Introduction
Lung cancer (LC) was the leading cause of mortality
in 2018 in males and the third in females worldwide (1). In Japan, recent mortality statistics
(2021) and incidence rate (2019) revealed that LC accounts for the
highest mortality and fourth highest incidence rate in males, and
the second highest mortality and third highest incidence rate in
females (2). However, LC treatment
has improved, which is mainly attributed to the increased detection
of early-stage LC, especially in cases where tumors are ≤2 cm in
size (3). Low-exposure CT and MRI
have majorly contributed to the diagnosis of early LC (4). However, the prognosis of LC remains
poor. In Japan, the Act for Health and Safety in Labors strictly
requires all workers to undergo a chest X-ray examination to
prevent tuberculosis and pulmonary diseases due to occupational
hazards. However, chest X-ray examination is not suitable for LC
detection because it is difficult to diagnose early-stage LC using
plain radiography; the specificity and sensitivity of chest X-ray
for LC is also insufficient (5).
Hence, there remains a need for develop a novel LC screening test
with easy access, high quality, and low price, for the early
detection of early-stage patients with LC having high sensitivity
and specificity at the work-place and community health centers.
We have previously reported a novel breast-cancer
screening method using a combination of microRNA-21 (miRNA-21) and
matrix metalloproteinase-1 (MMP-1) in urinary exosomes capable of
detecting breast cancer in 95% cases before metastasis (6). All cells secrete exosomes that are
small vesicles (30–150 nm in size) containing DNA, mRNA, miRNAs,
oncogenic genes, proteins, and lipids among others (7–9). These
exosomes relay important cell information between cells, migrate
through the blood vessels or other routes, and adhere to the
surface of distant cells (7–10).
Therefore, they are crucial for maintaining harmony with other
organs, not only for physiological growth, development, and cell
death, but also for pathogenesis. Exosomes from the cancer cells
can invade the distant cells of other organs, eventually resulting
in metastasis (7–10). Therefore, urinary exosomes contain
sufficient information on the oncogenic genes and their related
proteins. However, LC screening using urinary exosomes has not been
reported to date. Furthermore, MMP-1 is one of the key enzymes of
collagen metabolism (11) involved
in the carcinogenesis in LC (12–15).
An increased expression of MMP-1 evidenced in early stage of LC
(15). However, MMP-1 has not been
investigated to serve as a biomarker for LC screening.
We selected miRNA-21 and miRNA-486-5p as candidate
miRNAs to serve as biomarkers for LC screening in the present study
(16–21). Several studies have reported the
usefulness of miRNA-21 and miRNA-486-5p in diagnosing LC using the
circulating plasma exosomes (16,20).
Both miRNA-21 and miRNA-486-5p have multi-faced functions in cancer
diagnosis, tumorigenesis, and prognosis (16,17,20).
The function of miRNA-21 inhibits several tumor suppressor genes
such as PTEN and HOXD10 (17–19).
MiRNA-486-5p targeting the PIK3R1 gene is involved in the
suppression of tumor cell growth (21).
Herein, we analyze the expression of MMP-1/CD63 as
well as miRNA-21 and miRNA-486-5p in urinary exosomes of patients
with LC and determine their application in early screening and
detection of LC in patients.
Materials and methods
Study population
This study was conducted at the International
University of Health and Welfare (IUHW) Hospital (Nasu-Shiobara
City, Tochigi Japan) and the Kitasato University Medical Center
(Kitamoto City, Saitama, Japan) between October 1, 2018 and October
31, 2022. The study was approved by the ethics committee of the two
abovementioned institutions. The details were described in
Declarations.
Thirty-five patients with LC (24 males and 11
females; mean age, 71.0±8.4 and 64.8±9.5 years, respectively)
included in this study were admitted to the Center for Respiratory
Diseases, Department of Chest Surgery, IUHW Hospital. The patients
were diagnosed with LC after performing chest X-ray, chest CT,
chest MRI, and subsequent needle biopsy before surgery, if
necessary. The final pathological diagnosis was performed on the
basis of surgical resection at the IUHW Hospital. The clinical
stage was classified according to the eighth edition of the TNM
classification of LC (22).
Urine samples were collected before surgical
treatment in the morning before breakfast and stored at −80°C until
the exosomes were separated. All samples were collected before
patients received surgery, chemotherapy, or radiation.
The control urine samples were obtained from 40
healthy controls (20 males and 20 females) selected among 533
persons (351 males and 182 females) who visited the Department of
Preventive Medicine, IUHW Hospital for a health check from January
20, 2020 to February 8, 2020, because only a few visitors were over
70 years of age. The number of selected individuals in the
40-year-old, 50-year-old, 60-year-old, and >70-year-old groups
in each sex group was more than five. All selected controls had no
complaints or abnormal physical findings such as obesity,
hypertension, abnormal peripheral blood examination and blood
chemistry, abnormal ECG findings, or abnormal findings on chest
radiography, upper GI endoscopy, or abdominal echography. There was
no history of cancer, signs of dysplasia, inflammatory disease,
autoimmune disease, or chronic diseases such as cardiac, liver, or
kidney diseases. All participants provided written informed
consent.
Classification of LC clinical
stage
The clinical stage was classified according to the
eighth edition of the TNM classification of LC (22). The TNM classification of the 35
patients with LC is listed in Table
I.
 | Table I.Lung cancer cases, TNM
classification, and characteristics. |
Table I.
Lung cancer cases, TNM
classification, and characteristics.
| No. |
Age/Sexa | BMI,
kg/m2 | Smoking Habit | TNMb | Stagec | Pathod |
MMP1/CD63e | miR-21,
2ΔΔCqf | miR-486-5p,
2ΔΔCqf | Family
historyg |
|---|
| 1 | 60/M | 22.3 | Current | T1aNxM0 | IA1 | AC | 1.25 | 0.00 | 2.87 | LC |
| 2 | 85/M | 19.6 | Former | T2aNxM0 | IB | AC | 0.89 | 1.31 | 1.21 | None |
| 3 | 70/M | 22.4 | Never | T2bN0M0 | IIA | AC | 1.80 | 0.33 | 5.65 | None |
| 4 | 76/M | 25.7 | Former | T1cN0M0 | IA3 | AC | 1.92 | 1.47 | 5.23 | None |
| 5 | 72/F | 20.1 | Never | T1bN0M0 | IA2 | AC | 4.03 | 0.12 | 0.37 | GC |
| 6 | 70/F | 21.5 | Never | T1aN0M0 | IA1 | AC | 2.35 | 0.00 | 0.32 | LiC |
| 7 | 65/M | 19.5 | Current | T2bN1M0 | IIB | SC | 2.42 | 0.41 | 0.84 | None |
| 8 | 60/M | 24.9 | Never | T2aN0M0 | IB | AC | 4.29 | 0.01 | 2.46 | LC |
| 9 | 72/M | 21.4 | Former | T2aN1M0 | IB | AC | 2.92 | 1.32 | 2.17 | None |
| 10 | 63/M | 21.7 | Current | TisN0M0 | 0 | AC | 0.91 | 0.00 | 2.42 | LC |
| 11 | 67/M | 25.4 | Current | T1cN0M0 | IB | SC | 0.81 | 0.00 | 2.67 | None |
| 12 | 69/M | 28.1 | Former | T1cN0M0 | IA3 | AC | 0.82 | 0.04 | 2.14 | None |
| 13 | 80/M | 19.9 | Never | T1cN1M1a | IVa | AC | 0.41 | 0.00 | 0.30 | None |
| 14 | 51/M | 19.5 | Current | T1cN1M1b | IVb | AC | 0.40 | 1.85 | 1.21 | None |
| 15 | 82/M | 23.0 | Current | T1aNxM0 | IA2 | SC | 0.89 | 0.00 | 4.17 | LC.GC |
| 16 | 58/F | 18.0 | Never | T1cNxM0 | IA3 | AC | 2.32 | 0.59 | 0.01 | LC |
| 17 | 83/M | 31.2 | Never | T4N0M1c | IVb | AC | 4.01 | 10.66 | 7.59 | GC |
| 18 | 72/M | 26.9 | Current | T1cN2M1b | IVb | SCLC | 5.03 | 1.81 | 5.52 | None |
| 19 | 74/M | 21.3 | Current | T2aN2M0 | IIIa | SCLC | 0.35 | 1.55 | 2.83 | None |
| 20 | 69/M | 21.5 | Current | T2aN1M0 | IIB | SC | 4.41 | 3.69 | 1.80 | None |
| 21 | 62/F | 19.2 | Former | T2aN3M1c | IVB | AC | 0.27 | 44.43 | 11.70 | None |
| 22 | 65/F | 32.7 | Current | T1cN0M0 | IA2 | SC | 1.34 | 2.40 | 0.06 | None |
| 23 | 50/F | 19.9 | Never | T3N2M0 | IIIA | AC | 6.69 | 0.01 | 0.46 | None |
| 24 | 71/M | 23.4 | Former | T3N0M0 | IIB | Pleom | 1.49 | 25.8 | 6.70 | None |
| 25 | 74/F | 24.0 | Never | T1miN0M0 | IA1 | AC | 1.97 | 6.23 | 2.08 | None |
| 26 | 70/F | 22.7 | Never | T1aN0M0 | IA1 | AC/SC | 1.97 | 1.87 | 0.91 | Le |
| 27 | 70/F | 17.6 | Former | T1miNM0 | IA1 | AC | 0.91 | 1.55 | 0.45 | GC.CC |
| 28 | 64/M | 23.1 | Former | T1bN0M0 | IA2 | AC | 0.59 | 0.31 | 0.07 |
|
| 29 | 65/M | 23.6 | Former | T1bN1M1b | IVb | AC | 0.39 | 1.30 | 4.42 | LC |
| 30 | 69/M | 25.1 | Current | T1miN0M0 | IA1 | AC | 0.43 | 1.87 | 2.37 | None |
| 31 | 75/M | 18.9 | Former | T4N3M0 | IIIB | SCLC | 1.01 | 0.65 | 3.27 | None |
| 32 | 82/M | 18.8 | Current | T3N0M1a | IVa | AC | 0.88 | 1.53 | 5.44 | None |
| 33 | 47/F | 22.2 | Never | T1bN0M0 | IA2 | AC | 1.91 | 1.94 | 4.01 | None |
| 34 | 75/F | 22.7 | Current | T1bN0M0 | IA2 | SCLC | 4.03 | 0.56 | 0.84 | CC.LC |
| 35 | 79/M | 21.7 | Current | T3N0M0 | IIB | LCC | 1.11 | 1.05 | 1.12 | None |
|
|
|
|
|
| Median |
| 1.34 | 1.30 | 2.17 |
|
|
|
|
|
|
| (95% CI) |
| (0.89–1.97) | (0.33–1.55) | (1.12–1.86) |
|
Isolation of the urinary exosomes
All urine samples were transferred to Kitasato
University Medical Center at −80°C. A uniform volume of 2 ml urine
was collected from all participants for exosome isolation. Before
exosome isolation, the thawed urine sample was centrifuged at 3,000
× g for 15 min at 4°C and passed through a 0.22-µm nylon filter.
The urinary exosomes isolated using the Exosome Isolation Kit (Cat.
130-110-912, Miltenyi Biotec, Bergisch Gladbach, Germany) were
subjected to western blotting using SDS-PAGE and anti-CD63
biotin-conjugated antibody (BioLegend Inc., San Diego, CA, USA) and
identified using transmission electron microscopy (Fig. S1) as reported previously (6). The pellet of isolated exosomes was
placed over the Transmission Electron Microscopy (TEM) grid coated
with carbon/formvar (Oken-Shoji Co., Tokyo, Japan), stained by the
2% uranyl acetate for 2 min at room temperature, and finally
exosomes were observed by transmission electron microscopy (Hitachi
H-7600, Hitachi Ltd., Tochigi, Japan).
MMP-1/CD63 expression ratio in urinary
exosomes
The levels of MMP-1 and CD63 were determined with
western blotting using an anti-MMP-1 antibody (Cat. ab134184, Abcam
plc., Cambridge, UK) and biotin-conjugated anti-CD 63 antibodies
(Cat. 353017, BioLegend Inc., CA, USA). One-sixth of the urinary
exosomes extracted from 2 ml was applied to the wells. Anti-MMP-1
antibody (1:1,000) and anti-CD63 antibody (1:1,000) were used. The
ratio of MMP-1/CD63 was pixelated in patients with LC and compared
to that in healthy controls (Fig.
S2). The analysis was performed using the ImageJ 1.52a software
(NIH, Bethesda, MD, USA) (23). To
validate reproducibility, all experiments were performed twice, and
the average or median values were calculated, as described
previously (6).
Analysis of miRNA-21 and miRNA-486-5p
extracted from the urinary exosomes
RNA for analyzing miRNAs (miRNA-21 and miRNA-486-5p)
was extracted from the isolated exosomes using a Total Exosome RNA
and Protein Isolation Kit (Thermo Fisher Scientific, Waltham, MA,
USA); the isolated miRNA was reverse-transcribed to cDNA using the
TaqMan MicroRNA Reverse Transcription Kit (Cat. 4366596, Thermo
Fisher Scientific), according to the manufacturer's protocol.
RT-qPCR was performed on a Step One Plus® thermal cycler
(Applied Biosystems, Foster City, CA, USA) for miRNAs extracted
from the urinary exosomes. For determining the miRNA-21 and
miRNA-486-5p expression respectively, assays were performed using a
commercial miRNA-21 assay kit (Cat. 4427975, Assay ID: 000397,
Applied Biosystems) and miRNA-486-5p assay kit (Cat. 4427975, Assay
ID 001278, Applied Biosystems) respectively according to the
manufacturer's instructions. The assays were performed in duplicate
for each sample, as described previously (6). In this study, instead of internal
controls, miRNAs were extracted from urinary exosomes and reverse
transcription reactions were performed using 5 ng of the recovered
miRNAs to correct for miRNA levels between samples, and expression
analysis was performed. This method has been proven in previous
reports (6).
Relative expression levels of miRNA-21
and miRNA-486-5p in the urine exosomes
Based on the mean number of miRNA-21 and
miRNA-486-5p copies in the healthy controls, the copy number of
miRNA-21 and miRNA-486-5p in patients with LC was calculated as the
relative expression grade, as described previously (6). The ΔΔCq value was determined by
subtracting the mean CT value of miRNA-21 and miRNA-486-5p in the
healthy controls from the individual CT value of miRNA-21 and
miRNA-486-5p in patients with LC. In the present study, the number
of RT-PCR cycles in miRNA-486-5p varied between healthy male and
healthy female controls, hence miRNA-486-5p was analyzed by gender.
Finally, the copy number of miRNA-21 and miRNA-486-5p were compared
as the value of 2ΔΔCq.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD) or median [95% confidence interval (CI)]. All statistical
analyses were performed using the PRISM 9 software for Mac OS
(GraphPad, Inc., La Jolla, CA, USA). Data were tested for normality
and equal variance to confirm the appropriateness of the parametric
tests, and those that followed a normal distribution were analyzed
using the unpaired Student's t-test. Mann-Whitney U-tests were used
to compare differences between groups, and Kruskal-Wallis test
followed by Dunn's post hoc test were used to compare differences
among three or more groups. P<0.05 was considered to indicate a
statistically significant difference.
Logistic regression analysis was performed with the
presence of lung cancer as dependent variable, and the expression
ratio for MMP-1/CD63, and relative expression of miRNA-486-5p as
independent variable adjusted with age, sex, and smoking status as
covariates. ROC analysis was applied for the prediction of logistic
regression model, and cut-off value was calculated by the Youden
index. Logistic regression analysis and its ROC analysis were
performed by IBM SPSS ver 26.0.
Results
Clinical characteristics and
histopathological findings in patients with LC
The included patients with LC were relatively
early-detected cases since most have visited the Department of
Preventive Medicine of IUHW Hospital for health checkups, including
LC screening including chest X-ray and cytological examination on
sputum, and their suspicious lesions were examined using lung CT
and MRI followed by the further examinations (Tables I and SI).
The pathological findings in the surgically resected
tissues were as follows: non-small cell lung cancer was evident in
30 patients, adenocarcinoma (AC) in 24 (15 males and 9 females),
squamous cell carcinoma (SC) in 5 (4 males and 1 female), and large
cell carcinoma (LCC) in 1 (1 male and 0 female). Small cell lung
cancer (SCLC) was evident in 4 (3 males and 1 female); pleomorphic
carcinoma was evident in one male.
Among the 35 patients with LC, stage 0 was seen in
one man, stage I in 19 (10 males and 9 females), stage II in 5 men,
stage III in 3 (2 males and 1 female), and stage IV in 7 (6 males
and 1 female).
Comparison of expression ratio of
MMP-1/CD63 in patients with LC and those in healthy controls
The individual expression ratios of MMP-1/CD63 in
patients with LC and healthy controls are listed in Tables I and SII, respectively. The MMP-1/CD63
expression ratio in both male and female healthy controls showed
the same distribution trend, and those in both male and female
patients with LC was increased (Fig.
1A-C). The median expression ratio in patients with LC was
1.342 (95% CI: 0.890–1.974), which was significantly higher than
that in the healthy controls, 0.600 (95% CI: 0.490–0.900)
(P<0.0001) (Tables I and
SII; Fig. 1A).
The MMP-1/CD63 expression ratio in female healthy
controls who are under 60 years of age was not significant
different from those who are older (Table IIA). Male patients with LC showed
higher ratio tendency in both under 60 years and over 60 years of
age, especially in those under 60 years patients, than in both
healthy males under and over 60 years of age (Table IIA). A significantly higher ratio
in both female patients under and over 60 years was observed
compared with the females healthy controls (P<0.01 and
P<0.05, respectively; Table
IIA). The MMP-1/CD63 expression ratio of SCLC and those of
NSCLC was not statistically different in the limited
population.
 | Table II.Expression ratio of MMP-1/CD63 and
relative expression of miRNA-21 and miRNA-486-5p in 40 healthy
controls and 35 lung cancer patients. |
Table II.
Expression ratio of MMP-1/CD63 and
relative expression of miRNA-21 and miRNA-486-5p in 40 healthy
controls and 35 lung cancer patients.
| A, Expression ratio
of MMP-1/CD63 |
|---|
|
|---|
|
| Healthy
control | LC |
|---|
| Characteristic
Age |
|
|
|---|
| Total | Male | Female | Total | Male | Female |
|---|
| ≤60 years | 0.87
(0.48–1.22) | 0.63
(0.46–1.04) | 0.56
(0.43–0.91) | 2.12
(1.04–4.89)a | 1.25
(0.40–4.29) | 2.32
(1.91–6.69)b |
| >60 years | 0.57
(0.39–1.00) | 0.63
(0.42–1.02) | 0.50
(0.35–1.03) | 1.11
(0.82–2.39)b | 0.91
(0.70–2.17) | 1.97
(1.02–3.61)a |
| Total | 0.60
(0.08–1.62) | 0.63
(0.46–1.01) | 0.56
(0.43–0.91) | 1.34
(0.89–1.97)c | 0.96
(0.81–1.92)d | 1.97
(0.91–4.01)c |
|
| B, Relative
expression of miRNA-21 |
|
|
| Healthy
control | LC |
| Characteristic
Age |
|
|
| Total | Male | Female | Total | Male | Female |
|
| ≤60 years | 1.08
(0.48–1.94) | 1.22
(0.89–1.95) | 0.87
(0.43–3.07) | 0.30
(0.01–1.87) | 0.001 (0–1.85) | 0.59
(0.01–1.94) |
| >60 years | 1.23
(0.50–2.25) | 1.39
(0.39–2.68) | 0.90
(0.52–1.75) | 1.31
(0.22–1.87) | 1.30
(0.17–1.68) | 1.71
(0.23–5.27) |
| Total | 1.16 (0–12.8) | 1.29
(0.75–1.99) | 0.87
(0.49–1.68) | 1.30
(0.33–1.55) | 1.18
(0.04–1.55) | 1.55
(0.01–6.23) |
|
| C, Relative
expression of miRNA-486-5p |
|
|
| Healthy
control | LC |
| Characteristic
Age |
|
|
| Total | Male | Female | Total | Male | Female |
|
| ≤60 years | 1.11
(0.77–2.24) | 0.85
(0.57–0.94) | 2.51
(1.32–3.27)e | 1.84
(0.35–3.16) | 2.46
(1.21–2.87)a | 0.46
(0.01–4.01) |
| >60 years | 0.96
(0.32–1.63) | 1.52
(0.83–2.35) | 0.44
(0.18–1.06)f,g | 2.17
(0.84–4.83)a | 2.67
(1.51–5.34)a | 0.65
(0.33–1.78)g |
| Total | 1.04
(0.02–8.13) | 0.93
(0.79–1.53) | 1.25
(0.47–2.51) | 2.17
(1.12–1.86) | 2.565
(1.80–4.42)d | 0.46
(0.06–4.01) |
Expression ratio of MMP-1/CD63 in
patients with LC based on the cancer stage
The expression ratio of MMP-1/CD63 in 20 patients
with stage 0 and I was 1.342 (95% CI: 0.888–2.319), which was
significantly higher than that in 40 healthy controls (P=0.0074).
The expression ratio of MMP-1/CD63 in 5 patients with stage II was
1.795 (95% CI: 1.105–4.414), which was higher than that in 40
healthy controls (P=0.0192), as depicted in Fig. 1D (all), 1E (males), and 1F
(females). These data suggest that the expression ratio of
MMP-1/CD63 is a useful biomarker for early stage patients with LC
including both sex.
Expression ratio of MMP-1/CD63 in 35
patients with LC based on the cancer metastasis
Two cases (No. 13 and 32) revealed M1a, their
expression ratio of MMP-1/CD63 were 0.41 and 0.88. Three M1b cases
(No. 14, 18 and 29) showed 0.40, 5.03 and 0.39, respectively. The
rest two M1c cases (No. 17 and 21) revealed 4.01 and 0.27. Among 7
metastasis cases, two cases showed the marked increased value.
Expression ratio of MMP-1/CD63 based
on pathology and smoking habits
The expression ratio of MMP-1/CD63 in 11 patients
who were never-smokers was higher (2.32; 95% CI: 1.80–4.29) than
that in 14 current smokers (1.01; 95% CI: 0.43–4.03) and in 10
former smokers (0.90; 95% CI: 0.39–1.92), as presented in Table IIIA, Fig. 1G (all), Fig. 1H (males), and Fig. 1I (females), although our preliminary
study did not show the statistical significance.
 | Table III.Comparison of MMP-1/CD63 and
miRNA-486-5p expression based on smoking status and cancer type in
lung cancer patients. |
Table III.
Comparison of MMP-1/CD63 and
miRNA-486-5p expression based on smoking status and cancer type in
lung cancer patients.
| A, Comparison of
MMP-1/CD63 expression based on smoking status and cancer type in
lung cancer patients |
|---|
|
|---|
|
| Total | Current | Former | Never |
|---|
|
|
|
|
|
|
|---|
| Types of lung
cancer | n | Median (95%
CI) | n | Median (95%
CI) | n | Median (95%
CI) | n | Median (95%
CI) |
|---|
| Non-small cell lung
cancer | 30 | 1.30
(0.84–2.35) | 10 | 1.01
(0.88–1.32) | 8 | 0.85
(0.54–1.16) | 12 | 2.15
(1.88–4.01) |
|
Adenocarcinoma | 23 | 1.25
(0.71–2.34) | 4 | 0.90
(0.76–0.99) | 8 | 0.85
(0.54–1.16) | 11 | 2.32
(1.85–4.02) |
|
Squamous cell carcinoma | 6 | 1.66
(1.01–2.31) | 5 | 1.34
(0.89–2.42) | 0 |
| 1 | 1.97 |
| Large
cell carcinoma | 1 | 1.11 | 1 | 1.11 | 0 |
| 0 |
|
| Small cell
carcinoma | 4 | 2.52
(0.84–4.28) | 3 | 4.03
(2.19–4.53) | 1 | 1.01 | 0 |
|
| Sarcoma
(pleomorphic) | 1 | 1.41
(0.98–2.82) | 0 |
| 1 | 1.49 | 0 |
|
|
| B, Comparison of
miRNA-486-5p expression based on smoking status and cancer type in
lung cancer patients |
|
|
| Total | Current | Former | Never |
|
|
|
|
|
|
| Types of lung
cancer | n | Median (95%
CI) | n | Median (95%
CI) | n | Median (95%
CI) | n | Median (95%
CI) |
|
| Non-small cell lung
cancer | 30 | 2.11
(0.56–3.72) | 10 | 2.11
(1.14–2.82) | 8 | 2.16
(1.02–4.62) | 12 | 1.50
(0.36–2.85) |
| Adenocarcinoma | 23 | 2.17
(0.45–4.21) | 4 | 2.64
(2.11–3.51) | 8 | 2.16
(1.02–4.62) | 11 | 2.08
(0.35–3.23) |
| Squamous cell
carcinoma | 6 | 1.36
(0.86–2.45) | 5 | 1.80
(0.84–2.67) | 0 |
| 1 | 0.91 |
| Large cell
carcinoma | 1 | 1.12 | 1 | 1.12 | 0 |
| 0 |
|
| Small cell
carcinoma | 4 | 3.05
(2.33–3.83) | 3 | 2.83
(1.83–4.17) | 1 | 3.27 | 0 |
|
| Sarcoma
(pleomorphic) | 1 | 6.70 | 0 |
| 1 | 6.70 | 0 |
|
Eleven never-smokers (4 males and 7 females), among
whom the expression ratio of MMP-1/CD63 was higher than 1.25 in 9
patients (3 males and 6 females). Interestingly, all had AC and 67%
(6/9) were female patients with AC. The ratio was slightly higher
in the AC group than that in the SC group (Table IIIA).
Relative expression levels of miRNA-21
and miRNA-486-5p in patients with LC and healthy controls
We selected miRNA-21 and miRNA-486-5p as a candidate
screening biomarker as they have been reported to be effective in
diagnosis/screening for LC using the circulating plasma exosomes
(16–21).
Relative expression levels of miRNA-21
in patients with LC and healthy controls
The relative expression level of miRNA-21 in healthy
controls was slightly higher in both of male and female over 60
years healthy controls compared with those in both sex under 60
years healthy controls, although not significantly; the expression
level was higher in healthy males than females, although not
significantly (Table IIB).
The expression level of miRNA-21 in male patients
with LC was not significantly different from those in male healthy
controls (Table IIB). The same
tendency was seen in female patients with LC compared with those in
female healthy controls (Fig. 2A-C;
Table IIB). The expression levels
of miRNA-21 were not significantly different from those of healthy
controls for pathology and smoking habits (Fig. 2D-I).
Relative expression levels
miRNA-486-5p in patients with LC and healthy controls
The relative expression level of miRNA-486-5p in
under 60 years healthy males was significantly lower than that in
under 60 years healthy females (P<0.01), while significantly
higher in over 60 years healthy males than their healthy females
counterparts (P<0.05; Table
IIC).
The distribution pattern of the relative expression
levels of miRNA-486-5p in male patients with LC compared with those
in healthy males was up-regulated, while those in females patients
with LC compared with those in healthy controls was down-regulated
(Fig. 3A-C). These data show the
sex difference in the relative expression levels of
miRNA-486-5p.
Then we measured the relative expression levels of
miRNA-486-5p in healthy controls and patients with LC by sex
separately; the individual relative expression levels of
miRNA-486-5p are listed in Tables I
and SII.
The median expression levels of miRNA-486-5p in male
patients with LC was 2.565 (95% CI: 1.800–4.420), which was
significantly higher than that in male healthy controls (1.254, 95%
CI: 0.790–1.530, P=0.0004). The median relative expression levels
in female patients with LC (0.460, 95% CI: 0.060–2.130) tending to
be lower than that in female healthy controls (1.250, 95% CI:
0.470–2.510, P=0.1566), as shown in Fig. 3A-C.
The results of miRNA-21 and miRNA-486-5p
significantly varied; we concluded that the relative expression of
miRNA-486-5p is a useful screening method for LC at work-place and
community health centers.
Signature of miRNA-486-5p in patients
with LC based on cancer stages
The expression levels of miRNA-486-5p in stage I and
IV all patients with LC were shown in Fig. 3D, and those of male patients
slightly higher than those in the healthy male controls (Fig. 3E), whereas the relative expression
of miRNA-486-5p tended to be lower in stage I female patients with
LC than in healthy female controls (Fig. 3F). That is, miRNA-486-5p showed
sex-difference, up-regulation in males and down-regulation in
female patients with LC.
Relative expression of miR-486-5p in
35 Patients with LC based on the cancer metastasis
Two cases (No. 13 and 32) revealed M1a, their
expression ratio of MMP-1/CD63 were 0.30 and 5.44. Three M1b cases
(No. 14, 18 and 29) showed 1.21, 5.52 and 4.42, respectively. The
rest two M1c cases (No. 17 and 21) revealed 7.59 and 11.7. Among 7
metastasis cases, six cases showed the extremely very high
increased value.
Expression levels of miRNA-486-5p
based on smoking habits
Never-smoker female patients with LC showed low
expression levels of miRNA-486-5p, except one case. In contrast,
among the 4 never-smoker male patients with LC, two had high and
two had low miRNA-486-5p expression levels. The relative expression
of miRNA-486-5p was not related to smoking habits (Fig. 3G-I).
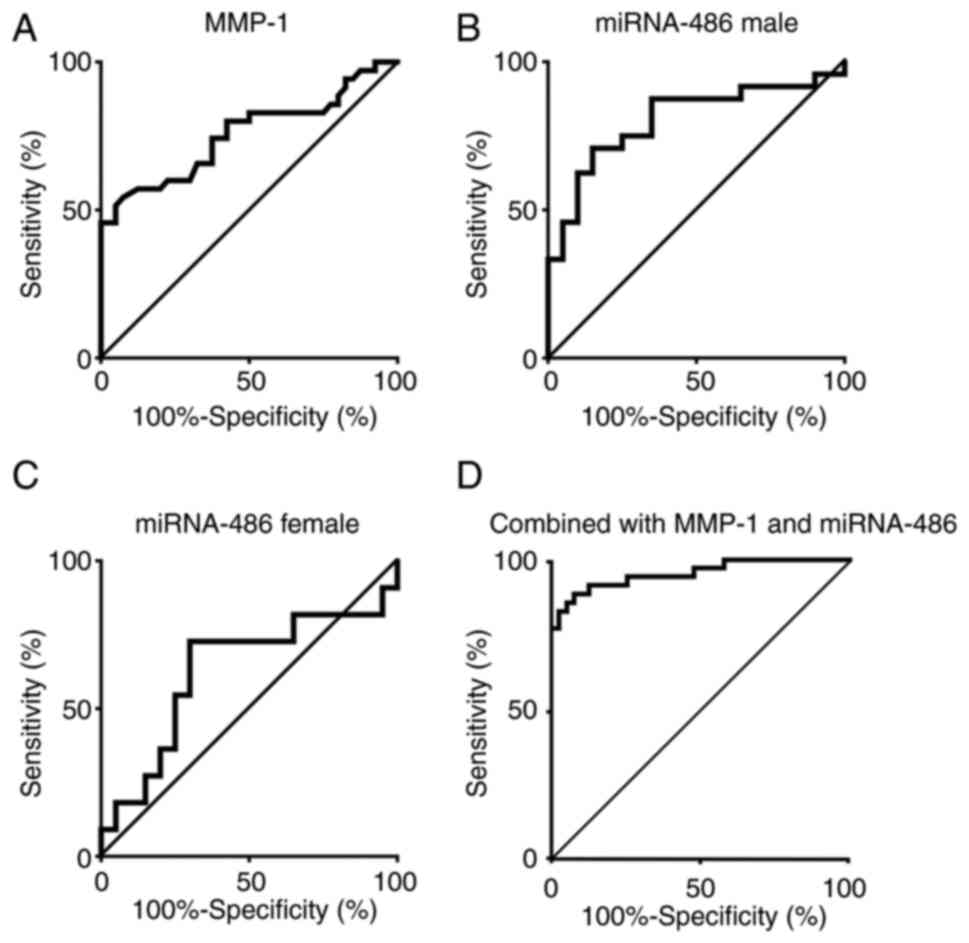
Receiver operating characteristics
analysis
The sensitivity and specificity of MMP-1/CD63 as
a primary screening approach for LC
The AUC of MMP-1/CD63 in the patients with LC was
0.755 (95% CI 0.640–0.871), and the cut-off value was 1.25
(Fig. 4A). The sensitivity and
specificity of MMP-1/CD63 expression were 0.925 and 0.543,
respectively.
The sensitivity and specificity of
miRNA-486-5p as a primary screening approach for LC based on
sex
The AUC of miRNA-486-5p in male patients with LC was
0.801 (95% CI 0.669–0.939), that in female patients with LC was
0.627 (95% CI 0.396–0.858); the cutoff values were 2.14 and 0.91,
respectively (Fig. 4B and C). The
sensitivity and specificity of miRNA-486-5p in the male patients
with LC were 0.850 and 0.708, respectively, and those in the female
patients with LC were 0.700 and 0.727, respectively.
ROC analysis of combined biomarkers of
MMP-1/CD63 and miRNA-486-5p
Both the biomarkers of the expression ratio of
MMP-1/CD63 and the relative expression of miRNA-486-5p in the
urinary exosomes improved ROC analysis: 86% sensitivity and 85%
specificity calculated using the cut-off value of 1.25 for
MMP-1/CD63, 2.14 in males and 0.91 for females for miRNA-486-5p
against 35 patients with LC and 40 healthy controls in the present
study as one cohort.
Logistic regression analysis for the
usefulness of both biomarkers in LC screening: The association
between the presence of lung cancer and the expression ratio for
MMP-1/CD63, and relative expression of miRNA-486-5p
The association between the expression ratio for
MMP-1/CD63 and the presence of lung cancer showed an odds ratio
(OR)=31.70 (95% CI: 4.12–243.67), while that between the relative
expression of miRNA-486-5p and the presence of lung cancer had an
OR=1.56 (95% CI:1.01–2.42) after adjusting with age, sex, and
smoking status (Table IV). AUROC
of the logistic regression model including MMP-1/CD63 and
miRNA-486-5p was 0.954 (95% CI: 0.908–1.000), with 89% sensitivity
and 88% specificity using the cut-off value of 0.48 (Fig. 4D).
 | Table IV.Logistic regression analysis between
lung cancer, and smoking status, MMP-1/CD63 and
microRNA-486-5p. |
Table IV.
Logistic regression analysis between
lung cancer, and smoking status, MMP-1/CD63 and
microRNA-486-5p.
| Characteristic | Regression
coefficient | SE | Wald | P-value | Odds ratio | 95% CI |
|---|
| Age (continuous
variable) | 0.108 | 0.045 | 5.677 | 0.017 | 1.11 | 1.02–1.22 |
| Sex (male) | −0.380 | 1.069 | 0.127 | 0.722 | 0.68 | 0.08–5.55 |
| Smoking status
(current and former smoking) | 4.158 | 1.361 | 9.327 | 0.002 | 63.94 | 4.44–921.85 |
| MMP-1/CD63
(continuous variable) | 3.456 | 1.041 | 11.031 | 0.001 | 31.70 | 4.12–243.67 |
| microRNA-486-5p
(continuous variable) | 0.444 | 0.224 | 3.932 | 0.047 | 1.56 | 1.01–2.42 |
| const | −13.663 | 3.935 | 12.053 | 0.001 | 0.000 |
|
The relative expression of miRNA-486-5p is a
supportive biomarker for the expression ratio of MMP-1/CD63, which
may contribute to the better specificity.
Discussion
To the best of our knowledge, this is the first
study that used urinary exosome-derived MMP-1 and miRNA for early
LC screening. We demonstrated that the combined screening for both
biomarkers of MMP-1/CD63 and miRNA-486-5p targeting urinary
exosomes is useful in detecting early-stage LC. Our purpose was to
develop a universal and practical screening method for early-stage
LC for job workers and community residents to enable a simple, easy
to access, cost-effective screening of LC and requires only a small
volume of urine.
The expression ratio of MMP-1/CD63 did not show a
sex-based difference in the present study; the ratio increased in
both male and female patients with LC, as observed in breast cancer
patients (6). In 1998, Rutter et
al reported a single nucleotide polymorphism (SNP) at −1607 bp
in the MMP-1 promoter region, where an additional guanine
(G) created an erythroblast transformation specific (Ets) binding
site, 5′-GGA-3′ (12). This SNP was
associated with transcriptional and protein/DNA binding activities;
hence its frequency was investigated in tumor cells. Subsequent
studies reported a close relationship between the MMP-1
promoter SNP and the risk of LC (13,14).
Sauter et al found that MMP-1 SNPs are related to an
increased risk of early onset of LC; this risk is further worsened
by smoking in patients who are younger than 51 years of age
(15). Moreover, increased MMP-1
levels in serum or plasma (24–26)
and increased expression of MMP-1 in the tumor tissues of patients
with LC was observed by immunohistochemical staining (26).
Therefore, we consider that the expression ratio of
MMP-1/CD 63 in the urinary exosomes of the patients with LC is a
useful biomarker for LC screening, and the present study reports
the upregulation of MMP-1 in the urinary exosomes in early-stage
patients with LC. MMP-1 upregulation in LC can be affected by
environmental and/or genetic factors, such as signal transducer and
activator of transcription 3 (27),
extracellular signal-regulated kinases/mitogen-activated protein
kinases pathways in the epidermal growth factor receptor (EGFR)
ligands, and their signaling pathways (28). The Capicua (CIC) repressor (29–34) is
a similar factor. The missense, nonsense, insertion, deletion, and
splice sites for the CIC repressor have been reported (29–34),
but the direct relationship between CIC and upregulation of MMP-1
expression in LC has not yet been determined. CIC is a high
mobility group-box transcriptional repressor, and its target genes
include the polyoma enhancer activator 3, whose group Ets
translocation variants (ETVs) have been well characterized
(29–32). A recent report revealed the
progression of hepatocellular carcinoma related to the
CIC-ETV4-MMP-1 axis (33). We have
previously reported the increased expression ratio of MMP-1/CD63 in
breast cancer patients including early-stage of cancer (6), while the data after operation were
low, although high value was observed in the case with metastasis
after operation (6). The CIC
repressor involvement in Ewing sarcoma, melanoma, prostate-,
breast-, lung-, gastric-, or colon cancer has not been fully
verified. Since CIC in Drosophila plays an important role in
terminal and dorsoventral patterning (30), similar genes are supposed in human
(33,34).
Saito et al demonstrated that the
EGFR-tyrosine kinase inhibitor (TKI)-resistant AC showed strong
MMP-1-positive staining with a significantly independent poor
prognosis (35). However, once
MMP-1 expression is upregulated, the cell phenotype may change in
many organs associated with the hedgehog protein and/or
epithelial-mesenchymal transition, thereby leading to
carcinogenesis (35,36). This suggests that MMP-1-positive
cancer patients have a poor prognosis, as reported in the case of
colorectal cancer (37). Our
screening tests for early-stage LC illustrated the usefulness of
urinary exosomes and can clarify the relationship between CIC and
LC occurrence and metastases in future studies. Furthermore,
combined screening of MMP-1/CD 63 and miRNA-486-5p using urinary
exosomes as a target could detect LC more accurately.
The miRNA-21 was useful for breast cancer screening
and can modulate the expression of cancer suppressor genes such as
PTEN, HOXD10, TPM1 and mapin (a tumor-suppressing
serpin protein) (6,17–19).
However, several patients with LC are over the age of 70 years;
therefore; hence, relative expression of miRNA-21 in such patients
could not be discriminated from those of the healthy controls among
the aged individuals. Therefore, we focused on miRNA-486-5p, which
targets the PIK3R1 gene, a suppressor of tumor growth
(20,21). Surprisingly, miRNA-486-5p showed sex
differences here but not in previous reported studies. The obtained
signature of miRNA-486-5p in this study is not consistent with the
literature (20,21). ElKhouly et al (20) reported that miRNA-486-5p is
multifaceted, thereby reflecting the disease conditions on the date
of examination. These high-age limitation have not been previously
reported in studies on miRNAs in patients with LC (38–44).
However, recent reports on miRNA-486 suggest that dysregulation of
miRNAs may affect sex hormone signaling via modulation of estrogen
receptors and through miRNAs (45,46).
Our results suggest that the relative expression of miRNA-486-5p
was a useful biomarker for LC screening at work-place and community
health centers, with a sensitivity and specificity of nearly 90%.
Moreover, the relative expression of miRNA-486-5p increased at the
early stage of LC. Although miRNA-486-5p was increased in AC and
SCC, the association with smoking habits was not expected. Saito
et al reported that the number of cases with high MMP-1
immunoreactivity was significantly higher in smokers (26/54 cases)
than non-smokers (8/27 cases) (35), which is inconsistent with our
findings, showing higher expression of MMP-1 in never-smoker
patients with AC. However, Pao et al reported frequently
observed point mutations in codon 858 (exon 21) in
EGFR-TKI-sensitive never-smokers patients (47). The results of the ROC analysis for
logistic regression model suggests that MMP-1/CD63 and miNA-486-5p
could predict lung cancer, although further studies are
warranted.
Isolation of exosomes from urine and miRNA analysis
may be less methodologically established than isolation from serum
or plasma. Hence, it may be difficult to normalize for
urine-derived exosomes. One reason for this is the difficulty in
selecting appropriate miRNAs as controls for body fluid samples,
including urine (48,49). In this study, we did not use
internal controls such as small RNAs such as U6 and 5S rRNA, which
are commonly used in miRNA analysis (50,51).
Since these small RNAs are structurally different from miRNAs in
urinary exosome-derived miRNA expression analysis, it is unclear
whether they can be used as generic internal controls like GAPDH
and β-actin in mRNA expression analysis. In our preliminary study,
the measurement of internal standard miRNAs in exosomes did not
work well. We next investigated the use of externally added miRNAs
as internal standards. However, since RNA added externally as an
internal control was not derived from exosomes extracted from
patients but from RNA synthesized during RNA extraction (50,51),
we considered the external addition of RNA to be insufficient as a
control in this study. For example, U6 RNA is not appropriate in
patient with liver fibrosis, suggesting that it may not function as
an intrinsic control depending on the type of disease complicating
the patient (52). Furthermore, we
believe that externally added miRNAs cannot eliminate the effects
of contamination and the risk of degradation. Furthermore, simple
analysis using an electron microscope revealed no differences in
the shape, size, or number of urine-derived exosomes per urine
volume. Therefore, we believe that there are no major differences
in the number of exosomes or the variety of miRNA pools between
each sample. Therefore, in this study, exosomes were extracted from
2 ml of urine and all RNA was recovered; 5 ng of RNA was used for
reverse transcription, after which the CT values of the target
miRNAs were measured; individual samples from LC and healthy
controls were normalized using the mean CT values of healthy
controls. In other words, they were compared to patients based on
the healthy control mean expression levels of the target miRNAs.
Although this method has been reported in previous reports
(6), this study, which did not use
internal controls for miRNAs, is more controversial in the
normalization of urine-derived exosomes. It is hoped that more
appropriate methods for internal control of urinary exosomes will
be established in the future.
This study has several limitations. The study is
preliminary, the population is not large, and it has not been
conducted on exosome characteristics other than EM. Future studies
should identify more precise characteristics on exosomes.
Global statistics estimate that approximately 25% of
patients with LC are never smokers (53). Therefore, our screening method can
help identify numbers of the early-staged patients with LC that are
never-smokers but exposed to passive smoking. In Asian countries,
there are never-smoker female patients with LC, which is a major
challenge that our novel screening method can address.
Our study proposed a new screening method useful for
workers who cannot visit a health-check clinic for cancer screening
due to busy work schedules and/or economic limitations.
We are presently investigating the collection of
information on the false-positive cases in at least 500 visitors
for the annual health checks. Further studies with a larger cohort
of patients with LC are needed to validate our findings.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Japanese Society for the
Promotion of Science (Kakenhi grant no. 18K08797) given to SI and
YK.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WA, MS, TU, SI and IO designed and conducted the
study. WA and MS analyzed the data. WA, MS and IO confirm the
authenticity of all the raw data. HY confirmed morphological
analysis. WA and MS performed statistical analysis. Logistic
regression analysis was done by HF. WA, MS, SI, HY, YK, KO, TN, SE,
HT, and IO interpreted and discussed the data. WA, MS, HF and IO
wrote the draft of this manuscript. SI, HY, YK, KO, TN, SE and HT
edited the paper for English grammar and writing. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by ‘The Ethics Committee of
IUHW Hospital’ (#13-B-299 IUHW on May 24, 2018; last updated August
7, 2020), and ‘The ethical committee of Kitasato University Medical
Center’ (#28–51 Kitasato University Medical Center on March 27,
2017) and conformed to the principles of the 1975 Declaration of
Helsinki, as reflected by its prior approval by the institution's
human research committee. All the study participants provided
written informed consent. All participants signed informed consent
for participation in this study.
Patient consent for publication
All participants signed informed consent to publish
the results in peer review journal.
Authors' information
IO published a research paper in Nature (1974)
entitled ‘Collagenase activity in experimental hepatic fibrosis’,
and continued matrix metalloproteinase study. The year before last
year he published original paper as a first author entitled
‘Sequential matrix metalloproteinase-1 expression triggered by
infiltrating monocytic lineage cells modulates pathophysiological
aspects of human nonalcoholic steatohepatitis, Metalloproteinases
in Medicine 2020:7 1–13’.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AC
|
adenocarcinoma
|
|
AUC
|
area under the receiver operating
characteristic curve
|
|
CIC
|
Capicua
|
|
EGFR
|
epidermal growth factor receptor
|
|
Ets
|
erythroblast transformation
specific
|
|
ETVs
|
Ets translocation variants
|
|
LC
|
lung cancer
|
|
LCC
|
large cell carcinoma
|
|
miRNA
|
microRNA
|
|
MMP-1
|
matrix metalloproteinase-1
|
|
OR
|
odds ratio
|
|
ROC
|
receiver operating characteristic
|
|
RT-PCR
|
reverse transcription polymerase
chain reaction
|
|
SCLC
|
small cell lung cancer
|
|
SC
|
squamous cell carcinoma
|
|
TKI
|
tyrosine kinase inhibitor
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Cancer Center, . Latest Cancer
Statistics. https://ganjoho.jp/reg_stat/statistics/stat/summary.htmlFebruary
24–2023(In Japanese).
|
|
3
|
Koike T, Yamato Y, Asamura H, Tsuchiya R,
Sohara Y, Eguchi K, Mori M, Nakanishi Y, Goya T, Koshiishi Y, et
al: Improvements in surgical results for lung cancer from 1989 to
1999 in Japan. J Thorac Oncol. 4:1364–1369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wood DE, Kazerooni EA, Baum SL, Eapen GA,
Ettinger DS, Hou L, Jackman DM, Klippenstein D, Kumar R, Lackner
RP, et al: Lung cancer screening, version 3.2018, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
16:412–441. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gavelli G and Giampalma E: Sensitivity and
specificity of chest X-ray screening for lung cancer: Review
article. Cancer. 89 (11 Suppl):S2453–S2456. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ando W, Kikuchi K, Uematsu T, Yokomori H,
Takaki T, Sogabe M, Kohgo Y, Otori K, Ishikawa S and Okazaki I:
Novel breast cancer screening: Combined expression of miR-21 and
MMP-1 in urinary exosomes detects 95% of breast cancer without
metastasis. Sci Rep. 9:135952019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anastasiadou E and Slack FJ: Cancer.
Malicious exosomes. Science. 346:1459–1460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalluri R: The biology and function of
exosomes in cancer. J Clin Invest. 126:1208–1215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L and Yu D: Exosomes in cancer
development, metastasis, and immunity. Biochim Biophys Acta Rev
Cancer. 1871:455–468. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okazaki I and Nabeshima K: Introduction:
MMPs, ADAMs/ADAMTSs research products to achieve big dream.
Anticancer Agents Med Chem. 12:688–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rutter JL, Mitchell TI, Butticè G, Meyers
J, Gusella JF, Ozelius LJ and Brinckerhoff CE: A single nucleotide
polymorphism in the matrix metalloproteinase-1 promoter creates an
Ets binding site and augments transcription. Cancer Res.
58:5321–5325. 1998.PubMed/NCBI
|
|
13
|
Zhu Y, Spitz MR, Lei L, Mills GB and Wu X:
A single nucleotide polymorphism in the matrix metalloproteinase-1
promoter enhances lung cancer susceptibility. Cancer Res.
61:7825–7829. 2001.PubMed/NCBI
|
|
14
|
Su L, Zhou W, Park S, Wain JC, Lynch TJ,
Liu G and Christiani DC: Matrix metalloproteinase-1 promoter
polymorphism and lung cancer risk. Cancer Epidemiol Biomarkers
Prev. 14:567–570. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sauter W, Rosenberger A, Beckmann L, Kropp
S, Mittelstrass K, Timofeeva M, Wölke G, Steinwachs A, Scheiner D,
Meese E, et al: Matrix metalloproteinase 1 (MMP1) is associated
with early-onset lung cancer. Cancer Epidemiol Biomarkers Prev.
17:1127–1135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen J, Liu Z, Todd NW, Zhang H, Liao J,
Yu L, Guarnera MA, Li R, Cai L, Zhan M and Jiang F: Diagnosis of
lung cancer in individuals with solitary pulmonary nodules by
plasma microRNA biomarkers. BMC Cancer. 11:3742011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu LF, Wu ZP, Chen Y, Zhu QS, Hamidi S and
Navab R: MicroRNA-21 (miR-21) regulates cellular proliferation,
invasion, migration, and apoptosis by targeting PTEN, RECK and
Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS One.
9:e1036982014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu S, Si ML, Wu H and Mo YY: MicroRNA-21
targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol
Chem. 282:14328–14336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Frankel LB, Christoffersen NR, Jacobsen A,
Lindow M, Krogh A and Lund AH: Programmed cell death 4 (PDCD4) is
an important functional target of the microRNA miR-21 in breast
cancer cells. J Biol Chem. 283:1026–1033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
ElKhouly AM, Youness RA and Gad MZ:
MicroRNA-486-5p and microRNA-486-3p: Multifaceted pleiotropic
mediators in oncological and non-oncological conditions. Noncoding
RNA Res. 5:11–21. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian F, Wang J, Ouyang T, Lu N, Lu J, Shen
Y, Bai Y, Xie X and Ge Q: MiR-486-5p serves as a good biomarker in
nonsmall cell lung cancer and suppresses cell growth with the
involvement of a target PIK3R1. Front Genet. 10:6882019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH image to ImageJ: 25 Years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schutz A, Schneidenbach D, Aust G,
Tannapfel A, Steinert M and Wittekind C: Differential expression
and activity status of MMP-1, MMP-2 and MMP-9 in tumor and stromal
cells of squamous cell carcinomas of the lung. Tumour Biol.
23:179–184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
An HJ, Lee YJ, Hong SA, Kim JO, Lee KY,
Kim YK, Park JK and Kang JH: The prognostic role of tissue and
serum MMP-1 and TIMP-1 expression in patients with non-small cell
lung cancer. Pathol Res Pract. 212:357–364. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li M, Xiao T, Zhang Y, Feng L, Lin D, Liu
Y, Mao Y, Guo S, Han N, Di X, et al: Prognostic significance of
matrix metalloproteinase-1 levels in peripheral plasma and tumour
tissues of lung cancer patients. Lung Cancer. 69:341–347. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schütz A, Röser K, Klitzsch J, Lieder F,
Aberger F, Gruber W, Mueller KM, Pupyshev A, Moriggl R and
Friedrich K: Lung adenocarcinomas and lung cancer cell lines show
association of MMP-1 expression with STAT3 activation. Transl
Oncol. 8:97–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park S, Jung HH, Park YH, Ahn JS and Im
YH: ERK/MAPK pathways play critical roles in EGFR ligands-induced
MMP1 expression. Biochem Biophys Res Commun. 407:680–686. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanaka M, Yoshimoto T and Nakamura T: A
double-edged sword: The world according to Capicua in cancer.
Cancer Sci. 108:2319–2325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiménez G, Shvartsman SY and Paroush Z:
The Capicua repressor-a general sensor of RTK signaling in
development and disease. J Cell Sci. 125:1383–1391. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee Y, Fryer JD, Kang H, Crespo-Barreto J,
Bowman AB, Gao Y, Kahle JJ, Hong JS, Kheradmand F, Orr HT, et al:
ATXN1 protein family and CIC regulate extracellular matrix
remodeling and lung alveolarization. Dev Cell. 21:746–757. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okimoto RA, Breitenbuecher F, Olivas VR,
Wu W, Gini B, Hofree M, Asthana S, Hrustanovic G, Flanagan J,
Tulpule A, et al: Inactivation of Capicua drives cancer metastasis.
Nat Genet. 49:87–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim E, Kim D, Lee JS, Yoe J, Park J, Kim
CJ, Jeong D, Kim S and Lee Y: Capicua suppresses hepatocellular
carcinoma progression by controlling the ETV4-MMP1 axis.
Hepatology. 67:2287–2301. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiménez G, Guichet A, Ephrussi A and
Casanova J: Relief of gene repression by torso RTK signaling: Role
of capicua in Drosophila terminal and dorsoventral
patterning. Genes Dev. 14:224–231. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saito R, Miki Y, Ishida N, Inoue C,
Kobayashi M, Hata S, Yamada-Okabe H, Okada Y and Sasano H: The
significance of MMP-1 in EGFR-TKI-resistant lung adenocarcinoma:
Potential for therapeutic targeting. Int J Mol Sci. 19:6092018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Niland S, Riscanevo AX and Eble JA: Matrix
metalloproteinases shape the tumor microenvironment in cancer
progression. Int J Mol Sci. 23:1462021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Murray GI, Duncan ME, O'Neil P, Melvin WT
and Fothergill JE: Matrix metalloproteinase-1 is associated with
poor prognosis in colorectal cancer. Nat Med. 2:461–462. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cazzoli R, Buttitta F, Di Nicola M,
Malatesta S, Marchetti A, Rom WN and Pass HI: microRNAs derived
from circulating exosomes as noninvasive biomarkers for screening
and diagnosing lung cancer. J Thorac Oncol. 8:1156–1162. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou X, Wen W, Shan X, Zhu W, Xu J, Guo R,
Cheng W, Wang F, Qi LW, Chen Y, et al: A six-microRNA panel in
plasma was identified as a potential biomarker for lung
adenocarcinoma diagnosis. Oncotarget. 8:6513–6525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang L, Shan X, Wang J, Zhu J, Huang Z,
Zhang H, Zhou X, Cheng W, Shu Y, Zhu W and Liu P: A three-microRNA
signature for lung squamous cell carcinoma diagnosis in Chinese
male patients. Oncotarget. 8:86897–86907. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dejima H, Iinuma H, Kanaoka R, Matsutani N
and Kawamura M: Exosomal microRNA in plasma as a non-invasive
biomarker for the recurrence of non-small cell lung cancer. Oncol
Lett. 13:1256–1263. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shan X, Zhang H, Zhang L, Zhou X, Wang T,
Zhang J, Shu Y, Zhu W, Wen W and Liu P: Identification of four
plasma microRNAs as potential biomarkers in the diagnosis of male
lung squamous cell carcinoma patients in China. Cancer Med.
7:2370–2381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Feng M, Zhao J, Wang L and Liu J:
Upregulated expression of serum exosomal micrornas as diagnostic
biomarkers of lung adenocarcinoma. Ann Clin Lab Sci. 48:712–718.
2018.PubMed/NCBI
|
|
44
|
Zhang Y, Zhang Y, Yin Y and Li S:
Detection of circulating exosomal miR-17-5p serves as a novel
non-invasive diagnostic marker for non-small cell lung cancer
patients. Pathol Res Pract. 215:1524662019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang R, Bhat-Nakshatri P, Zhong X, Zimmers
T and Nakshatri H: Hormonally regulated myogenic miR-486 influences
sex-specific differences in cancer-induced skeletal muscle defects.
Endocrinology. 162:bqab1422021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Thakur L and Thakur S: The interplay of
sex steroid hormones and microRNAs in endometrial cancer: Current
understanding and future directions. Front Endocrinol (Lausanne).
14:11669482023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pao W, Miller V, Zakowski M, Doherty J,
Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al:
EGF receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Matsuzaki K, Fujita K, Jingushi K,
Kawashima A, Ujike T, Nagahara A, Ueda Y, Tanigawa G, Yoshioka I,
Ueda K, et al: MiR-21-5p in urinary extracellular vesicles is a
novel biomarker of urothelial carcinoma. Oncotarget. 8:24668–24678.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yazarlou F, Mowla SJ, Oskooei VK,
Motevaseli E, Tooli LF, Afsharpad M, Nekoohesh L, Sanikhani NS,
Ghafouri-Fard S and Modarressi MH: Urine exosome gene expression of
cancer-testis antigens for prediction of bladder carcinoma. Cancer
Manag Res. 10:5373–5381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Occhipinti G, Giulietti M, Principato G
and Piva F: The choice of endogenous controls in exosomal microRNA
assessments from biofluids. Tumour Biol. 37:11657–11665. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu D, Di K, Fan B, Wu J, Gu X, Sun Y, Khan
A, Li P and Li Z: MicroRNAs in extracellular vesicles: Sorting
mechanisms, diagnostic value, isolation, and detection technology.
Front Bioeng Biotechnol. 10:9489592022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Benz F, Roderburg C, Vargas Cardenas D,
Vucur M, Gautheron J, Koch A, Zimmermann H, Janssen J,
Nieuwenhuijsen L, Luedde M, et al: U6 is unsuitable for
normalization of serum miRNA levels in patients with sepsis or
liver fibrosis. Exp Mol Med. 45:e422013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Okazaki I, Ishikawa S, Ando W and Sohara
Y: Lung adenocarcinoma in never smokers: Problems of primary
prevention from aspects of susceptible genes and carcinogens.
Anticancer Res. 36:6207–6224. 2016. View Article : Google Scholar : PubMed/NCBI
|