|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar
|
|
3
|
Vogel A, Cervantes A, Chau I, Daniele B,
Llovet JM, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, et al:
Hepatocellular carcinoma: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 29 (Suppl
4):iv238–iv255. 2018. View Article : Google Scholar
|
|
4
|
Dimitroulis D, Damaskos C, Valsami S,
Davakis S, Garmpis N, Spartalis E, Athanasiou A, Moris D,
Sakellariou S, Kykalos S, et al: From diagnosis to treatment of
hepatocellular carcinoma: An epidemic problem for both developed
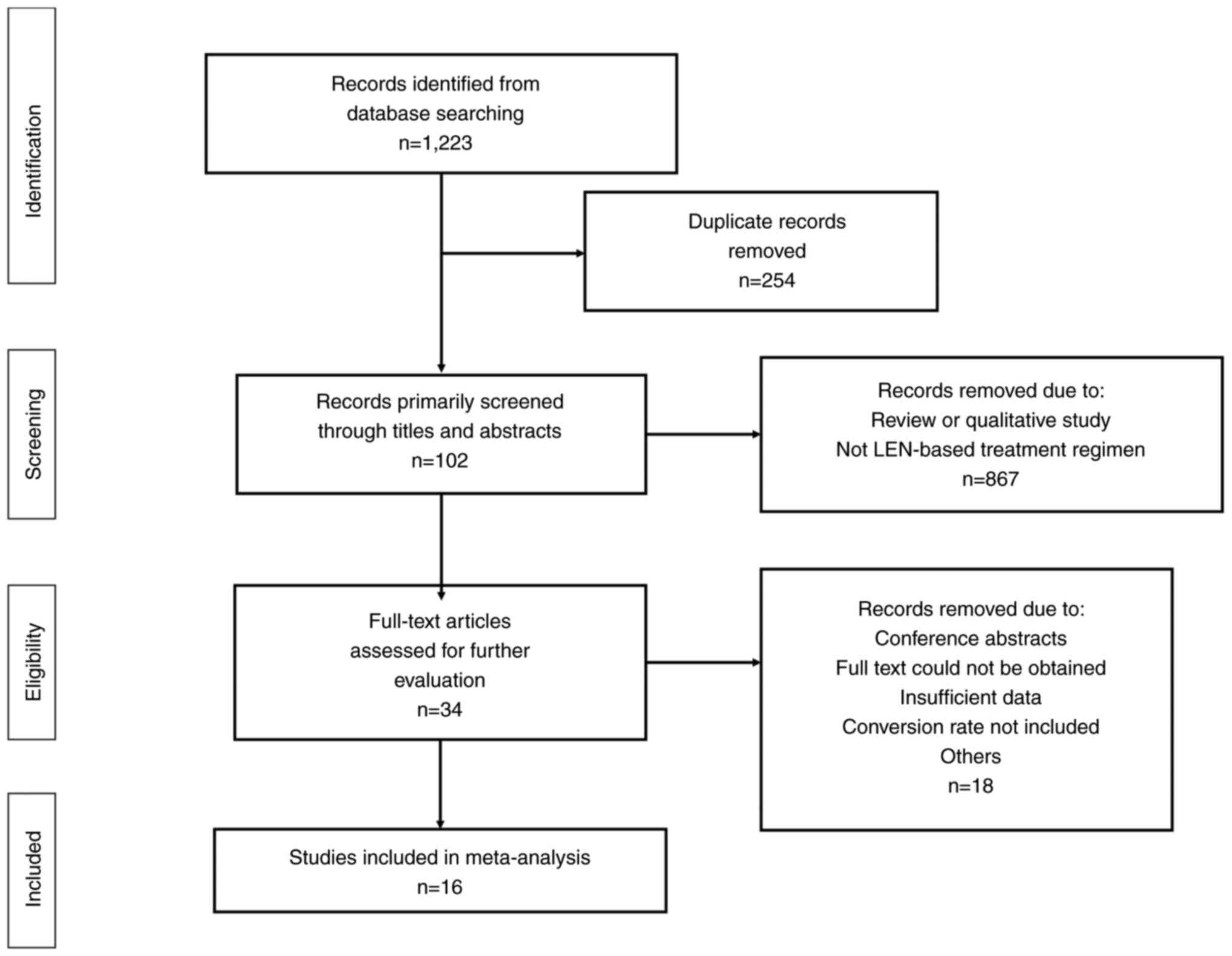
and developing world. World J Gastroenterol. 23:5282–5294. 2017.
View Article : Google Scholar
|
|
5
|
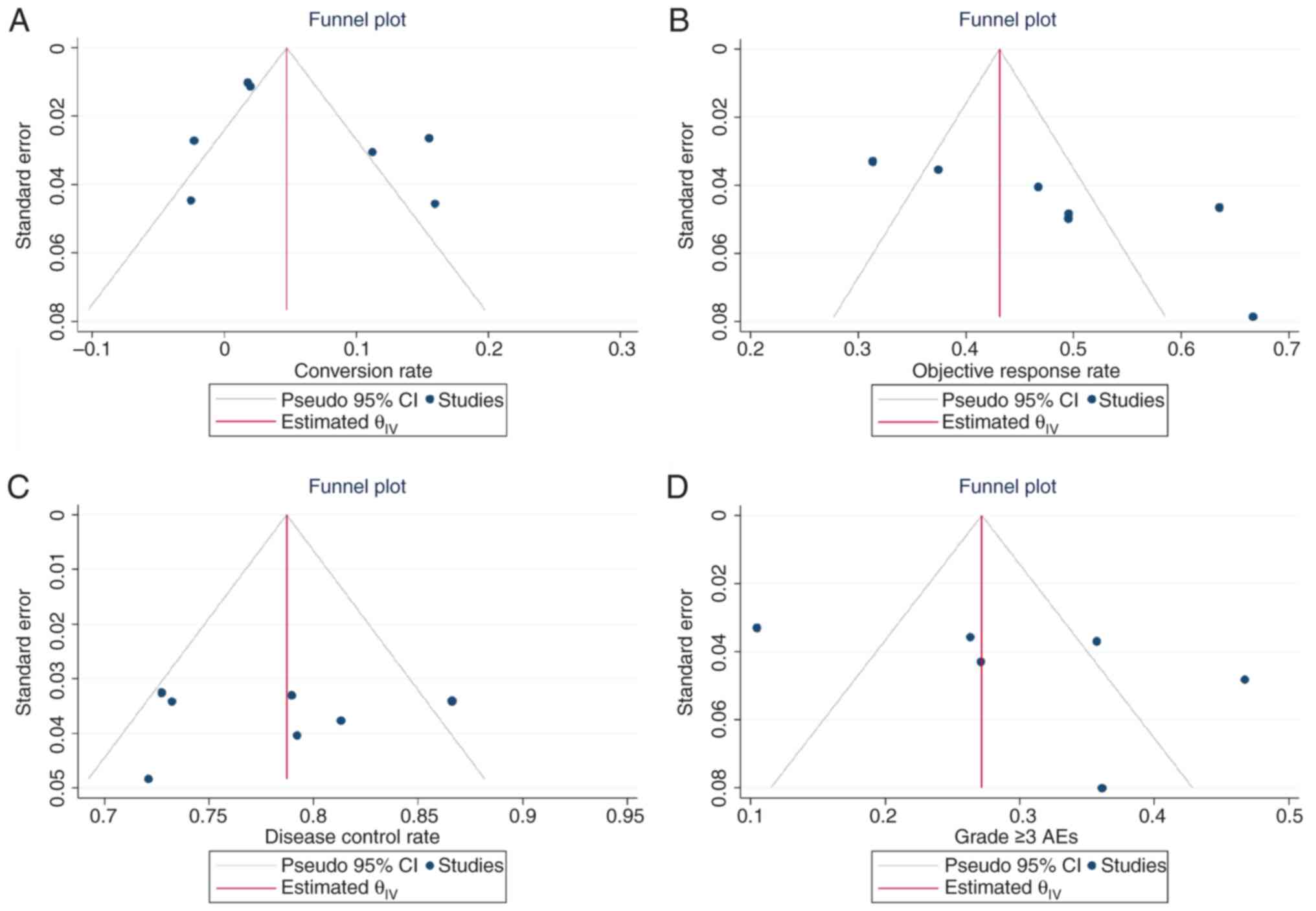
Llovet JM, De Baere T, Kulik L, Haber PK,
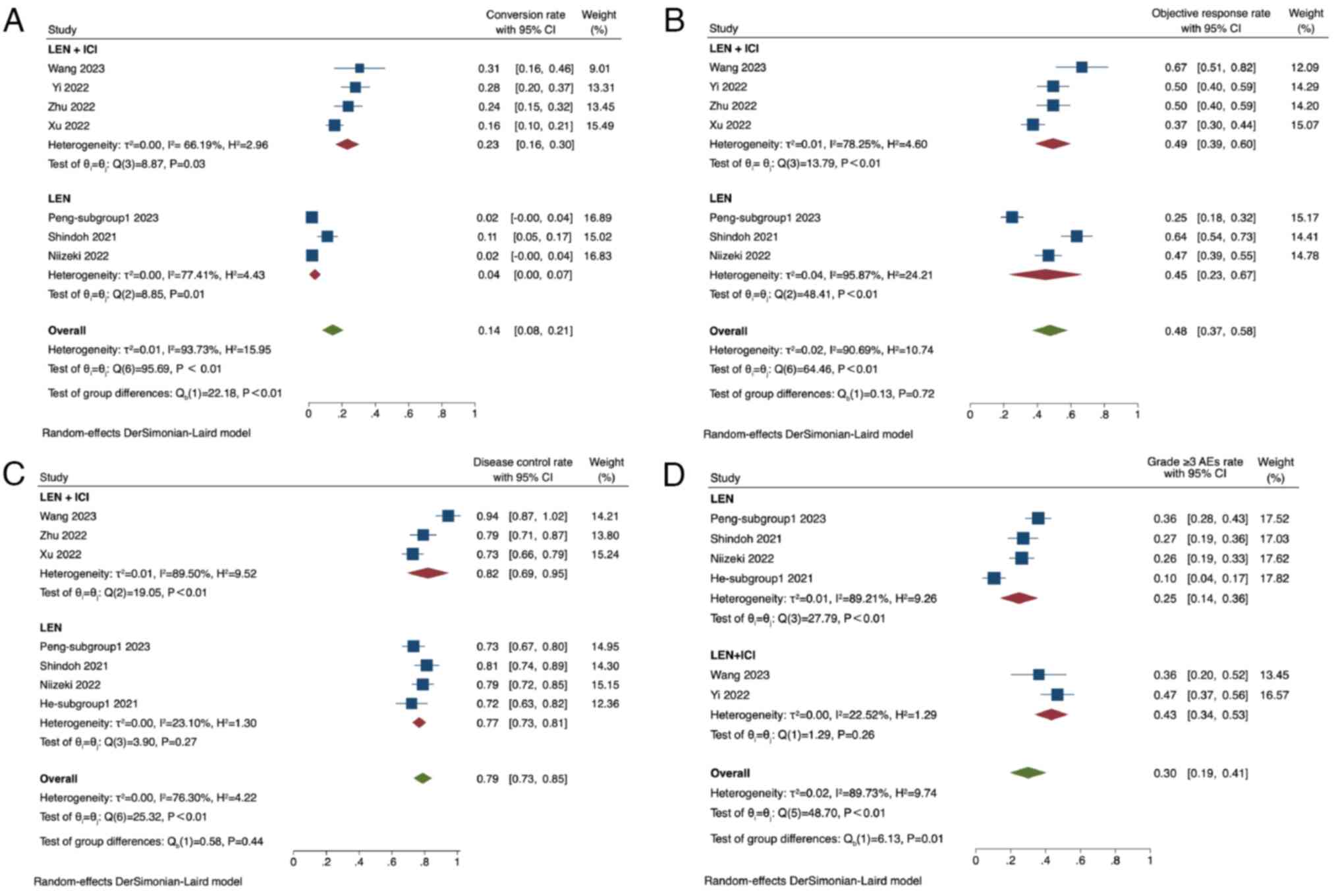
Greten TF, Meyer T and Lencioni R: Locoregional therapies in the
era of molecular and immune treatments for hepatocellular
carcinoma. Nat Rev Gastroenterol Hepatol. 18:293–313. 2021.
View Article : Google Scholar
|
|
6
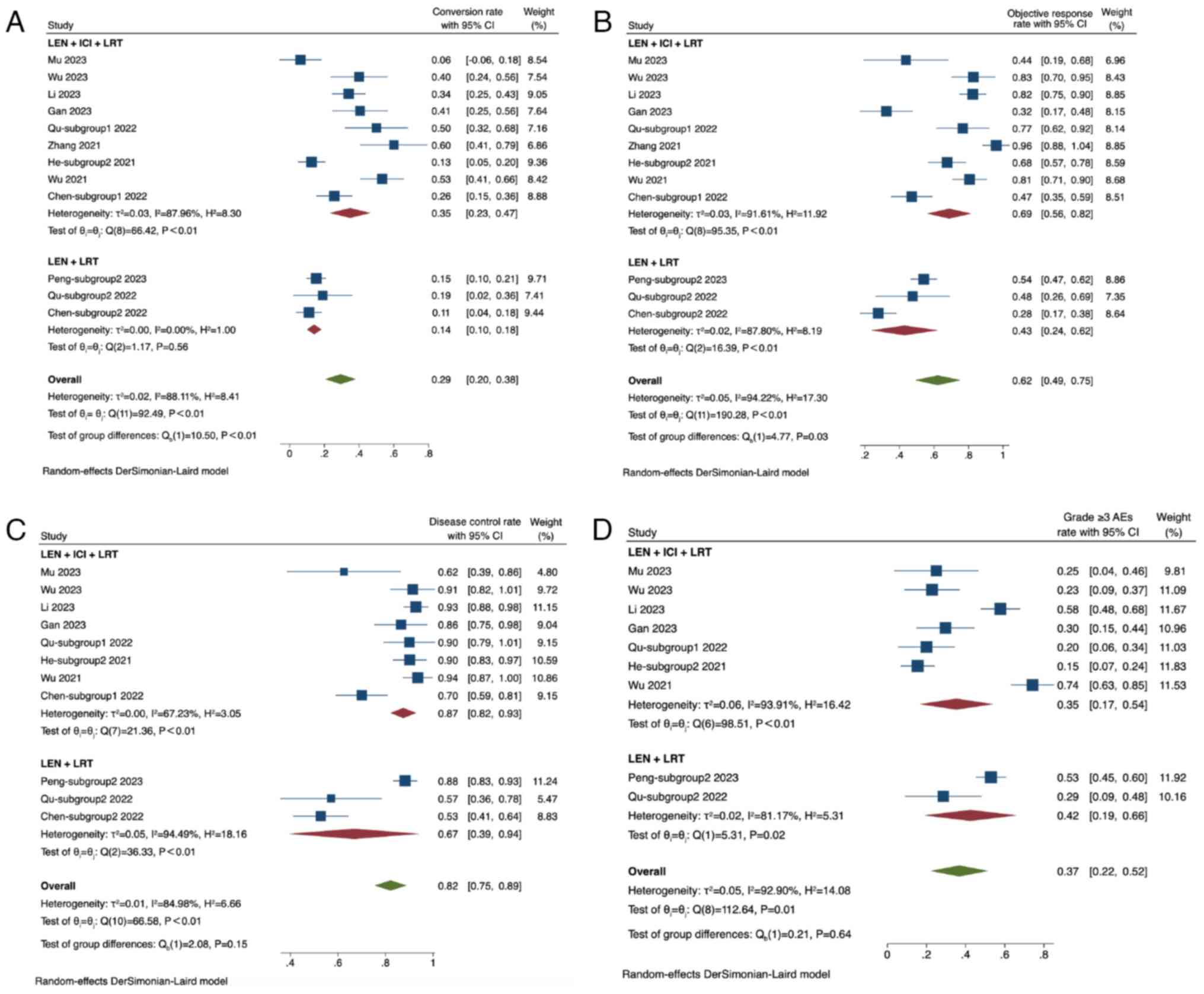
|
Huang A, Yang XR, Chung WY, Dennison AR
and Zhou J: Targeted therapy for hepatocellular carcinoma. Signal
Transduct Target Ther. 5:1462020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brown ZJ, Tsilimigras DI, Ruff SM, Mohseni
A, Kamel IR, Cloyd JM and Pawlik TM: Management of hepatocellular
carcinoma: A review. JAMA Surg. 158:410–420. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rizzo A, Ricci AD and Brandi G: Systemic
adjuvant treatment in hepatocellular carcinoma: Tempted to do
something rather than nothing. Future Oncol. 16:2587–2589. 2020.
View Article : Google Scholar
|
|
9
|
Matsuki R, Kogure M, Hasui N, Momose H,
Suzuki Y and Sakamoto Y: Development of conversion therapy for
advanced hepatocellular carcinoma. Hepatobiliary Surg Nutr.
12:453–456. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kudo M: Atezolizumab plus bevacizumab
followed by curative conversion (ABC conversion) in patients with
unresectable, TACE-unsuitable intermediate-stage hepatocellular
carcinoma. Liver Cancer. 11:399–406. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiang CL, Chiu KWH, Chan KSK, Lee FAS, Li
JCB, Wan CWS, Dai WC, Lam TC, Chen W, Wong NSM, et al: Sequential
transarterial chemoembolisation and stereotactic body radiotherapy
followed by immunotherapy as conversion therapy for patients with
locally advanced, unresectable hepatocellular carcinoma
(START-FIT): A single-arm, phase 2 trial. Lancet Gastroenterol
Hepatol. 8:169–178. 2023. View Article : Google Scholar
|
|
12
|
Qu WF, Ding ZB, Qu XD, Tang Z, Zhu GQ, Fu
XT, Zhang ZH, Zhang X, Huang A, Tang M, et al: Conversion therapy
for initially unresectable hepatocellular carcinoma using a
combination of toripalimab, lenvatinib plus TACE: Real-world study.
BJS Open. 6:zrac1142022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Z and Zhang E: Conversion therapy
for advanced hepatocellular carcinoma with vascular invasion: A
comprehensive review. Front Immunol. 14:10735312023. View Article : Google Scholar
|
|
14
|
Benson AB, D'Angelica MI, Abbott DE, Anaya
DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, et
al: Hepatobiliary cancers, version 2.2021, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 19:541–565. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
European Association for the Study of the
Liver. lectronic address, . simpleeasloffice@easloffice.eu;
European Association for the Study of the Liverr: EASL clinical
practice guidelines: Management of hepatocellular carcinoma. J
Hepatol. 69:182–236. 2018. View Article : Google Scholar
|
|
16
|
Lee JJX, Tai DWM and Choo SP: Locoregional
therapy in hepatocellular carcinoma: When to start and when to stop
and when to revisit. ESMO Open. 6:1001292021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Llovet JM, Pinyol R, Kelley RK,
El-Khoueiry A, Reeves HL, Wang XW, Gores GJ and Villanueva A:
Molecular pathogenesis and systemic therapies for hepatocellular
carcinoma. Nat Cancer. 3:386–401. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rizzo A, Ricci AD and Brandi G:
Trans-arterial chemoembolization plus systemic treatments for
hepatocellular carcinoma: An update. J Pers Med. 12:17882022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar
|
|
20
|
Abou-Alfa GK, Meyer T, Cheng AL,
El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park
JW, et al: Cabozantinib in patients with advanced and progressing
hepatocellular carcinoma. N Engl J Med. 379:54–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu AX, Kang YK, Yen CJ, Finn RS, Galle
PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, et al:
Ramucirumab after sorafenib in patients with advanced
hepatocellular carcinoma and increased α-fetoprotein concentrations
(REACH-2): A randomised, double-blind, placebo-controlled, phase 3
trial. Lancet Oncol. 20:282–296. 2019. View Article : Google Scholar
|
|
22
|
Al-Salama ZT, Syed YY and Scott LJ:
Lenvatinib: A review in hepatocellular carcinoma. Drugs.
79:665–674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamashita T, Kudo M, Ikeda K, Izumi N,
Tateishi R, Ikeda M, Aikata H, Kawaguchi Y, Wada Y, Numata K, et
al: REFLECT-a phase 3 trial comparing efficacy and safety of
lenvatinib to sorafenib for the treatment of unresectable
hepatocellular carcinoma: An analysis of Japanese subset. J
Gastroenterol. 55:113–122. 2020. View Article : Google Scholar
|
|
24
|
He MK, Liang RB, Zhao Y, Xu YJ, Chen HW,
Zhou YM, Lai ZC, Xu L, Wei W, Zhang YJ, et al: Lenvatinib,
toripalimab, plus hepatic arterial infusion chemotherapy versus
lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv
Med Oncol. 13:175883592110027202021. View Article : Google Scholar
|
|
25
|
Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang
F, Zhuang W, Chen X, Chen H, Xu B, et al: Lenvatinib plus TACE with
or without pembrolizumab for the treatment of initially
unresectable hepatocellular carcinoma harbouring PD-L1 expression:
A retrospective study. J Cancer Res Clin Oncol. 148:2115–2125.
2022. View Article : Google Scholar
|
|
26
|
Mu C, Shen J, Zhu X, Peng W, Zhang X and
Wen T: The efficacy and safety of lenvatinib plus transarterial
chemoembolization in combination with PD-1 antibody in treatment of
unresectable recurrent hepatocellular carcinoma: A case series
report. Front Oncol. 13:10969552023. View Article : Google Scholar
|
|
27
|
Mollica V, Rizzo A, Marchetti A, Tateo V,
Tassinari E, Rosellini M, Massafra R, Santoni M and Massari F: The
impact of ECOG performance status on efficacy of immunotherapy and
immune-based combinations in cancer patients: The MOUSEION-06
study. Clin Exp Med. 23:5039–5049. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rizzo A, Mollica V, Tateo V, Tassinari E,
Marchetti A, Rosellini M, De Luca R, Santoni M and Massari F:
Hypertransaminasemia in cancer patients receiving immunotherapy and
immune-based combinations: The MOUSEION-05 study. Cancer Immunol
Immunother. 72:1381–1394. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sangro B, Sarobe P, Hervás-Stubbs S and
Melero I: Advances in immunotherapy for hepatocellular carcinoma.
Nat Rev Gastroenterol Hepatol. 18:525–543. 2021. View Article : Google Scholar
|
|
30
|
Pei Y, Li W, Wang Z and Liu J: Successful
conversion therapy for unresectable hepatocellular carcinoma is
getting closer: A systematic review and meta-analysis. Front Oncol.
12:9788232022. View Article : Google Scholar
|
|
31
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate healthcare
interventions: Explanation and elaboration. BMJ. 339:b27002009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cumpston M, Li T, Page MJ, Chandler J,
Welch VA, Higgins JP and Thomas J: Updated guidance for trusted
systematic reviews: A new edition of the cochrane handbook for
systematic reviews of interventions. Cochrane Database Syst Rev.
10:ED0001422019.PubMed/NCBI
|
|
33
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Slim K, Nini E, Forestier D, Kwiatkowski
F, Panis Y and Chipponi J: Methodological index for non-randomized
studies (minors): Development and validation of a new instrument.
ANZ J Surg. 73:712–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo B, Moga C, Harstall C and Schopflocher
D: A principal component analysis is conducted for a case series
quality appraisal checklist. J Clin Epidemiol. 69:199–207.e2. 2016.
View Article : Google Scholar
|
|
36
|
Higgins JPT and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang L, Wang H, Cui Y, Liu M, Jin K, Xu D,
Wang K and Xing B: Sintilimab plus Lenvatinib conversion therapy
for intermediate/locally advanced hepatocellular carcinoma: A phase
2 study. Front Oncol. 13:11151092023. View Article : Google Scholar
|
|
39
|
Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao
C, Huang F, Tang R, Cheng Y, Huang Z, et al: Lenvatinib combined
with transarterial chemoembolization as first-line treatment for
advanced hepatocellular carcinoma: A phase III, randomized clinical
trial (LAUNCH). J Clin Oncol. 41:117–127. 2023. View Article : Google Scholar
|
|
40
|
Yi Y, Sun BY, Weng JL, Zhou C, Zhou CH,
Cai MH, Zhang JY, Gao H, Sun J, Zhou J, et al: Lenvatinib plus
anti-PD-1 therapy represents a feasible conversion resection
strategy for patients with initially unresectable hepatocellular
carcinoma: A retrospective study. Front Oncol. 12:10465842022.
View Article : Google Scholar
|
|
41
|
Zhu XD, Huang C, Shen YH, Xu B, Ge NL, Ji
Y, Qu XD, Chen L, Chen Y, Li ML, et al: Hepatectomy after
conversion therapy using tyrosine kinase inhibitors plus anti-PD-1
antibody therapy for patients with unresectable hepatocellular
carcinoma. Ann Surg Oncol. 30:2782–2790. 2023. View Article : Google Scholar
|
|
42
|
Shindoh J, Kawamura Y, Kobayashi Y,
Kobayashi M, Akuta N, Okubo S, Suzuki Y and Hashimoto M: Prognostic
impact of surgical intervention after lenvatinib treatment for
advanced hepatocellular carcinoma. Ann Surg Oncol. 28:7663–7672.
2021. View Article : Google Scholar
|
|
43
|
Xu B, Zhu XD, Shen YH, Zhu JJ, Liu J, Li
ML, Tang PW, Zhou J, Fan J, Sun HC and Huang C: Criteria for
identifying potentially resectable patients with initially
oncologically unresectable hepatocellular carcinoma before
treatment with envatinib plus an anti-PD-1 antibody. Front Immunol.
13:10167362022. View Article : Google Scholar
|
|
44
|
Niizeki T, Tokunaga T, Takami Y, Wada Y,
Harada M, Shibata M, Nakao K, Sasaki R, Hirai F, Shakado S, et al:
Comparison of efficacy and safety of atezolizumab plus bevacizumab
and lenvatinib as first-line therapy for unresectable
hepatocellular carcinoma: A propensity score matching analysis.
Target Oncol. 17:643–653. 2022. View Article : Google Scholar
|
|
45
|
Wu SJ, Ruan DD, Wu QY, Tang Y, Zhang JH,
Cai SL, Zhou YF, Luo JW and Fang ZT: Safety and efficacy of
drug-eluting bead transarterial chemoembolization combined with
lenvatinib and anti-PD-1 antibodies for unresectable hepatocellular
carcinoma: A retrospective analysis. J Hepatocell Carcinoma.
10:807–820. 2023. View Article : Google Scholar
|
|
46
|
Li X, Chen J, Wang X, Bai T, Lu S, Wei T,
Tang Z, Huang C, Zhang B, Liu B, et al: Outcomes and prognostic
factors in initially unresectable hepatocellular carcinoma treated
using conversion therapy with lenvatinib and TACE plus PD-1
inhibitors. Front Oncol. 13:11106892023. View Article : Google Scholar
|
|
47
|
Gan L, Lang M, Tian X, Ren S, Li G, Liu Y,
Han R, Zhu K, Li H, Wu Q, et al: A retrospective analysis of
conversion therapy with lenvatinib, sintilimab, and
arterially-directed therapy in patients with initially unresectable
hepatocellular carcinoma. J Hepatocell Carcinoma. 10:673–686. 2023.
View Article : Google Scholar
|
|
48
|
Zhang J, Zhang X, Mu H, Yu G, Xing W, Wang
L and Zhang T: Surgical conversion for initially unresectable
locally advanced hepatocellular carcinoma using a triple
combination of angiogenesis inhibitors, anti-PD-1 antibodies, and
hepatic arterial infusion chemotherapy: A retrospective study.
Front Oncol. 11:7297642021. View Article : Google Scholar
|
|
49
|
Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ,
Wang SJ, Zhou JY, Li YN, Qiu FN, Li B and Yan ML: Lenvatinib
combined with anti-PD-1 antibodies plus transcatheter arterial
chemoembolization for unresectable hepatocellular carcinoma: A
multicenter retrospective study. J Hepatocell Carcinoma.
8:1233–1240. 2021. View Article : Google Scholar
|
|
50
|
Zhao Y, Zhang YN, Wang KT and Chen L:
Lenvatinib for hepatocellular carcinoma: From preclinical
mechanisms to anti-cancer therapy. Biochim Biophys Acta Rev Cancer.
1874:1883912020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cai M, Huang W, Huang J, Shi W, Guo Y,
Liang L, Zhou J, Lin L, Cao B, Chen Y, et al: Transarterial
chemoembolization combined with lenvatinib plus PD-1 inhibitor for
advanced hepatocellular carcinoma: A retrospective cohort study.
Front Immunol. 13:8483872022. View Article : Google Scholar
|
|
52
|
Hatanaka T, Yata Y, Naganuma A and
Kakizaki S: Treatment strategy for intermediate-stage
hepatocellular carcinoma: Transarterial chemoembolization, systemic
therapy, and conversion therapy. Cancers (Basel). 15:17982023.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hermann RE and Lonsdale D: Chemotherapy,
radiotherapy, and hepatic lobectomy for hepatoblastoma in an
infant: Report of a survival. Surgery. 68:383–388. 1970.PubMed/NCBI
|
|
54
|
Tsurusaki M and Murakami T: Surgical and
locoregional therapy of HCC: TACE. Liver Cancer. 4:165–175. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sergio A, Cristofori C, Cardin R, Pivetta
G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A and
Farinati F: Transcatheter arterial chemoembolization (TACE) in
hepatocellular carcinoma (HCC): The role of angiogenesis and
invasiveness. Am J Gastroenterol. 103:914–921. 2008. View Article : Google Scholar
|
|
56
|
Forner A, Llovet JM and Bruix J:
Chemoembolization for intermediate HCC: Is there proof of survival
benefit? J Hepatol. 56:984–986. 2012. View Article : Google Scholar
|
|
57
|
Fan J, Tang ZY, Yu YQ, Wu ZQ, Ma ZC, Zhou
XD, Zhou J, Qiu SJ and Lu JZ: Improved survival with resection
after transcatheter arterial chemoembolization (TACE) for
unresectable hepatocellular carcinoma. Dig Surg. 15:674–678. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lau WY, Ho SKW, Yu SCH, Lai ECH, Liew C
and Leung TWT: Salvage surgery following downstaging of
unresectable hepatocellular carcinoma. Ann Surg. 240:299–305. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yamamura K and Beppu T: Conversion surgery
for hepatocellular carcinoma after multidisciplinary treatment
including lenvatinib. Hepatol Res. 51:1029–1030. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sun HC, Zhou J, Wang Z, Liu X, Xie Q, Jia
W, Zhao M, Bi X, Li G, Bai X, et al: Chinese expert consensus on
conversion therapy for hepatocellular carcinoma (2021 edition).
Hepatobiliary Surg Nutr. 11:227–252. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lei Q, Yan X, Zou H, Jiang Y, Lai Y, Ung
COL and Hu H: Efficacy and safety of monotherapy and combination
therapy of immune checkpoint inhibitors as first-line treatment for
unresectable hepatocellular carcinoma: A systematic review,
meta-analysis and network meta-analysis. Discov Oncol. 13:952022.
View Article : Google Scholar
|
|
62
|
Arita J, Ichida A, Nagata R, Mihara Y,
Kawaguchi Y, Ishizawa T, Akamatsu N, Kaneko J and Hasegawa K:
Conversion surgery after preoperative therapy for advanced
hepatocellular carcinoma in the era of molecular targeted therapy
and immune checkpoint inhibitors. J Hepatobiliary Pancreat Sci.
29:732–740. 2022. View Article : Google Scholar
|
|
63
|
Chen J, Zhang D and Yuan Y:
Anti-PD-1/PD-L1 immunotherapy in conversion treatment of locally
advanced hepatocellular carcinoma. Clin Exp Med. 23:579–590. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Killock D: Novel ICI-TKI combination
improves HCC outcomes. Nat Rev Clin Oncol. 20:7332023. View Article : Google Scholar
|
|
65
|
Kudo M: A novel treatment strategy for
patients with intermediate-stage HCC who are not suitable for TACE:
Upfront systemic therapy followed by curative conversion. Liver
Cancer. 10:539–544. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yamamoto Y, Matsui J, Matsushima T,
Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A,
Hoshi SS, et al: Lenvatinib, an angiogenesis inhibitor targeting
VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft
models associated with microvessel density and pericyte coverage.
Vasc Cell. 6:182014. View Article : Google Scholar
|
|
67
|
Kato Y, Tabata K, Kimura T,
Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y,
Matsuki M, et al: Lenvatinib plus anti-PD-1 antibody combination
treatment activates CD8+ T cells through reduction of
tumor-associated macrophage and activation of the interferon
pathway. PLoS One. 14:e02125132019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kimura T, Kato Y, Ozawa Y, Kodama K, Ito
J, Ichikawa K, Yamada K, Hori Y, Tabata K, Takase K, et al:
Immunomodulatory activity of lenvatinib contributes to antitumor
activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci.
109:3993–4002. 2018. View Article : Google Scholar
|
|
69
|
Deng H, Kan A, Lyu N, Mu L, Han Y, Liu L,
Zhang Y, Duan Y, Liao S, Li S, et al: Dual vascular endothelial
growth factor receptor and fibroblast growth factor receptor
inhibition elicits antitumor immunity and enhances programmed cell
death-1 checkpoint blockade in hepatocellular carcinoma. Liver
Cancer. 9:338–357. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jiang H, Meng Q, Tan H, Pan S, Sun B, Xu R
and Sun X: Antiangiogenic therapy enhances the efficacy of
transcatheter arterial embolization for hepatocellular carcinomas.
Int J Cancer. 121:416–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pinato DJ, Murray SM, Forner A, Kaneko T,
Fessas P, Toniutto P, Mínguez B, Cacciato V, Avellini C, Diaz A, et
al: Trans-arterial chemoembolization as a loco-regional inducer of
immunogenic cell death in hepatocellular carcinoma: implications
for immunotherapy. J Immunother Cancer. 9:e0033112021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018. View Article : Google Scholar
|
|
73
|
Llovet JM, Castet F, Heikenwalder M, Maini
MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX and Finn RS:
Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol.
19:151–172. 2022. View Article : Google Scholar
|
|
74
|
Sung MW, Finn RS, Qin S, Han KH, Ikeda K,
Cheng AL, Kudo M, Tateishi R, Ikeda M, Breder V, et al: Association
between overall survival and adverse events with lenvatinib
treatment in patients with hepatocellular carcinoma (REFLECT). J
Clin Oncol. 37 (Suppl):S3172019. View Article : Google Scholar
|