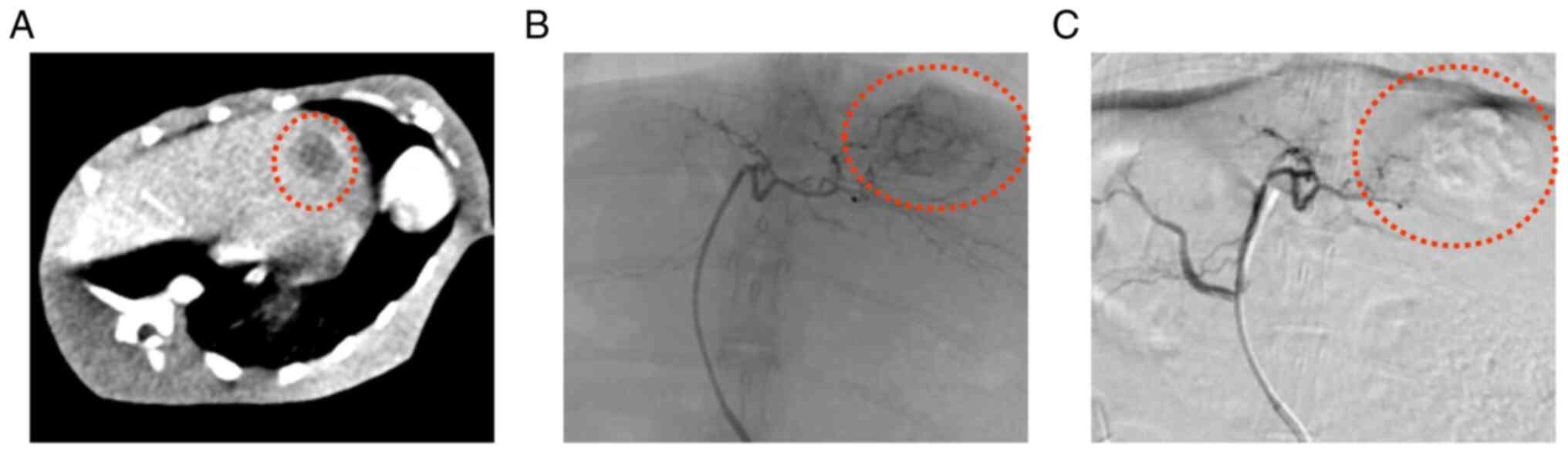
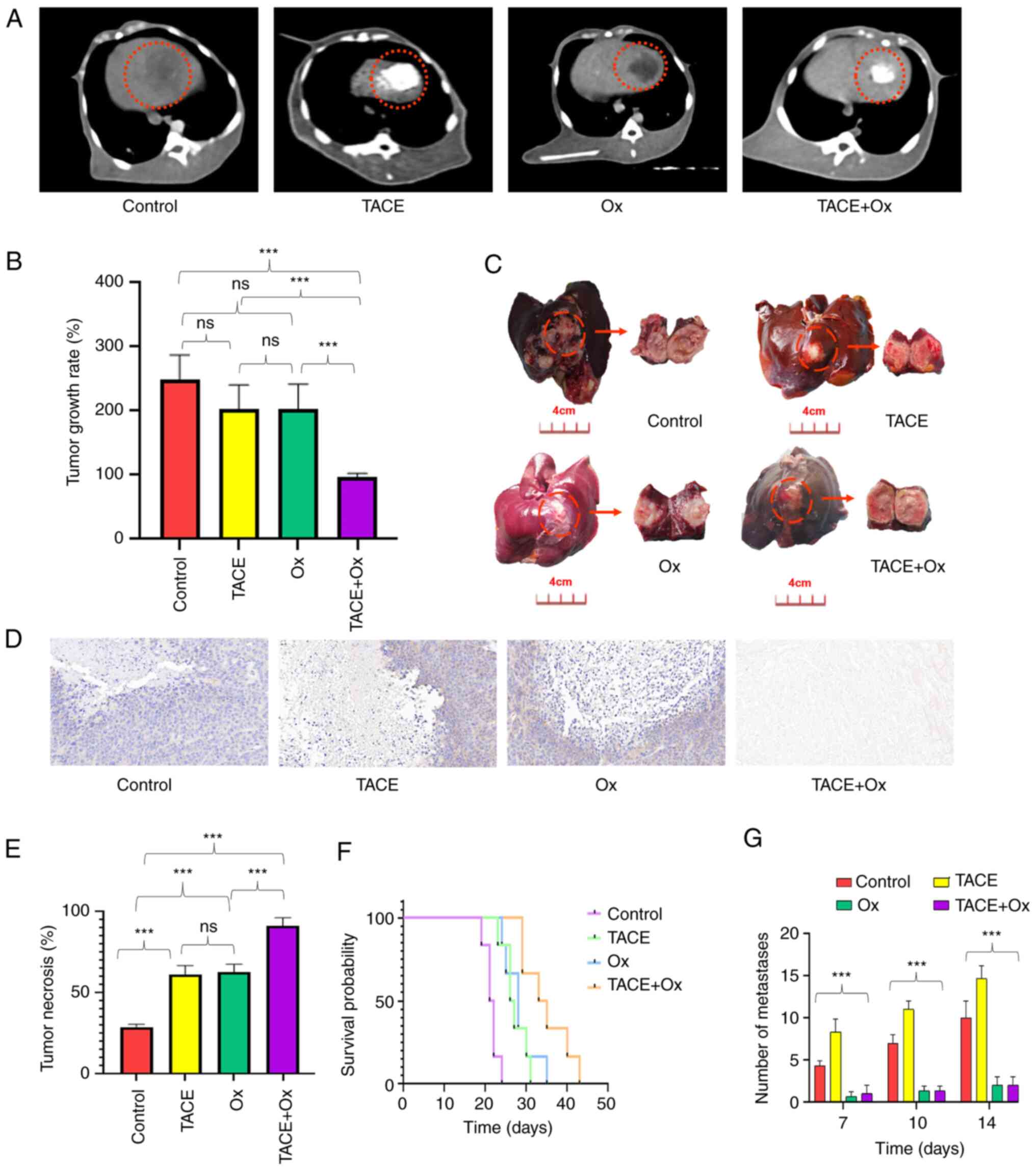
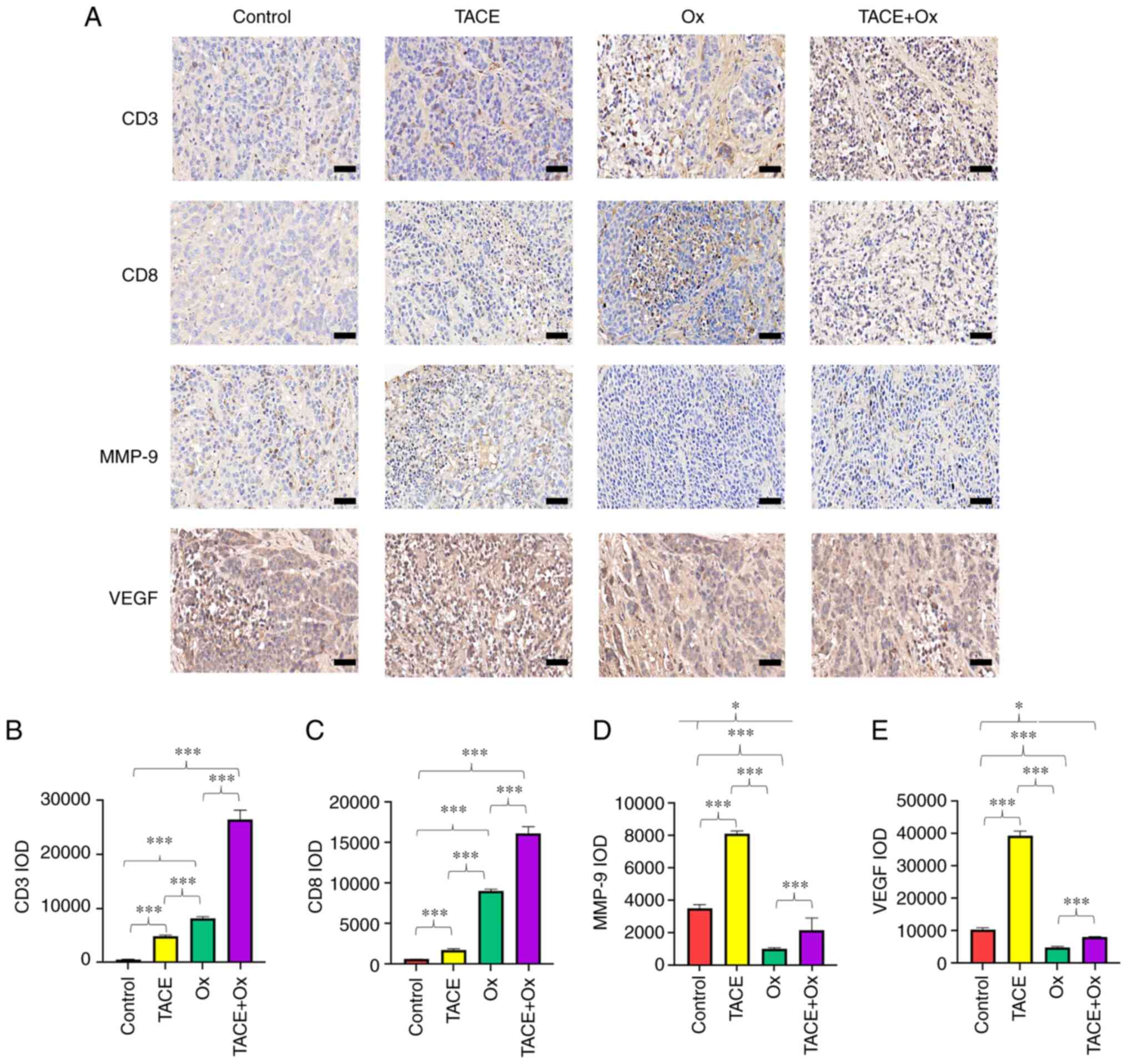
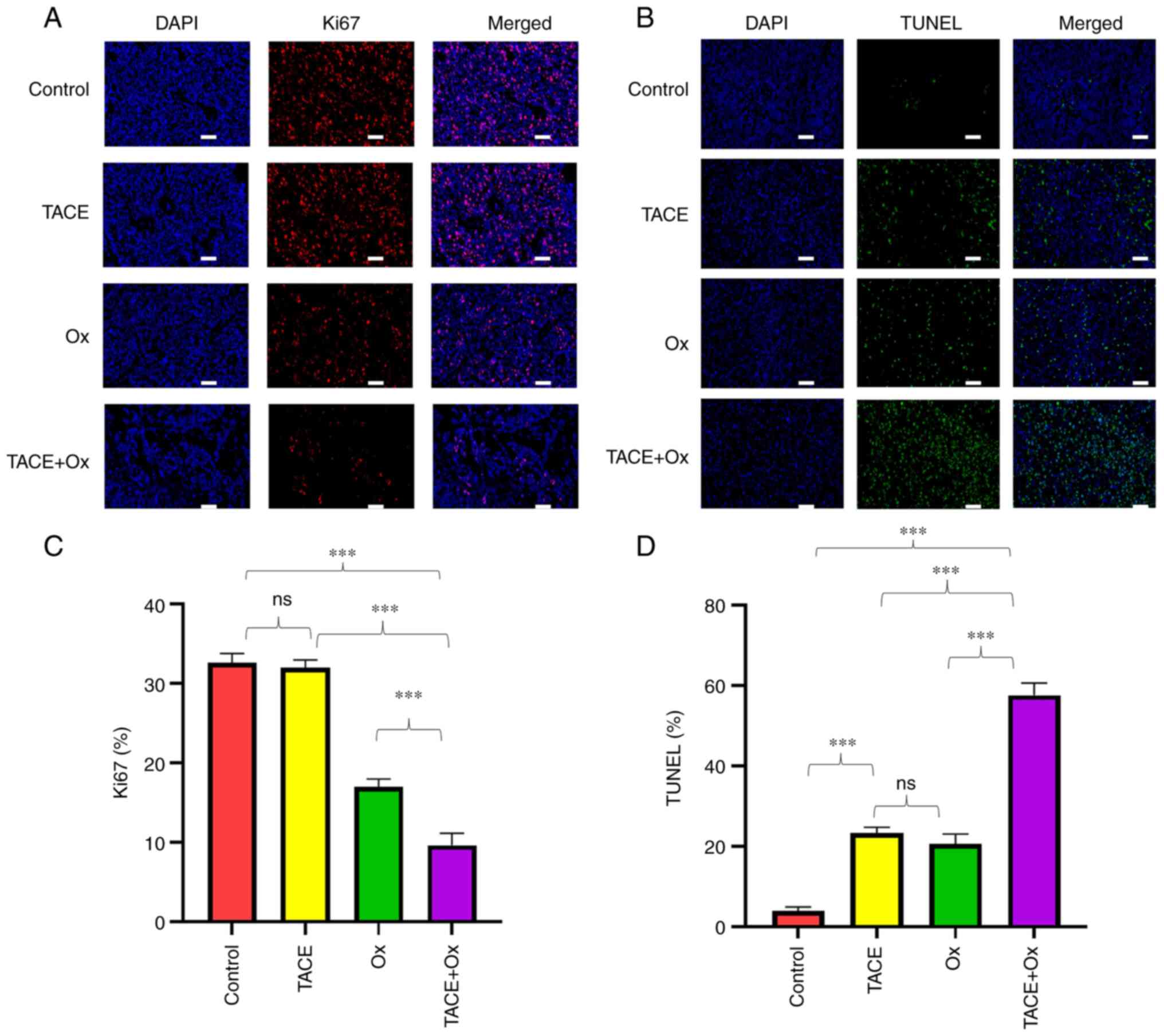
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Global Burden of Disease Cancer
Collaboration, . Fitzmaurice C, Allen C, Barber RM, Barregard L,
Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al:
Global, regional, and national cancer incidence, mortality, years
of life lost, years lived with disability, and disability-adjusted
life-years for 32 cancer groups, 1990 to 2015: A systematic
analysis for the global burden of disease study. JAMA Oncol.
3:524–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arnold M, Abnet CC, Neale RE, Vignat J,
Giovannucci EL, McGlynn KA and Bray F: Global burden of 5 major
types of gastrointestinal cancer. Gastroenterology.
159:335–349.e15. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sieghart W, Hucke F and Peck-Radosavljevic
M: Transarterial chemoembolization: Modalities, indication, and
patient selection. J Hepatol. 62:1187–1195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forner A, Gilabert M, Bruix J and Raoul
JL: Treatment of intermediate-stage hepatocellular carcinoma. Nat
Rev Clin Oncol. 11:525–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun B, Zhang L, Sun T, Ren Y, Cao Y, Zhang
W, Zhu L, Guo Y, Gui Y, Liu F, et al: Safety and efficacy of
lenvatinib combined with camrelizumab plus transcatheter arterial
chemoembolization for unresectable hepatocellular carcinoma: A
two-center retrospective study. Front Oncol. 12:9829482022.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: Cancer's Achilles' heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng J: Energy metabolism of cancer:
Glycolysis versus oxidative phosphorylation (review). Oncol Lett.
4:1151–1157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robey IF, Stephen RM, Brown KS, Baggett
BK, Gatenby RA and Gillies RJ: Regulation of the Warburg effect in
early-passage breast cancer cells. Neoplasia. 10:745–756. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iansante V, Choy PM, Fung SW, Liu Y, Chai
JG, Dyson J, Del Rio A, D'Santos C, Williams R, Chokshi S, et al:
PARP14 promotes the Warburg effect in hepatocellular carcinoma by
inhibiting JNK1-dependent PKM2 phosphorylation and activation. Nat
Commun. 6:78822015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miao P, Sheng S, Sun X, Liu J and Huang G:
Lactate dehydrogenase A in cancer: A promising target for diagnosis
and therapy. IUBMB Life. 65:904–910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martinez-Outschoorn UE, Peiris-Pagés M,
Pestell RG, Sotgia F and Lisanti MP: Cancer metabolism: A
therapeutic perspective. Nat Rev Clin Oncol. 14:11–31. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee GH, Yan C, Shin SJ, Hong SC, Ahn T,
Moon A, Park SJ, Lee YC, Yoo WH, Kim HT, et al: BAX inhibitor-1
enhances cancer metastasis by altering glucose metabolism and
activating the sodium-hydrogen exchanger: The alteration of
mitochondrial function. Oncogene. 29:2130–2141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gottfried E, Kunz-Schughart LA, Ebner S,
Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A and Kreutz M:
Tumor-derived lactic acid modulates dendritic cell activation and
antigen expression. Blood. 107:2013–2021. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brand A, Singer K, Koehl GE, Kolitzus M,
Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et
al: LDHA-associated lactic acid production blunts tumor
immunosurveillance by T and NK cells. Cell Metab. 24:657–671. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Le A, Cooper CR, Gouw AM, Dinavahi R,
Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL and Dang
CV: Inhibition of lactate dehydrogenase A induces oxidative stress
and inhibits tumor progression. Proc Natl Acad Sci USA.
107:2037–2042. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huynh KN, Rao S, Roth B, Bryan T, Fernando
DM, Dayyani F, Imagawa D and Abi-Jaoudeh N: Targeting
hypoxia-inducible factor-1α for the management of hepatocellular
carcinoma. Cancers (Basel). 15:27382023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldman RD, Kaplan NO and Hall TC: Lactic
dehydrogenase in human neoplastic tissues. Cancer Res. 24:389–399.
1964.PubMed/NCBI
|
|
20
|
Zhai X, Yang Y, Wan J, Zhu R and Wu Y:
Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and
increases radiosensitivity in nasopharyngeal carcinoma cells. Oncol
Rep. 30:2983–2991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beckner ME, Stracke ML, Liotta LA and
Schiffmann E: Glycolysis as primary energy source in tumor cell
chemotaxis. J Natl Cancer Inst. 82:1836–1840. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cassim S, Raymond VA, Dehbidi-Assadzadeh
L, Lapierre P and Bilodeau M: Metabolic reprogramming enables
hepatocarcinoma cells to efficiently adapt and survive to a
nutrient-restricted microenvironment. Cell Cycle. 17:903–916. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu H, Savaraj N, Priebe W and Lampidis
TJ: Hypoxia increases tumor cell sensitivity to glycolytic
inhibitors: a strategy for solid tumor therapy (model C). Biochem
Pharmacol. 64:1745–1751. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qian K, Ma Y, Wan J, Geng S, Li H, Fu Q,
Peng X, Kan X, Zhou G, Liu W, et al: The studies about
doxorubicin-loaded p(N-isopropyl-acrylamide-co-butyl
methylacrylate) temperature-sensitive nanogel dispersions on the
application in TACE therapies for rabbit VX2 liver tumor. J Control
Release. 212:41–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Shi D, Ren Y, Li L, Zhao Y, Zheng C
and Yang X: The immune-chemo-embolization effect of temperature
sensitive gold nanomedicines against liver cancer. Nano Res.
16:2749–2761. 2023. View Article : Google Scholar
|
|
26
|
Li X, Yu H, Huang Y, Chen Y, Wang J, Xu L,
Zhang F, Zhuge Y and Zou X: Preparation of microspheres
encapsulating sorafenib and catalase and their application in
rabbit VX2 liver tumor. Biomed Pharmacother. 129:1105122020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pouysségur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Swietach P, Vaughan-Jones RD and Harris
AL: Regulation of tumor pH and the role of carbonic anhydrase 9.
Cancer Metastasis Rev. 26:299–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patra KC and Hay N: The pentose phosphate
pathway and cancer. Trends Biochem Sci. 39:347–354. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hermans D, Gautam S, García-Cañaveras JC,
Gromer D, Mitra S, Spolski R, Li P, Christensen S, Nguyen R, Lin
JX, et al: Lactate dehydrogenase inhibition synergizes with IL-21
to promote CD8+ T cell stemness and antitumor immunity. Proc Natl
Acad Sci USA. 117:6047–6055. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang YX, Zhao YY, Shen J, Sun X, Liu Y,
Liu H, Wang Y and Wang J: Nanoenabled modulation of acidic tumor
microenvironment reverses anergy of infiltrating T cells and
potentiates anti-PD-1 therapy. Nano Lett. 19:2774–2783. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Daneshmandi S, Wegiel B and Seth P:
Blockade of lactate dehydrogenase-A (LDH-A) improves efficacy of
anti-programmed cell death-1 (PD-1) therapy in melanoma. Cancers
(Basel). 11:4502019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Colegio OR, Chu NQ, Szabo AL, Chu T,
Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC,
Phillips GM, et al: Functional polarization of tumour-associated
macrophages by tumour-derived lactic acid. Nature. 513:559–563.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang AC, Postow MA, Orlowski RJ, Mick R,
Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al: T-cell
invigoration to tumour burden ratio associated with anti-PD-1
response. Nature. 545:60–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang H: Matrix metalloproteinase-9
(MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent
advances. Sensors (Basel). 18:32492018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mondal S, Adhikari N, Banerjee S, Amin SA
and Jha T: Matrix metalloproteinase-9 (MMP-9) and its inhibitors in
cancer: A minireview. Eur J Med Chem. 194:1122602020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Owyong M, Chou J, van den Bijgaart RJ,
Kong N, Efe G, Maynard C, Talmi-Frank D, Solomonov I, Koopman C,
Hadler-Olsen E, et al: MMP9 modulates the metastatic cascade and
immune landscape for breast cancer anti-metastatic therapy. Life
Sci Alliance. 2:e2018002262019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nass RD, Wagner M, Surges R and
Holdenrieder S: Time courses of HMGB1 and other inflammatory
markers after generalized convulsive seizures. Epilepsy Res.
162:1063012020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Morse MA, Sun W, Kim R, He AR, Abada PB,
Mynderse M and Finn RS: The role of angiogenesis in hepatocellular
carcinoma. Clin Cancer Res. 25:912–920. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Raoul JL, Forner A, Bolondi L, Cheung TT,
Kloeckner R and de Baere T: Updated use of TACE for hepatocellular
carcinoma treatment: How and when to use it based on clinical
evidence. Cancer Treat Rev. 72:28–36. 2019. View Article : Google Scholar : PubMed/NCBI
|