Introduction
Hepatocellular carcinoma (HCC) is the second leading
cause of cancer-related mortality worldwide and the most common
pathological subtype of liver cancer (1,2). The
prognosis of HCC remains unsatisfactory, with a 5-year survival
rate of ~18% (3). A previous study
suggested that transarterial chemoembolization (TACE) can control
intermediate-stage HCC and improve the 5-year survival rate of
patients (4). Therefore, TACE has
been recommended by several authorities as a first-line therapy for
treating patients with HCC (5).
However, due to the high rate of incomplete embolization, TACE
remains inadequate in controlling HCC growth as a palliative
treatment approach (6).
Several studies on tumor metabolism have highlighted
the association between energy availability and cell proliferation,
and the critical role of metabolic reprogramming in neoplastic
cells (7). Metabolism is abnormal
in solid tumors and is characterized by a higher glycolytic rate
and glucose absorption, thus promoting the production of high
concentrations of lactate; this process is widely known as the
Warburg effect or aerobic glycolysis (8). Unlike normal cells, neoplastic cells
display an enhanced metabolism to maintain a reduction-oxidation
balance and provide enough energy for cancer cell proliferation and
growth (9). The aforementioned
features have been reported in several types of solid tumors,
including HCC, colon cancer and breast cancer (10,11).
Lactate dehydrogenase A (LDHA) is a key enzyme in
the aerobic glycolytic pathway, as it converts lactate to pyruvate
(12). During the Warburg effect,
ATP generation is more rapid in cancer cells compared with that in
normal cells (13). In addition, a
study reported that cancer cells can secrete lactate into the
extracellular matrix, thus enhancing cell motility, invasion and
metastasis via upregulating metalloproteases (14). Moreover, lactate has been reported
to decrease the activation and differentiation of dendritic cells
(15), and inhibit tumor
surveillance via T and natural killer cells (16). It has also been reported that
hypoxia-inducible factor-1α (HIF-1α), as a key factor in hypoxic
adaptation, can activate the expression of glycolytic enzymes,
including LDHA (17). Another study
demonstrated that TACE can upregulate HIF-1α due to the high rate
of incomplete embolization, thus further increasing the expression
of LDHA in residual tumors (18).
Furthermore, LDHA overexpression has been reported to be associated
with tumor development, particularly in disseminated types of
cancer, including hypoxic carcinoma and metastatic cancer cells,
thus leading to a poor prognosis (19). Additionally, a previous study
indicated that LDHA inhibition can suppress tumor growth (20). Therefore, targeting metabolic
reprogramming to directly inhibit glycolysis and ATP generation
could be a feasible protocol for patients with HCC treated with
TACE.
Sodium oxamate (Ox) is an analog of pyruvate, which
acts as a competitive inhibitor. Emerging evidence has demonstrated
that Ox can inhibit LDHA and the Warburg effect (21–23).
Therefore, the current study assessed the therapeutic efficacy of a
combination of Ox and TACE in a rabbit VX2 liver tumor model via
the evaluation of tumor growth. To assess the mechanism underlying
the antitumor effect of Ox + TACE therapy, immune responses, cell
metastasis and angiogenesis were evaluated in the tumor
microenvironment by immunohistochemical analysis. In addition, to
assess the biocompatibility of the combination therapy, changes in
hepatorenal function were measured.
Materials and methods
Materials
Ox (cat. no. sc-215880) was purchased from Santa
Cruz Biotechnology, Inc., whilst iohexol (Omnipaque™),
ethiodized oil (Lipiodol®) and polyvinyl alcohol (PVA)
foam embolization particles (Ivalon®; 180–300 mm) were
purchased from Guerbet Laboratories Ltd., B. Braun Melsungen AG and
Cook Medical, respectively. All reagents were of chemical pure or
analytical grade, and were used as purchased without any further
purification.
New Zealand white rabbits (n=48; weight, 2.5-3 kg;
24 male and 24 female rabbits; age, 10–12 weeks) were obtained from
Liaoning Changsheng Biotechnology Co., Ltd. All animal experiments
were carried out according to the Chinese National Animal Law on
the use of laboratory animals. All animal welfare considerations
were taken, including efforts to minimize suffering and distress,
use of analgesics or anaesthetics and special housing conditions
[20°C; ventilation rate, 2–3 m3/h; 14/10-h light/dark
cycle; humidity, 60–65%; pellet feed, 150 g/day, twice a day;
automatic water supply system (free access)]. All rabbits were
euthanized by intraperitoneal injection of 100 mg/kg pentobarbital
sodium. At 20 min after the injection, death was confirmed by
checking respiration, heartbeat, pupil and nerve reflex. All
procedures were approved by the Animal Care and Use Committee of
Huazhong University of Science and Technology (Wuhan, China; 2023;
approval no. 3380).
Establishment of the rabbit VX2 liver
tumor model
All interventional procedures were performed by a
radiologist with ≥15 years of experience. The rabbit VX2 liver
tumor model was established as previously described (24). A 12-week-old tumor-bearing male
rabbit was purchased from the Animal Center of Huazhong University
of Science and Technology; the VX2 tumor was collected at 14 days
since xenograft implantation, and the tumor was collected after the
rabbit was sacrificed by intraperitoneal injection of 100 mg/kg
pentobarbital sodium. At 20 min after the injection, death was
confirmed by checking respiration, heartbeat, pupil and nerve
reflex. Then, the VX2 tumor was sliced into 1.0 mm3
pieces and, under gas anesthesia (3–4% isoflurane used for
induction and 2% for maintenance), a piece of the VX2 tissue was
implanted into the left liver lobe of the rabbits (24). To avoid infection, the wounds were
topically treated with 2.0×104 IU gentamycin, while
rabbits were also administered an intramuscular injection of
1.0×104 IU ampicillin (25). No adverse effects were observed
after injection of ampicillin.
A total of 24 VX2-tumor-bearing rabbits were
randomly divided into the following four groups (n=6 rabbits/group;
treatment duration, 14 days): Control group, normal saline was
injected into the tumor-feeding arteries (control group); TACE
group, TACE was performed via delivering a mixture of 0.3 ml
Lipiodol mixed with 6 mg doxorubicin into the tumor-feeding
arteries. The arteries were then occluded with PVA foam
embolization particles; Ox group Ox, the tumor-feeding arteries
were injected with an intra-arterial bolus of Ox saline (5 mg/kg; 5
min); and TACE + Ox group, the tumor-feeding arteries were injected
with an intra-arterial bolus of Ox saline (5 mg/kg; 5 min),
followed by TACE. When the VX2 tumor volume reached 1.61
cm3, TACE was performed in the TACE and TACE + Ox groups
using a 2.7F microcatheter (Terumo Corporation), guided by digital
subtraction angiography (Siemens Healthineers). The tumor-feeding
arteries were continuously monitored during TACE until the tumor
blood supply was completely occluded (no delivery of contrast agent
was visible in tumoral and peritumoral vessels), with the normal
hepatic artery unobstructed; the contrast agent used for
angiography was Iohexol.
Tumor growth and rabbit survival
follow-up
A 320-row spiral computed tomography (CT; Siemens
Healthineers) was used for plain scan and contrast-enhanced CT. The
contrast-enhanced CT was performed to assess the therapeutic
effects of different treatments at 14 days. CT was repeated at days
3, 7, 10 and 14 to record the metastasis in the four groups. Tumor
volume was calculated using CT (80 kV; 100 mA; 1-mm slice
thickness; 1.1 pitch; 200×200-mm2 field of view) with
the following formula: V=a × b × c × π/6, where a, b and c indicate
the anteroposterior, transverse and axial diameters, respectively.
In addition, the survival of all treated rabbits (an additional 6
rabbits/group) was recorded. Animal health and behaviour were
monitored every day and the rabbits were euthanized when the tumor
weight reached ≤10% of their weight or when pain could not be
effectively controlled during observation. The rabbits were
considered to be in pain when they were abnormally vocal, or when
they licked, bit, scratched or shook the painful area. The time of
euthanasia or death was recorded, which was used to indicate
mortality.
Sample collection
Blood samples were collected from the auricular vein
of the rabbits under anesthesia (3–4% isoflurane used for induction
and 2% for maintenance) in each treatment group (2 ml/collection;
once daily on days 0, 1, 3, 7 and 14). The 14-day blood samples
were collected before euthanasia. Subsequently, the samples were
centrifuged (4°C, 1,000 × g, 10 min) to obtain serum, which was
then stored at −80°C until use. The tumor and peritumoral tissues
from each group were fixed in 4% formalin overnight at 25°C and
paraffin-embedded at 52–54°C. The paraffin-embedded tissues were
then cut into 5.0-µm slices for histological analysis.
Biochemical analyses
To measure the levels of biochemical markers in
serum in vivo, the blood samples from all four groups were
collected and centrifuged at 1,000 × g for 10 min at 4°C to collect
serum. The levels of the biochemical markers alanine
aminotransferase (ALT), aspartate aminotransferase (AST), blood
urea nitrogen (BUN) and creatinine (CRE) in serum were measured
using a biochemical auto-analyzer (Model DXC8000; Beckman Coulter,
Inc.).
Histological analysis
For rehydration, tissue sections were immersed in a
descending ethanol series. Subsequently, antigen retrieval in 1 mM
EDTA (pH 8.0) was performed at 98°C for 15 min followed by washing
twice in PBS and distilled water. For permeabilization, the
Permeabilization Wash Buffer (cat. no. 40403ES64; Shanghai Yeasen
Biotechnology Co., Ltd.) was used, after which, tissues were
blocked in 10% normal goat serum (cat. no. C0265; Beyotime
Institute of Biotechnology) for 20 min at 25°C.
H2O2 (3%, 50 µl) was used to block endogenous
peroxidase. The tissue sections were then incubated with
anti-matrix metalloproteinase-9 (MMP-9; 1:1,000; cat. no.
GB12132-100; Wuhan Servicebio Technology Co., Ltd.), anti-CD3
(1:100; cat. no. GB12014-100; Wuhan Servicebio Technology Co.,
Ltd.), anti-CD8 (1:100; cat. no. GB12068-100; Wuhan Servicebio
Technology Co., Ltd.) and anti-vascular endothelial growth factor
(VEGF; 1:150; cat. no. AB1876-I; MilliporeSigma) primary antibodies
at 4°C overnight. Subsequently, the sections were incubated with an
anti-mouse HRP (IgG H&L) secondary antibody (cat. no. ab97040;
Abcam; 1:1,000) at 37°C for 1 h. DAB was used for chromogen
detection. Tumor tissues also underwent hematoxylin and eosing
(H&E) staining, as follows: Sections were stained with
hematoxylin at 25°C for 15 min and eosin at 25°C for 5 min. In
addition, the tumor necrosis ratio (TNR) was calculated using the
following formula: TNR=N/(N + T), where N indicates the necrotic
areas of the tumor and T indicates the living tumor areas of the
tumor (detected by H&E assay). A total of six sections per
tumor tissue were observed and the integrated optical density (IOD)
sum in five randomly selected fields (magnification, ×400; light
microscope) was calculated using ImageJ version 22 (National
Institutes of Health). The mean values from the five randomly
selected fields were calculated and used for statistical
analysis.
Immunofluorescence assay
Cell apoptosis was assessed using a Terminal
Deoxynucleotidyl Transferase mediated dUTP Nick-End Labeling
(TUNEL) assay (cat. no. 12156792910; MilliporeSigma) according to
the manufacturer's instructions. In addition, a Ki67 assay
(dilution, 1:200; Wuhan Servicebio Technology Co., Ltd.) was used
to evaluate tumor cell proliferation according to the
manufacturer's instructions. Tumor cell nuclei were stained with
DAPI at 25°C for 20 min. In both fluorescence assays, images were
captured by excitation at 488 nm and emission at 560 nm (BX53;
Olympus Corporation). A total of six sections were assessed per
tumor sample. The IOD sum of the images was calculated using
ImageJ. The mean results from five randomly selected fields were
calculated and statistical analysis was performed.
Statistical analysis
All statistical analyses were performed using SPSS
v24.0 (IBM Corp.) and GraphPad Prism (version 8.0; Dotmatics)
software. All data are expressed as the mean ± standard deviation.
Kaplan-Meier analysis was used to plot the overall survival curves,
and the significance was calculated using the log-rank test.
Comparisons among multiple groups were performed using one-way
ANOVA, followed by Tukey's Honest Significant Difference test to
assess the differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Successful operations and
establishment of the rabbit VX2 liver tumor model
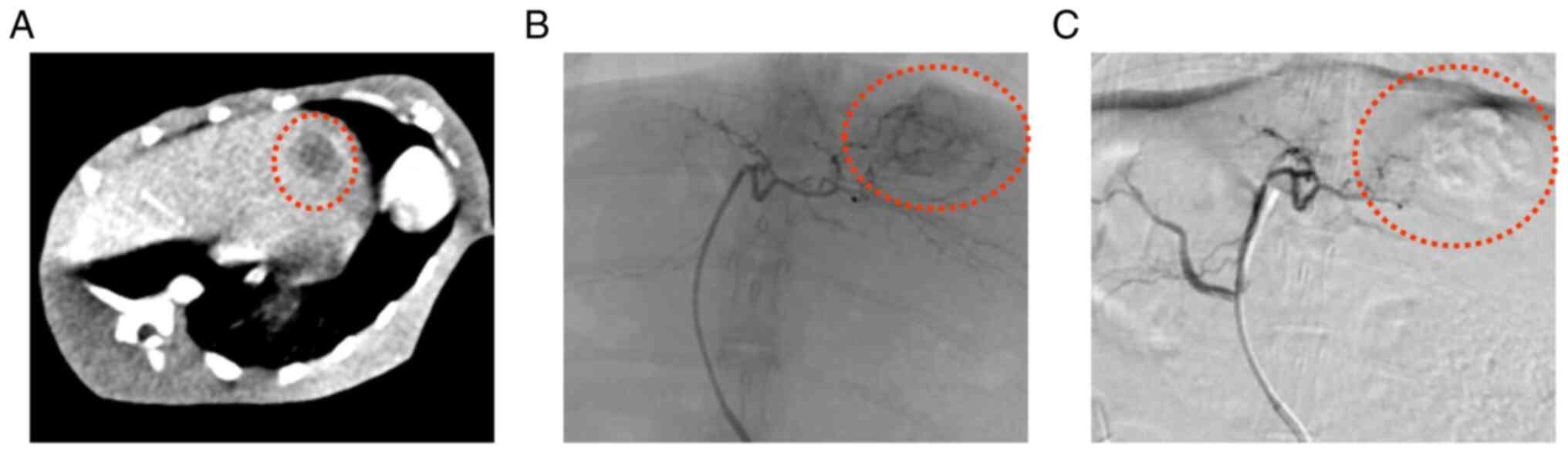
The liver tumor masses were clearly visible on CT
images (Fig. 1A). Prior to
treatment, tumor volumes were 1,628.5±20.3, 1,601.8±22.3,
1,608.9±29.8 and 1,630.2±19.2 mm3 in the control, TACE,
Ox and TACE + Ox groups, respectively. No statistically significant
difference in tumor volume was demonstrated among the different
groups. The TACE procedure is shown in Fig. 1B and C. No rabbits were euthanized
or found dead before the end of the experiment and all tumor
tissues and serum samples were successfully collected.
Tumor response and survival of treated
rabbits
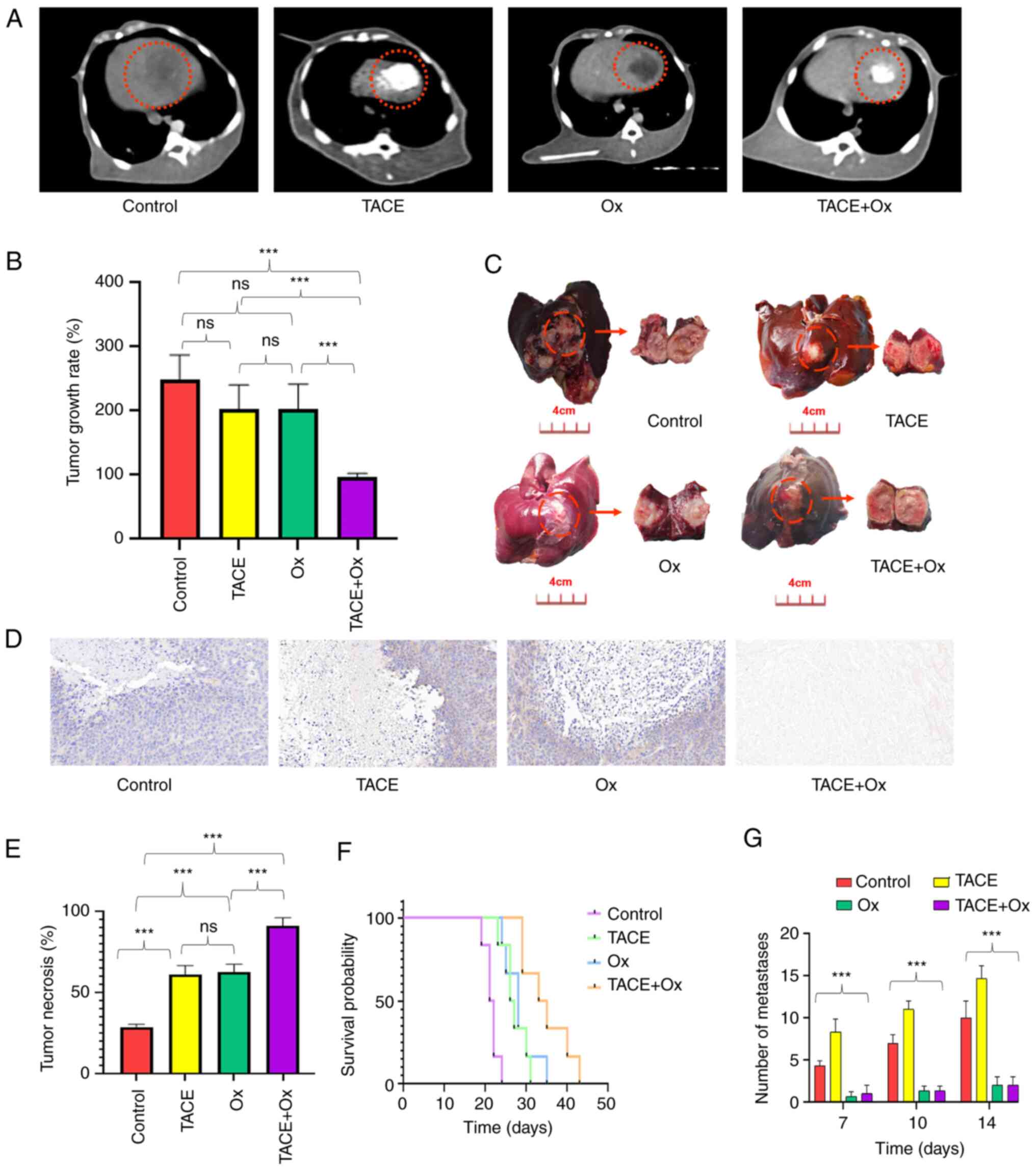
A representative CT image from each treatment group
at 14 days after the operation is shown in Fig. 2A. Tumor growth rate was notably
reduced in the TACE (202.7±46.23%) and Ox (203.0±54.47%) groups,
and significantly reduced in the TACE + Ox (96.8±20.31%) group
compared with that of the control group (248.2±45.50%; Fig. 2B). Rabbits in the TACE + Ox group
demonstrated a significantly improved tumor response compared with
those in the TACE (P<0.001) and Ox (P<0.001) groups. No
statistically significant difference was observed between the TACE
and Ox groups (P>0.999; Fig.
2B). Representative tumor tissues of each group after treatment
for 14 days are presented in Fig.
2C. The majority (94.7%) of tumor tissues in the TACE + Ox
group were necrotic. Furthermore, compared with in the control
group (28.12±8.91%), TNR was significantly enhanced in the TACE
(60.25±12.25%; P<0.001), Ox (62.23±9.32%; P<0.001) and TACE +
Ox (90.62±4.96%; P<0.001) groups. Notably, the combination
demonstrated a significantly better TNR than that of the Ox group;
the combination group demonstrated the greatest TNR (Fig. 2D and E). Subsequently, to assess the
survival benefits of TACE, Ox and TACE + Ox therapy in VX2-bearing
rabbits, the ability of the different treatments to promote
survival was compared. Significant survival benefits were observed
in rabbits in the combination group compared with those in the
control (P<0.001), TACE (P<0.001) and Ox (P<0.001) groups
(Fig. 2F). The number of metastases
during follow-up is demonstrated in Fig. 2G. The metastases occurred at day 7
and gradually increased in the control and TACE groups. Conversely,
Ox exhibited an inhibition of metastasis. The volume of
metastasized tumors was 263.7±44.4, 342.1±32.2, 52.1±23.8 and
51.6±18.9 mm3 in the control, TACE, Ox and TACE + Ox
groups after 14 days of treatments (P<0.001), repectively.
Changes in tumor microenvironment
after treatment
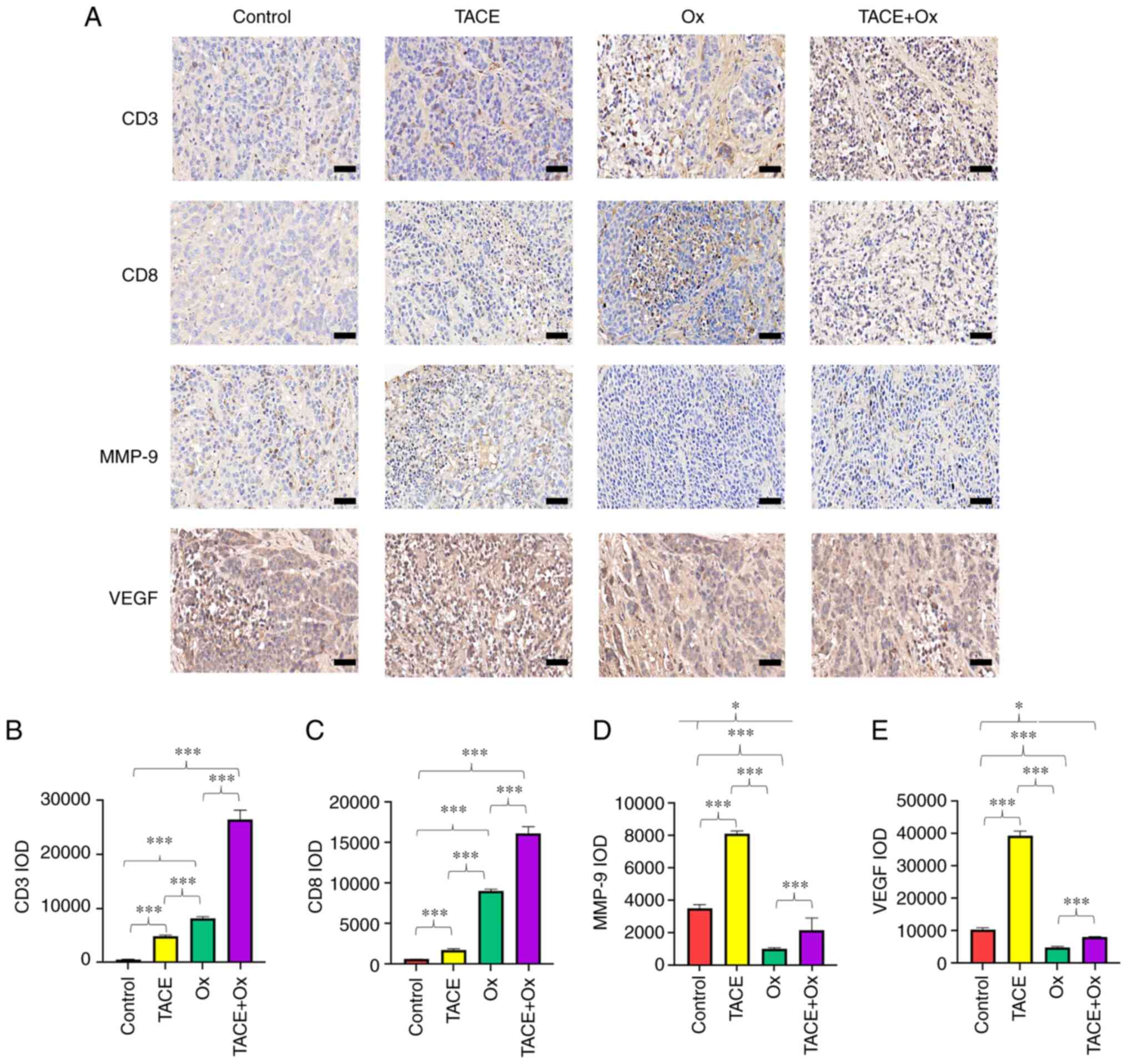
Semi-quantitative analysis of IHC staining revealed
that the administration of Ox significantly enhanced the
infiltration of CD3+ and CD8+ cells in tumor
tissues compared with that in the control group. Although TACE
alone also significantly increased the infiltration of the
aforementioned cells compared with that in the control group, its
capacity was notably attenuated compared with the Ox and TACE + Ox
groups (Fig. 3A-C). In addition,
although the application of TACE significantaly upregulated MMP-9
in the tumor tissues, treatment with Ox significantly decreased the
expression of MMP-9 in the combination group compared with that in
the control group (Fig. 3A and D).
Moreover, the results indicated that Ox significantly decreased the
expression of VEGF compared with that in the control group, and
VEGF expression was significantly reduced in the TACE + Ox group
compared with that in the control group (Fig. 3A and E). Furthermore, the
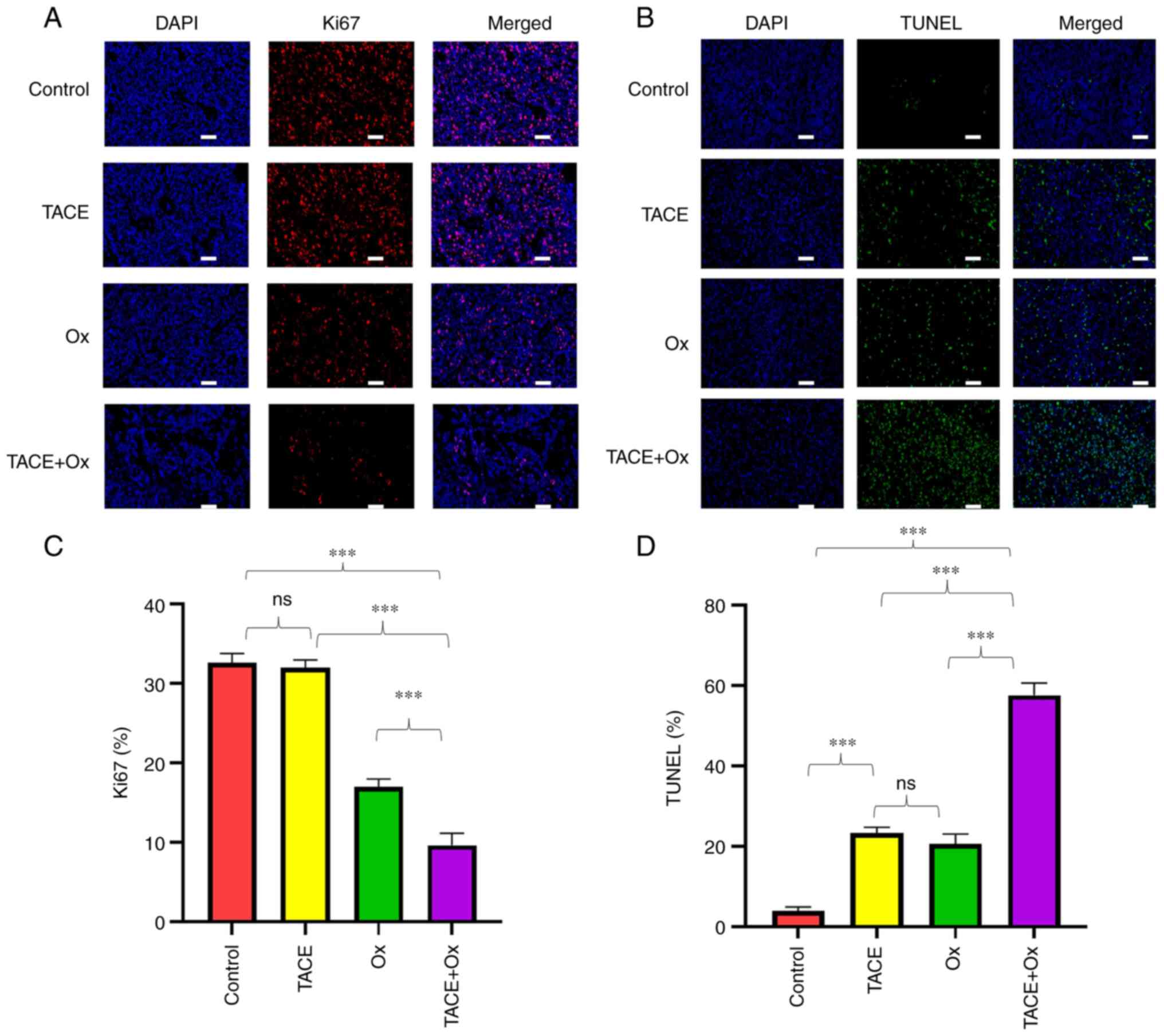
immunofluorescence staining results demonstrated that the protein
expression of Ki67 was significantly decreased in tumors in the
TACE + Ox group compared with that in the control, TACE and Ox
groups (Fig. 4A and C). Finally,
the number of TUNEL+ cells was markedly increased in the
TACE + Ox group compared with that in the other groups (Fig. 4A and D).
Changes of hepatorenal function
The changes in hepatorenal function are demonstrated
in Fig. 5. Except from in the
control group, rabbits in the TACE and combination groups had
transient liver function impairment; the levels of AST and ALT were
significantly higher than control on days 1, 3 and 7, and they then
returned to normal levels at day 14 (Fig. 5A and B). However, kidney function
was only slightly affected (Fig. 5C and
D). The levels of ALT, AST, BUN and CRE reached their peaks on
day 1 after treatment and gradually recovered (Fig. 5). These findings indicated that
combination therapy may cause a transient impairment in hepatorenal
function.
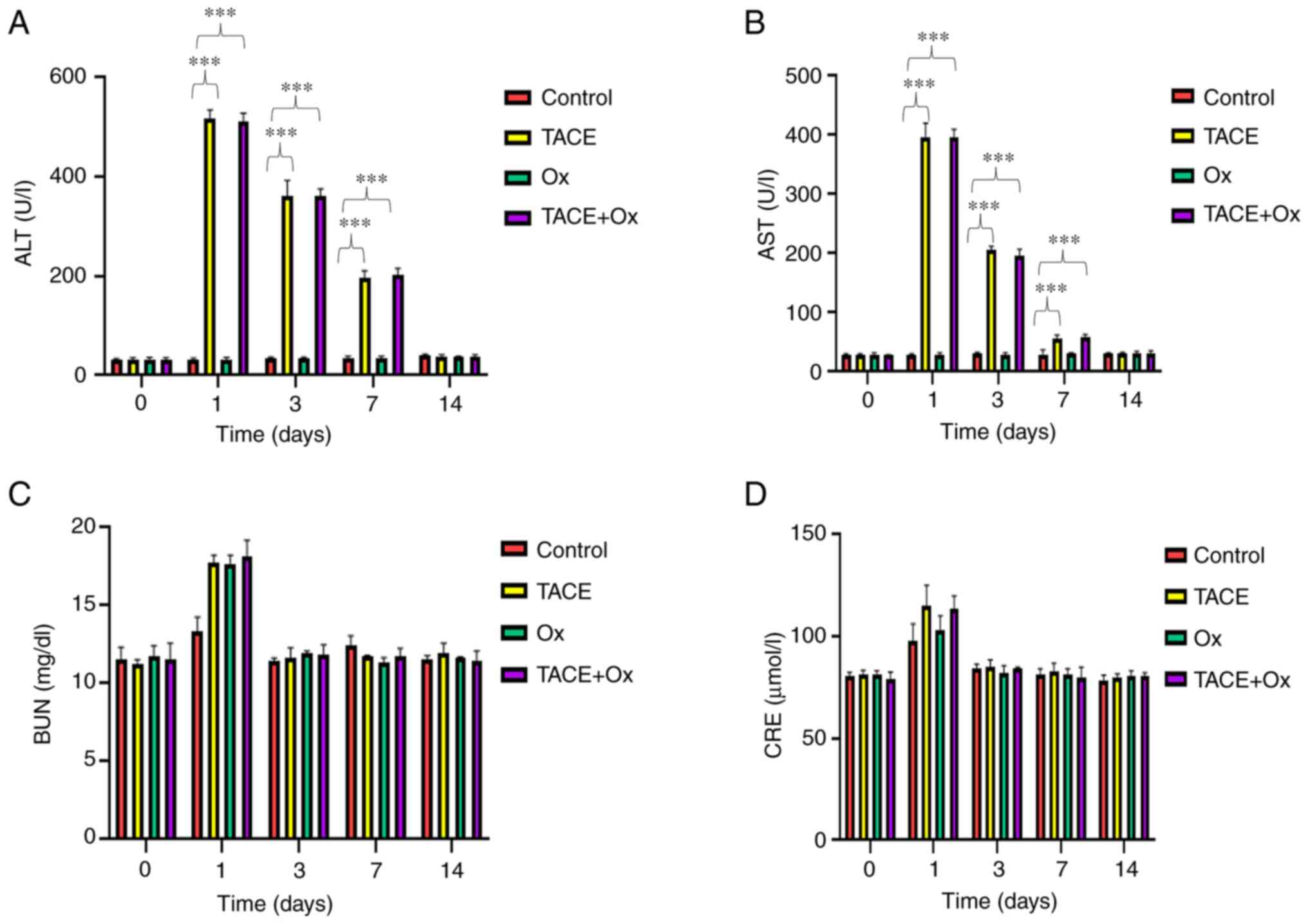 | Figure 5.Serum levels of (A) ALT, (B) AST, (C)
BUN and (D) CRE, measured at 0, 1, 3, 7 and 14 days after
treatment. ***P<0.001. ALT, alanine aminotransferase; AST,
aspartate aminotransferase; BUN, blood urea nitrogen; CRE,
creatinine; TACE, transarterial chemoembolization; Ox, sodium
oxamate. |
Discussion
It has been reported that the efficacy of TACE is
associated with local ischemic necrosis caused by embolic material
and the anticancer effects mediated by chemotherapy (26). However, hypoxia caused by incomplete
TACE may cause LDHA upregulation in tumors, which is the key enzyme
in aerobic glycolysis (27).
Furthermore, the enhancement of aerobic glycolysis in tumor tissues
may promote tumor growth due to the following reasons: i) Enhanced
anaerobic glycolysis may counteract the sharp oxygen fluctuation,
which could be fatal for cells that rely on oxygen or anaerobic
glycolysis for ATP production (28); ii) lactic acid generated via the
anaerobic glycolytic pathway could promote the invasion of tumor
cells via the upregulation of the monocarboxylate transporter
(MCT)1 and MCT2 (29); and iii)
tumor cells could simultaneously activate the pentose phosphate
pathway to satisfy their anabolic demands and combat oxidative
stress (30,31). Therefore, targeting metabolic
reprogramming after TACE may be key for the inhibition of tumor
growth, thus improving the therapeutic effect of TACE.
In the present study, the improvement of the
antitumor effect of TACE combined with the LDHA inhibitor, Ox, and
its underlying mechanism were assessed. Firstly, the results
demonstrated that the combination therapy enhanced the infiltration
of CD8+ cells. The increase in hypoxic necrosis may be
associated with the significantly greater infiltration rate of
CD8+ T cells, which is consistent with previous reports
(32,33). The findings from a previous study
may indicate why Ox increased the infiltration of CD8+ T
cells. This previous study indicated that a decrease in lactic acid
may downregulate the expression of programmed death-ligand 1,
leading to an elevation of pro-inflammatory antitumor responses,
such as increased infiltration and activity of CD8+
cytotoxic cells (34). Another
study reported that targeting lactic acid metabolism can reduce the
production of the angiogenic factor VEGF by downregulating HIF-1α,
which contributes to the normalization of tumor blood vessels, and
thus increases the infiltration of CD8+ T cells
(35). The specific reason for this
is worth exploring in-depth in future studies. Furthermore, a
previous study reported that the efficacy of immunotherapy was
positively associated with the CD8+ T cell to tumor
burden ratio (36). Therefore, TACE
combined with immunotherapy may be considered as a novel treatment
strategy. In the present study, Ox administration downregulated
MMP-9; notably, MMP-9 can degrade extracellular matrix components
and serves a significant role in several pathophysiological
processes, including metastasis, invasion and migration (37). Previous studies have reported that
MMP-9 overexpression and dysregulation are associated with the
onset of several diseases (38,39).
The aforementioned finding may be associated with the reduced
lactic acid levels reported in a previous study (40). Furthermore, the administration of Ox
could also downregulate VEGF after embolization, which serves a
significant role in angiogenesis and tumor cell immune escape
(41,42). Lastly, the results of the present
study demonstrated that Ox inhibited cell proliferation and
promoted apoptosis, which may be associated with a decrease in
energy supply to tumor cells and in lactic acid production. The
aforementioned mechanisms could result in an improved regulation of
the local tumor and increased survival of patients with HCC.
In the present study, although the tumor grew by
202.7±46.23% in the TACE group, the necrosis rate and survival
benefits were still greater than that of the control, which is also
consistent with the treatment of human liver cancer (43). Moreover, group C (Ox) and B (TACE)
demonstrated similar therapeutic results. However, the therapeutic
effect in group D was not simply the additive effect of both
components. The results demonstrated that the increase in
CD8+ T cells and TUNEL+ cells was more than
the sum of the other two groups, which may indicate that the
protocol had a synergistic therapeutic effect.
In terms of safety, the impairment of hepatorenal
function reached its peak at day 1 after treatment and then
gradually reduced to normal levels, mimicking TACE. Moreover, Ox
monotherapy demonstrated a similar effect to TACE alone without
damage to hepatorenal function, indicating that targeting
metabolism may be beneficial for patients with HCC. Overall, the
results suggested that the combination of Ox and TACE was safe and
may only lead to transient and reversable injuries.
However, the present study has certain limitations.
The assessment of changes in the tumor immune microenvironment
after treatment was insufficient due to the lack of antibody
reagents for rabbits, which is the most suitable model for
investigating TACE. Furthermore, the underlying mechanisms of the
effects of combination therapy need to be further investigated.
The current study demonstrated that the combination
of TACE and the LDHA inhibitor, Ox, resulted in a stronger
antitumor immune response than that of the control, TACE and Ox
groups inhibited angiogenesis and promoted increased survival in a
rabbit VX2 liver tumor model. These findings indicated that
targeting metabolic reprogramming could promote the efficacy of
TACE, thus providing novel insights into the future application of
a new treatment strategy in clinical practice for patients with
advanced HCC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ designed the research. YL and MY performed the
experiments. YL and YY analyzed the data. JZ and YL wrote the
paper. YL and JZ confirm the authenticity of all the raw data. All
the authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal studies were approved by the Animal Care
and Use Committee of Huazhong University of Science and Technology
(Wuhan, China; approval no. 3380) and were conducted in accordance
with the institutional guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Global Burden of Disease Cancer
Collaboration, . Fitzmaurice C, Allen C, Barber RM, Barregard L,
Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al:
Global, regional, and national cancer incidence, mortality, years
of life lost, years lived with disability, and disability-adjusted
life-years for 32 cancer groups, 1990 to 2015: A systematic
analysis for the global burden of disease study. JAMA Oncol.
3:524–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arnold M, Abnet CC, Neale RE, Vignat J,
Giovannucci EL, McGlynn KA and Bray F: Global burden of 5 major
types of gastrointestinal cancer. Gastroenterology.
159:335–349.e15. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sieghart W, Hucke F and Peck-Radosavljevic
M: Transarterial chemoembolization: Modalities, indication, and
patient selection. J Hepatol. 62:1187–1195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forner A, Gilabert M, Bruix J and Raoul
JL: Treatment of intermediate-stage hepatocellular carcinoma. Nat
Rev Clin Oncol. 11:525–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun B, Zhang L, Sun T, Ren Y, Cao Y, Zhang
W, Zhu L, Guo Y, Gui Y, Liu F, et al: Safety and efficacy of
lenvatinib combined with camrelizumab plus transcatheter arterial
chemoembolization for unresectable hepatocellular carcinoma: A
two-center retrospective study. Front Oncol. 12:9829482022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: Cancer's Achilles' heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng J: Energy metabolism of cancer:
Glycolysis versus oxidative phosphorylation (review). Oncol Lett.
4:1151–1157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robey IF, Stephen RM, Brown KS, Baggett
BK, Gatenby RA and Gillies RJ: Regulation of the Warburg effect in
early-passage breast cancer cells. Neoplasia. 10:745–756. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iansante V, Choy PM, Fung SW, Liu Y, Chai
JG, Dyson J, Del Rio A, D'Santos C, Williams R, Chokshi S, et al:
PARP14 promotes the Warburg effect in hepatocellular carcinoma by
inhibiting JNK1-dependent PKM2 phosphorylation and activation. Nat
Commun. 6:78822015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miao P, Sheng S, Sun X, Liu J and Huang G:
Lactate dehydrogenase A in cancer: A promising target for diagnosis
and therapy. IUBMB Life. 65:904–910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martinez-Outschoorn UE, Peiris-Pagés M,
Pestell RG, Sotgia F and Lisanti MP: Cancer metabolism: A
therapeutic perspective. Nat Rev Clin Oncol. 14:11–31. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee GH, Yan C, Shin SJ, Hong SC, Ahn T,
Moon A, Park SJ, Lee YC, Yoo WH, Kim HT, et al: BAX inhibitor-1
enhances cancer metastasis by altering glucose metabolism and
activating the sodium-hydrogen exchanger: The alteration of
mitochondrial function. Oncogene. 29:2130–2141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gottfried E, Kunz-Schughart LA, Ebner S,
Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A and Kreutz M:
Tumor-derived lactic acid modulates dendritic cell activation and
antigen expression. Blood. 107:2013–2021. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brand A, Singer K, Koehl GE, Kolitzus M,
Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et
al: LDHA-associated lactic acid production blunts tumor
immunosurveillance by T and NK cells. Cell Metab. 24:657–671. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Le A, Cooper CR, Gouw AM, Dinavahi R,
Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL and Dang
CV: Inhibition of lactate dehydrogenase A induces oxidative stress
and inhibits tumor progression. Proc Natl Acad Sci USA.
107:2037–2042. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huynh KN, Rao S, Roth B, Bryan T, Fernando
DM, Dayyani F, Imagawa D and Abi-Jaoudeh N: Targeting
hypoxia-inducible factor-1α for the management of hepatocellular
carcinoma. Cancers (Basel). 15:27382023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldman RD, Kaplan NO and Hall TC: Lactic
dehydrogenase in human neoplastic tissues. Cancer Res. 24:389–399.
1964.PubMed/NCBI
|
|
20
|
Zhai X, Yang Y, Wan J, Zhu R and Wu Y:
Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and
increases radiosensitivity in nasopharyngeal carcinoma cells. Oncol
Rep. 30:2983–2991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beckner ME, Stracke ML, Liotta LA and
Schiffmann E: Glycolysis as primary energy source in tumor cell
chemotaxis. J Natl Cancer Inst. 82:1836–1840. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cassim S, Raymond VA, Dehbidi-Assadzadeh
L, Lapierre P and Bilodeau M: Metabolic reprogramming enables
hepatocarcinoma cells to efficiently adapt and survive to a
nutrient-restricted microenvironment. Cell Cycle. 17:903–916. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu H, Savaraj N, Priebe W and Lampidis
TJ: Hypoxia increases tumor cell sensitivity to glycolytic
inhibitors: a strategy for solid tumor therapy (model C). Biochem
Pharmacol. 64:1745–1751. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qian K, Ma Y, Wan J, Geng S, Li H, Fu Q,
Peng X, Kan X, Zhou G, Liu W, et al: The studies about
doxorubicin-loaded p(N-isopropyl-acrylamide-co-butyl
methylacrylate) temperature-sensitive nanogel dispersions on the
application in TACE therapies for rabbit VX2 liver tumor. J Control
Release. 212:41–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Shi D, Ren Y, Li L, Zhao Y, Zheng C
and Yang X: The immune-chemo-embolization effect of temperature
sensitive gold nanomedicines against liver cancer. Nano Res.
16:2749–2761. 2023. View Article : Google Scholar
|
|
26
|
Li X, Yu H, Huang Y, Chen Y, Wang J, Xu L,
Zhang F, Zhuge Y and Zou X: Preparation of microspheres
encapsulating sorafenib and catalase and their application in
rabbit VX2 liver tumor. Biomed Pharmacother. 129:1105122020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pouysségur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Swietach P, Vaughan-Jones RD and Harris
AL: Regulation of tumor pH and the role of carbonic anhydrase 9.
Cancer Metastasis Rev. 26:299–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patra KC and Hay N: The pentose phosphate
pathway and cancer. Trends Biochem Sci. 39:347–354. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hermans D, Gautam S, García-Cañaveras JC,
Gromer D, Mitra S, Spolski R, Li P, Christensen S, Nguyen R, Lin
JX, et al: Lactate dehydrogenase inhibition synergizes with IL-21
to promote CD8+ T cell stemness and antitumor immunity. Proc Natl
Acad Sci USA. 117:6047–6055. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang YX, Zhao YY, Shen J, Sun X, Liu Y,
Liu H, Wang Y and Wang J: Nanoenabled modulation of acidic tumor
microenvironment reverses anergy of infiltrating T cells and
potentiates anti-PD-1 therapy. Nano Lett. 19:2774–2783. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Daneshmandi S, Wegiel B and Seth P:
Blockade of lactate dehydrogenase-A (LDH-A) improves efficacy of
anti-programmed cell death-1 (PD-1) therapy in melanoma. Cancers
(Basel). 11:4502019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Colegio OR, Chu NQ, Szabo AL, Chu T,
Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC,
Phillips GM, et al: Functional polarization of tumour-associated
macrophages by tumour-derived lactic acid. Nature. 513:559–563.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang AC, Postow MA, Orlowski RJ, Mick R,
Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al: T-cell
invigoration to tumour burden ratio associated with anti-PD-1
response. Nature. 545:60–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang H: Matrix metalloproteinase-9
(MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent
advances. Sensors (Basel). 18:32492018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mondal S, Adhikari N, Banerjee S, Amin SA
and Jha T: Matrix metalloproteinase-9 (MMP-9) and its inhibitors in
cancer: A minireview. Eur J Med Chem. 194:1122602020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Owyong M, Chou J, van den Bijgaart RJ,
Kong N, Efe G, Maynard C, Talmi-Frank D, Solomonov I, Koopman C,
Hadler-Olsen E, et al: MMP9 modulates the metastatic cascade and
immune landscape for breast cancer anti-metastatic therapy. Life
Sci Alliance. 2:e2018002262019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nass RD, Wagner M, Surges R and
Holdenrieder S: Time courses of HMGB1 and other inflammatory
markers after generalized convulsive seizures. Epilepsy Res.
162:1063012020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Morse MA, Sun W, Kim R, He AR, Abada PB,
Mynderse M and Finn RS: The role of angiogenesis in hepatocellular
carcinoma. Clin Cancer Res. 25:912–920. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Raoul JL, Forner A, Bolondi L, Cheung TT,
Kloeckner R and de Baere T: Updated use of TACE for hepatocellular
carcinoma treatment: How and when to use it based on clinical
evidence. Cancer Treat Rev. 72:28–36. 2019. View Article : Google Scholar : PubMed/NCBI
|