Introduction
Wilms tumor (WT), also known as nephroblastoma, is
the most common pediatric malignancy of the kidney (1), and originates from poorly
differentiated mesenchymal kidney stem cells (2). It accounts for >90% of pediatric
renal tumors and 7% of all childhood cancers (3). In addition, most patients are
diagnosed at <5 years of age (4).
The current treatment strategies for WT include
surgery, chemotherapy and radiotherapy. Surgical procedures include
upfront nephrectomy as recommended by the Children's Oncology Group
and nephrectomy following chemotherapy according to the
International Society of Pediatric Oncology guidelines (5). The selection of chemotherapy drugs and
radiotherapy depends on the risk stratification of the patient
(5). In the last several decades,
the overall survival (OS) of patients with WT has steadily improved
in high-income countries and is ~90%, compared with <50% in
low-income countries (6).
Several prognostic factors have been identified for
WT, such as the tumor volume after preoperative chemotherapy
(7). However, few studies have
reported on the prognostic significance of primary tumor size.
Therefore, the aim of the present study was to evaluate the
association of primary tumor size with the clinicopathological
characteristics and survival of patients with WT.
Materials and methods
Study population
The data of 2,443 patients with WT diagnosed between
January 2000 and December 2017, inclusively, were retrieved from
the Surveillance, Epidemiology, and End Results (SEER) database.
The cases were filtered according to the following exclusion
criteria: i) Incomplete information on primary tumor size and ii)
lack of surgery. Based on these criteria, 1,523 eligible cases were
selected for inclusion in the present retrospective study.
Study variables
The covariates for each patient included demographic
characteristics, namely age, ethnicity and sex, and
clinicopathological characteristics, namely primary tumor size,
lymph node status, distant metastasis, the retention or removal of
regional lymph nodes and the type of surgery. The main endpoints
were OS and cancer-specific survival (CSS). OS was calculated from
the date of WT diagnosis to the date of death from any cause or the
date of censoring. CSS was calculated from the date of WT diagnosis
to the date of death due to this malignancy or the date of
censoring (8). All patients were
followed up until the date of death or until December 31, 2017. The
cause of death for each patient was obtained from the death
certificate.
Statistical analysis
The data were extracted from the SEER database using
SEER*Stat Software version 8.4.0 (Information Management Services,
Inc.). The optimal cut-off point of primary tumor size was
determined by receiver operating characteristic (ROC) curve
analysis. Chi-square test was used to compare demographic and
clinicopathological variables. The Kaplan-Meier method was used to
identify the factors that had a significant association with OS and
CSS, and to calculate survival probabilities in different groups,
and the log-rank test was used to compare survival rates. The
significant indicators identified by the Kaplan-Meier analyses were
included in a Cox proportional hazards regression model for
multivariate analysis. Statistical significance was determined by
calculating the hazard ratio (HR) and 95% confidence intervals (95%
CI). Forest plots were drawn using Excel 2019 (Microsoft
Corporation). Two-sided P<0.05 was considered to indicate a
statistically significant result. All statistical analyses were
conducted using SPSS 22.0 (IBM Corp.).
Results
Cut-off point identification of
primary tumor size
The area under the curve for primary tumor size in
the ROC curve analysis was 0.592 (95% CI 0.536–0.647), and the
optimal cut-off value was 11.15 cm (sensitivity, 59.7%;
specificity, 55.8%; P=0.001; Fig.
1). A total of 1,523 eligible patients with WT were included in
the study, of which 838 (55%) patients had a tumor size of
<11.15 cm and 685 (45%) patients had a tumor size of ≥11.15 cm.
The median follow-up period was 74 months (range 0–167 months).
During the time period of the study, 119 (7.8%) patients died, and
the cause of death was associated with WT for 102 patients.
Demographic and clinicopathological
characteristics
The associations of primary tumor size with
demographic and clinicopathological characteristics are summarized
in Table I. The analysis revealed a
significant association of age and ethnicity with primary tumor
size, and indicated that children <5 years old (P<0.001) and
of white ethnicity (P=0.048) were more susceptible to WT. However,
no significant difference was found in the prevalence of WT between
male and female patients (P=0.718). Moreover, lymph node metastasis
(P<0.001) and distant metastasis (P<0.001) were more frequent
in patients with WT and a tumor size of ≥11.15 cm than in those
with tumors <11.15 cm. In addition, regional lymph node removal
(P=0.018) and radical surgery (P<0.001) were more frequently
performed in patients with larger tumors.
 | Table I.Association of primary tumor size with
demographic and clinicopathological characteristics in patients
with Wilms tumor. |
Table I.
Association of primary tumor size with
demographic and clinicopathological characteristics in patients
with Wilms tumor.
| Variables | All patients, n
(%) | Tumor size <11.15
cm, n (%) | Tumor size ≥11.15 cm,
n (%) | P-value |
|---|
| No. of patients | 1,523 (100.0) | 838 (55.0) | 685 (45.0) |
|
| Age, years |
|
|
| <0.001 |
|
<5 | 1,090 (71.6) | 642 (76.6) | 448 (65.4) |
|
| ≥5 | 433 (28.4) | 196 (23.4) | 237 (34.6) |
|
| Ethnicity |
|
|
| 0.048 |
|
White | 1,149 (75.4) | 645 (77.0) | 504 (73.6) |
|
|
Black | 270 (17.7) | 131 (15.6) | 139 (20.3) |
|
|
Other | 104 (6.8) | 62 (7.4) | 42 (6.1) |
|
| Sex |
|
|
| 0.718 |
|
Female | 826 (54.2) | 451 (53.8) | 375 (54.7) |
|
| Male | 697 (45.8) | 387 (46.2) | 310 (45.3) |
|
| Lymph node
status |
|
|
| <0.001 |
|
Negative | 1,012 (66.4) | 571 (68.1) | 441 (64.4) |
|
|
Positive | 245 (16.1) | 101 (12.1) | 144 (21.0) |
|
|
Unknown | 266 (17.5) | 166 (19.8) | 100 (14.6) |
|
| Distant
metastasis |
|
|
| <0.001 |
| No | 1,185 (77.8) | 698 (83.3) | 487 (71.1) |
|
| Yes | 318 (20.9) | 126 (15.0) | 192 (28.0) |
|
|
Unknown | 20 (1.3) | 14 (1.7) | 6 (0.9) |
|
| Regional lymph node
removal |
|
|
| 0.018 |
| No | 269 (17.7) | 169 (20.2) | 100 (14.6) |
|
| Yes | 1,244 (81.7) | 664 (79.2) | 580 (84.7) |
|
|
Unknown | 10 (0.6) | 5 (0.6) | 5 (0.7) |
|
| Surgery |
|
|
| <0.001 |
|
Non-radical | 332 (21.8) | 211 (25.2) | 121 (17.7) |
|
|
Radical | 1,191 (78.2) | 627 (74.8) | 564 (82.3) |
|
Kaplan-Meier analyses predicting OS
and CSS
As shown in Table
II, patients with a tumor size ≥11.5 cm had significantly worse
OS and CSS than those with tumor size <11.15 cm (P=0.001 and
P<0.001, respectively). Moreover, significant associations with
OS and CSS were also found for age (P=0.002 and P=0.001,
respectively), lymph node status (P<0.001 for both) and distant
metastasis (P<0.001 for both). In addition, regional lymph node
removal was also significantly associated with OS (P=0.017). These
factors were included in a Cox proportional hazards regression
model for multivariate analysis.
 | Table II.Kaplan-Meier predictions of the
overall survival and cancer-specific survival of patients with
Wilms tumor. |
Table II.
Kaplan-Meier predictions of the
overall survival and cancer-specific survival of patients with
Wilms tumor.
|
| 5-Year overall
survival | 5-Year
cancer-specific survival |
|---|
|
|
|
|
|---|
| Variables | Probability, %
(SEM) | P-value | Probability, %
(SEM) | P-value |
|---|
| Age, years |
| 0.002 |
| 0.001 |
|
<5 | 94.0 (0.8) |
| 95.0 (0.7) |
|
| ≥5 | 88.6 (1.6) |
| 89.3 (1.6) |
|
| Ethnicity |
| 0.422 |
| 0.931 |
|
White | 92.7 (0.8) |
| 93.1 (0.8) |
|
|
Black | 91.3 (1.8) |
| 93.2 (1.7) |
|
|
Other | 92.2 (2.8) |
| 94.7 (2.6) |
|
| Sex |
| 0.345 |
| 0.448 |
|
Female | 92.9 (0.9) |
| 93.6 (0.9) |
|
|
Male | 91.9 (1.1) |
| 93.1 (1.0) |
|
| Lymph node
status |
| <0.001 |
| <0.001 |
|
Negative | 95.2 (0.7) |
| 96.1 (0.7) |
|
|
Positive | 84.6 (2.4) |
| 85.3 (2.4) |
|
|
Unknown | 89.4 (2.0) |
| 90.5 (1.9) |
|
| Distant
metastasis |
| <0.001 |
| <0.001 |
| No | 95.3 (0.7) |
| 96.1 (0.6) |
|
|
Yes | 82.6 (2.2) |
| 83.5 (2.2) |
|
|
Unknown | 83.3 (8.9) |
| 88.0 (8.1) |
|
| Regional lymph node
removal |
| 0.017 |
| 0.052 |
| No | 89.1 (2.0) |
| 90.2 (1.9) |
|
|
Yes | 93.3 (0.8) |
| 94.1 (0.7) |
|
|
Unknown | 87.5 (11.7) |
| 87.5 (11.7) |
|
| Surgery |
| 0.181 |
| 0.084 |
|
Non-radical | 94.0 (1.4) |
| 94.9 (1.3) |
|
|
Radical | 92.0 (0.8) |
| 92.9 (0.8) |
|
| Tumor size |
| 0.001 |
| <0.001 |
|
<11.15 cm | 94.4 (0.9) |
| 95.3 (0.8) |
|
| ≥11.15
cm | 90.2 (1.2) |
| 91.1 (1.1) |
|
Stratified Kaplan-Meier analyses
predicting OS and CSS
The stratified Kaplan-Meier analyses revealed that
tumor size ≥11.15 cm was significantly associated with worse OS and
CSS in the following subgroups: age ≥5 years (P=0.012 and P=0.003,
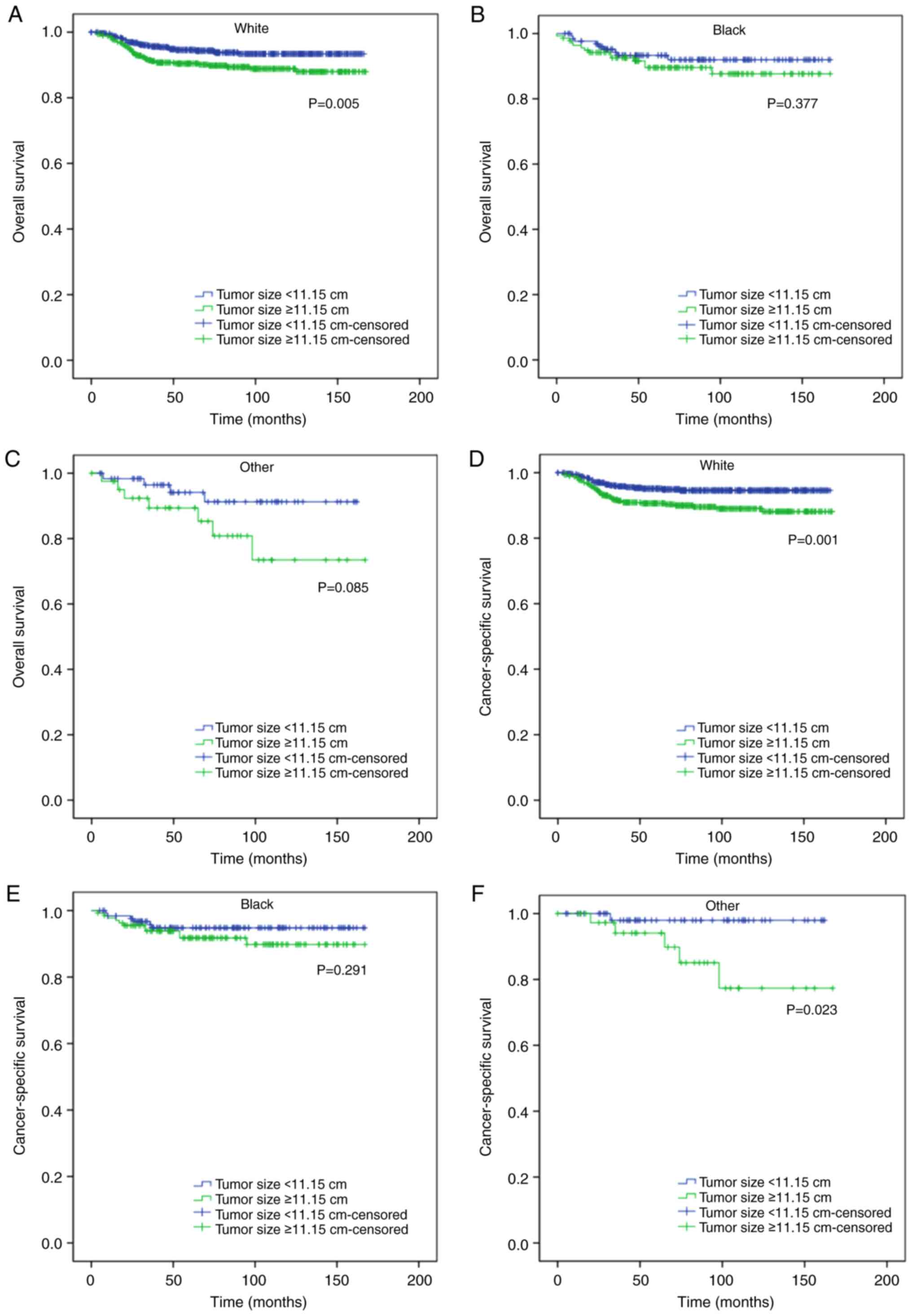
respectively; Fig. 2B and D), white
ethnicity (P=0.005 and P=0.001, respectively; Fig. 3A and D), male (P<0.001 for both;
Fig. 4B and D), no regional lymph
node removal (P=0.003 and P<0.001, respectively; Fig. 5A and C), regional lymph node removal
(P=0.027 and P=0.016, respectively; Fig. 5B and D) and radical surgery (P=0.001
and P<0.001; Fig. 6B and D). The
results also indicated that tumor size ≥11.15 cm was significantly
associated with worse CSS in patients <5 years of age (P=0.048;
Fig. 2C), of other ethnicities
(P=0.023; Fig. 3F), and with no
distant metastasis (P=0.017; Fig.
7C). In addition, tumor size ≥11.15 cm was significantly
associated with worse OS in patients with lymph node metastasis
(P=0.031; Fig. 8B). Moreover, the
removal of regional lymph nodes significantly improved OS (P=0.001;
Fig. 9B) and CSS (P<0.001;
Fig. 9D) compared with the
retention of regional lymph nodes in patients with tumors ≥11.15 cm
in size. By contrast, regional lymph node removal had no impact on
the OS (P=0.292; Fig. 9A) and CSS
(P=0.840; Fig. 9C) of patients with
a tumor size of <11.15 cm.
Multivariate analyses of the
predictors of OS and CSS
As shown in Figs.
10 and 11, primary tumor size
was an independent prognostic factor for OS (HR 1.478, P=0.044) and
CSS (HR 1.639, P=0.020). Moreover, lymph node status (P=0.001 and
P<0.001, respectively) and distant metastasis (P<0.001 for
both) were also independent predictors for OS and CSS. However, age
(HR 1.317, P=0.151 and HR 1.439, P=0.074, respectively) was not
significantly associated with OS or CSS, and regional lymph node
removal (P=0.131) was not identified as an independent prognostic
factor for OS.
Discussion
Despite advances in treatment strategies and the
favorable prognosis of most patients with WT, the mortality rate is
still 10% (9). Poor outcomes have
been reported for advanced, bilateral and recurrent WT (10). Therefore, it is critical to identify
novel prognostic factors for WT to guide the development of
individualized treatment strategies. The present retrospective
study demonstrated that patients with large WT (tumor size ≥11.15
cm) had worse OS and CSS than those with smaller tumors, and
primary tumor size was an independent prognostic factor for OS and
CSS.
Consistent with a previous study (4), the present study found that WT was
more prevalent in children <5 years old. In addition, patients
of white ethnicity were more susceptible to WT compared with other
racial groups. However, previous studies have shown that the
incidence of WT varies widely among different ethnic groups, with
black and East Asian populations having the highest and lowest
incidence rates respectively (11,12).
Moreover, no significant difference in the incidence of WT was
observed in the present study in terms of sex. By contrast,
Cunningham et al (13)
reported that WT was slightly more prevalent among female patients,
with the exception of those in Eastern Asia These discrepancies can
be explained by differences in the study populations.
In the present study, patients with WT larger tumors
had significantly worse OS and CSS compared with those with smaller
tumors, and were also more likely to develop lymph node metastasis.
Previous studies have shown that lymph node involvement portends a
poor prognosis in WT (14–16). Since the patients with WT and
positive lymph nodes had significantly lower 5-year OS and 5-year
CSS rates compared with those without lymph node involvement in the
Kaplan-Meier analyses, we hypothesize that lymph node metastasis is
a key cause of the poor prognosis of patients with large tumors.
Indeed, the results of the stratified Kaplan-Meier analyses showed
that larger tumors were associated with significantly worse OS
among patients with lymph node metastasis, whereas tumor size did
not affect the prognosis of patients without lymph node
involvement. These results further support this hypothesis.
The present study found that the removal of regional
lymph nodes significantly improved OS and CSS in the patients with
large tumors, while regional lymph node removal had no effect on
the survival of patients with smaller tumors. Thus, it is
recommended that regional lymphadenectomy should be considered for
patients with WT whose tumor is ≥11.15 cm in size to prolong
survival. Consistent with these findings, Zhuge et al
(17) also reported that patients
who had not undergone lymph node biopsy had a significantly lower
5-year OS, and the removal of lymph nodes increased the 5-year OS
of the patients.
The patients with WT in the present study who had
larger tumors were more likely to develop distant metastasis, and
distant metastasis was associated with significantly lower 5-year
OS and 5-year CSS rates when compared with those for patients
without distant metastasis. A previous study also reported a dismal
prognosis for patients with WT and distant metastasis (1). Moreover, Iaboni et al (18) found that the 5-year OS rate of
patients with WT and bone metastases was only 14.3% Thus, distant
metastasis is a risk factor in patients with WT who have large
tumors.
Reinhard et al (19) previously reported that a reduction
in tumor volume after preoperative chemotherapy was an effective
factor for the stratification of WT patients for postoperative
treatment. In addition, Provenzi et al (4) found that the tumor volume after
preoperative chemotherapy could independently predict poor
prognosis in patients with WT. However, the prognostic significance
of the primary tumor size of WT has not been thoroughly studied in
previous studies. The present study has demonstrated for the first
time, to the best of our knowledge, that primary tumor size is an
independent prognostic factor for WT, along with lymph node status
and distant metastasis.
Although the type of surgery was not found to be
significantly associated with the OS and CSS in the Kaplan-Meier
analyses, the curves of the stratified Kaplan-Meier analyses
indicated that tumor size <11.15 cm was associated with improved
OS and CSS in patients with WT who had undergone radical surgery.
By contrast, tumor size was shown to have no impact on the OS and
CSS of patients who underwent non-radical surgery. Therefore,
radical surgery may provide survival benefits for WT patients with
a tumor <11.15 cm in size.
In a previous study, Bahoush and Saeedi (20) showed that sex is not an independent
predictor of OS in patients with WT, which is consistent with the
findings of the present study. Nevertheless, male patients with
smaller tumors had significantly improved OS and CSS rates compared
with those with larger tumors, whereas no significant difference
was observed between the two tumor-size groups in female patients.
Differences in sex hormone levels may be an important reason for
this result. Similarly, a previous study showed that orchiectomy or
estradiol treatment significantly reduced tumor weight in male WT
model rats, while testosterone treatment significantly increased
tumor weight in female rats (21).
Based on these findings, it is recommended that the primary tumor
size of male patients with WT should be taken into consideration,
in order to provide more effective individualized treatment.
There are several limitations to this retrospective
study. Firstly, the SEER database does not include data on adjuvant
chemotherapy or comorbidities that may significantly affect
survival. In addition, the SEER database does not provide
information on whether the patients had undergone pre-operative
chemotherapy. Furthermore, all patients included in the study were
from the United States. Nevertheless, the present study has shown
for the first time that primary tumor size is an independent
prognostic factor for WT, which has potential clinical
applications.
Acknowledgements
Not applicable.
Funding
The study was supported by a grant from Suzhou Municipal Health
Commission (grant no. LCZX202010).
Availability of data and materials
Publicly datasets were used in the study and can be
found in the SEER database (https://seer.cancer.gov/).
Author's contributions
KL, HXY and CBF conceived and designed the study. KL
and KZ collected and analyzed the data. KL, HXY and CBF wrote the
manuscript. All authors read and approved the final version of the
manuscript. KL and CBF confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Institutional review board approval and informed
consent from patients are not required for the study of data from
the SEER database, as it is a de-identified public-use
database.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huang Y, Zhang W, Song H and Sun N: A
nomogram for prediction of distant metastasis in children with
Wilms tumor: A study based on SEER database. J Pediatr Urol.
16:473.e1–473.e9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spreafico F and Bellani FF: Wilms' tumor:
Past, present and (possibly) future. Expert Rev Anticancer Ther.
6:249–258. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Treger TD, Chowdhury T, Pritchard-Jones K
and Behjati S: The genetic changes of Wilms tumour. Nat Rev
Nephrol. 15:240–251. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Provenzi VO, Rosa RF, Rosa RC, Roehe AV,
dos Santos PP, Faulhaber FR, de Oliveira CA and Zen PR: Tumor size
and prognosis in patients with Wilms tumor. Rev Paul Pediatr.
33:82–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pater L, Melchior P, Rübe C, Cooper BT,
McAleer MF, Kalapurakal JA and Paulino AC: Wilms tumor. Pediatr
Blood Cancer. 68 Suppl 2:e282572021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ekenze SO, Okafor OC, Obasi AA, Okafor DC
and Nnabugwu II: Wilms tumor in Africa: A systematic review of
management challenges and outcome in two decades (2000–2019).
Pediatr Blood Cancer. 67:e286952020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Groenendijk A, Spreafico F, de Krijger RR,
Drost J, Brok J, Perotti D, van Tinteren H, Venkatramani R,
Godziński J, Rübe C, et al: Prognostic factors for Wilms tumor
recurrence: A review of the literature. Cancers (Basel).
13:31422021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li K, Wu G, Fan C and Yuan H: The
prognostic significance of primary tumor size in squamous cell
carcinoma of the penis. Discov Oncol. 12:222021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He Y, Cui X, Lin Y, Wang Y, Wu D and Fang
Y: Using elevated cholesterol synthesis as a prognostic marker in
Wilms' tumor: A bioinformatic analysis. Biomed Res Int.
2021:88262862021.PubMed/NCBI
|
|
10
|
Liu L, Song Z, Gao XD, Chen X, Wu XB, Wang
M and Hong YD: Identification of the potential novel biomarkers as
susceptibility gene for Wilms tumor. BMC Cancer. 21:3162021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Breslow NE and Beckwith JB:
Epidemiological features of Wilms' tumor: Results of the national
Wilms' tumor study. J Natl Cancer Inst. 68:429–436. 1982.PubMed/NCBI
|
|
12
|
Stiller CA and Parkin DM: International
variations in the incidence of childhood renal tumours. Br J
Cancer. 62:1026–1030. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cunningham ME, Klug TD, Nuchtern JG,
Chintagumpala MM, Venkatramani R, Lubega J and Naik-Mathuria BJ:
Global disparities in Wilms tumor. J Surg Res. 247:34–51. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fernandez CV, Mullen EA, Chi YY, Ehrlich
PF, Perlman EJ, Kalapurakal JA, Khanna G, Paulino AC, Hamilton TE,
Gow KW, et al: Outcome and prognostic factors in stage III
favorable-histology Wilms tumor: A report from the children's
oncology group study AREN0532. J Clin Oncol. 36:254–261. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ehrlich PF, Anderson JR, Ritchey ML, Dome
JS, Green DM, Grundy PE, Perlman EJ, Kalapurakal JA, Breslow NE and
Shamberger RC: Clinicopathologic findings predictive of relapse in
children with stage III favorable-histology Wilms tumor. J Clin
Oncol. 31:1196–1201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Honeyman JN, Rich BS, McEvoy MP, Knowles
MA, Heller G, Riachy E, Kobos R, Shukla N, Wolden SL, Steinherz PG,
et al: Factors associated with relapse and survival in Wilms tumor:
A multivariate analysis. J Pediatr Surg. 47:1228–1233. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhuge Y, Cheung MC, Yang R, Koniaris LG,
Neville HL and Sola JE: Improved survival with lymph node sampling
in Wilms tumor. J Surg Res. 167:e199–e203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iaboni DSM, Chi YY, Kim Y, Dome JS and
Fernandez CV: Outcome of Wilms tumor patients with bone metastasis
enrolled on national Wilms tumor studies 1–5: A report from the
children's oncology group. Pediatr Blood Cancer. 66:e274302019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reinhard H, Semler O, Bürger D, Bode U,
Flentje M, Göbel U, Gutjahr P, Leuschner I, Maass E, Niggli F, et
al: Results of the SIOP 93-01/GPOH trial and study for the
treatment of patients with unilateral nonmetastatic Wilms tumor.
Klin Padiatr. 216:132–140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bahoush G and Saeedi E: Outcome of
children with Wilms' tumor in developing countries. J Med Life.
13:484–489. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saroff J, Chu TM, Gaeta JF, Williams P and
Murphy GP: Characterization of a Wilms' tumor model. Invest Urol.
12:320–325. 1875.PubMed/NCBI
|