prasad S, Ramachandran S, Gupta N, Kaushik
I and Srivastava SK: Cancer cells stemness: A doorstep to targeted
therapy. Biochim Biophys Acta Mol Basis Dis. 1866:1654242020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ibragimova M, Tsyganov M and Litviakov N:
Tumour stem cells in breast cancer. Int J Mol Sci. 23:50582022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun HL, Men JR, Liu HY, Liu MY and Zhang
HS: FOXM1 facilitates breast cancer cell stemness and migration in
YAP1-dependent manner. Arch Biochem Biophys. 685:1083492020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Modi A, Purohit P, Roy D, Vishnoi JR,
Pareek P, Elhence P, Singh P, Sharma S, Sharma P and Misra S: FOXM1
mediates GDF-15 dependent stemness and intrinsic drug resistance in
breast cancer. Mol Biol Rep. 49:2877–2888. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsao AN, Chuang YS, Lin YC, Su Y and Chao
TC: Dinaciclib inhibits the stemness of two subtypes of human
breast cancer cells by targeting the FoxM1 and Hedgehog signaling
pathway. Oncol Rep. 47:1052022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang P, Xiao Z, Wang S, Zhang M, Wei Y,
Hang Q, Kim J, Yao F, Rodriguez-Aguayo C, Ton BN, et al: ZRANB1 Is
an EZH2 deubiquitinase and a potential therapeutic target in breast
cancer. Cell Rep. 23:823–837. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiao H, Claret FX and Shen Q: The novel
Jab1 inhibitor CSN5i-3 suppresses cell proliferation and induces
apoptosis in human breast cancer cells. Neoplasma. 66:481–486.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song Z, Tu X, Zhou Q, Huang J, Chen Y, Liu
J, Lee S, Kim W, Nowsheen S, Luo K, et al: A novel UCHL3 inhibitor,
perifosine, enhances PARP inhibitor cytotoxicity through inhibition
of homologous recombination-mediated DNA double strand break
repair. Cell Death Dis. 10:3982019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liao Y, Xia X, Liu N, Cai J, Guo Z, Li Y,
Jiang L, Dou QP, Tang D, Huang H, et al: Growth arrest and
apoptosis induction in androgen receptor-positive human breast
cancer cells by inhibition of USP14-mediated androgen receptor
deubiquitination. Oncogene. 37:1896–1910. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma A, Tang M, Zhang L, Wang B, Yang Z, Liu
Y, Xu G, Wu L, Jing T, Xu X, et al: USP1 inhibition destabilizes
KPNA2 and suppresses breast cancer metastasis. Oncogene.
38:2405–2419. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He J, Lee HJ, Saha S, Ruan D, Guo H and
Chan CH: Inhibition of USP2 eliminates cancer stem cells and
enhances TNBC responsiveness to chemotherapy. Cell Death Dis.
10:2852019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ahram M, Abdullah MS, Mustafa SA, Alsafadi
DB and Battah AH: Androgen downregulates desmocollin-2 in
association with induction of mesenchymal transition of breast
MDA-MB-453 cancer cells. Cytoskeleton (Hoboken NJ). 78:391–399.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liubomirski Y, Lerrer S, Meshel T,
Rubinstein-Achiasaf L, Morein D, Wiemann S, Körner C and Ben-Baruch
A: Tumor-stroma-inflammation networks promote pro-metastatic
chemokines and aggressiveness characteristics in triple-negative
breast cancer. Front Immunol. 10:7572019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pruteanu LL, Braicu C, Módos D, Jurj MA,
Raduly LZ, Zănoagă O, Magdo L, Cojocneanu R, Paşca S, Moldovan C,
et al: Targeting cell death mechanism specifically in triple
negative breast cancer cell lines. Int J Mol Sci. 23:47842022.
View Article : Google Scholar : PubMed/NCBI
|