Introduction
In the treatment of metastatic colorectal cancer
(mCRC), systemic chemotherapy comprising 5-fluorouracil plus
leucovorin combined with oxaliplatin (FOLFOX), or irinotecan
(FOLFIRI) in addition to molecular targeted agents, bevacizumab or
cetuximab or panitumumab, has achieved higher response rates and
prolonged patient survival (1,2). These
chemotherapy regimens have increased the conversion rates of
initially unresectable metastases to resectable ones in patients
with mCRC. Secondary resection following the downsizing of
unresectable metastases by chemotherapy has provided not only the
survival prolongation but also potential cure (3,4). Thus,
chemotherapy response for metastatic diseases plays an important
role on treatment decision-making of mCRC patients, because it is
one of the most predictive for overall survival of such patients
(3–5).
The molecular mechanisms of the above mentioned
chemotherapeutics have been explored by either in vitro or
in vivo studies (6–8). It still remains important to
understand how metastatic tumor cells and/or stromal cells respond
to chemotherapy on the metastatic organs, because the prognosis of
cancer patients depends on metastatic disease control. However,
there is no study of in vivo real-time visualization of
chemotherapy response at the cellular level on the microenvironment
of metastatic tumor in living animals.
Multiphoton microscopy including two-photon laser
scanning microscopy (TPLSM) has been widely used and become an
indispensable tool in the field of tumor biology (9,10).
TPLSM has made it possible to analyze the structural and functional
changes at the single cell level because of the ability of high
resolution and high magnification imaging. It has also extended
intravital imaging at a deeper tissue level with minimal
phototoxicity and photobleaching to living cells, compared with
single-photon confocal laser scanning microscopy (11,12).
Intravital TPLSM imaging has enabled us to study the dynamics of
cellular interactions in either two-dimensional (time-lapse) or
three-dimensional (z-stacks) environment for long time in living
animals.
We have established a method for in vivo
real-time TPLSM imaging of intra-abdominal gastrointestinal disease
using green fluorescent protein (GFP)-expressing mice (13–17).
Intravital TPLSM imaging with high resolution and high
magnification was accomplished by the reduction of motion artifact
due to cardiac and respiratory movement using an organ stabilizing
system (Japanese patent application number, P2007-129723).
Time-series (at multiple time points) intravital TPLSM imaging in
the same mice over the long experimental periods was also
accomplished by the prevention of abdominal adhesions using a
sodium hyaluronate and carboxymethylcellulose membrane.
In the present study, we visualized in vivo
real-time chemotherapy response for metastatic tumor xenografts
using intravital TPLSM to study the dynamic interactions between
metastatic tumor cells and host stromal cells on the
microenvironment of metastatic organs in living animals.
Materials and methods
GFP-expressing nude mice
GFP-expressing nude mice (C57BL/6-BALB/c-nu/nu-EGFP)
were purchased from AntiCancer Japan (Osaka, Japan). GFP nude mice
(20–22 g) were bred, housed in groups of six mice per cage and fed
with a pelleted basal diet (CE-7, CLEA Japan Inc., Tokyo, Japan).
Mice had free access to drinking water. They were kept in the
animal house facilities at Mie University School of Medicine under
standard conditions of humidity (50±10%), temperature (23±2˚C) and
light (12/12 h light/dark cycle), according to the Institutional
Animal Care Guidelines. The experimental protocols were reviewed
and approved by the Animal Care and Use Committee at Mie University
Graduate School of Medicine.
RFP-expressing human CRC cell line
The RFP-expressing human CRC cell line (RFP-HT29)
was purchased from AntiCancer Japan. RFP-HT29 cells were grown in
monolayer cultures in RPMI-1640 (Sigma-Aldrich, Inc., St. Louis,
MO, USA) supplemented with fetal bovine serum (10% (v/v), Gibco
BRL, Tokyo, Japan), glutamine (2 mM), penicillin (100,000 U/l),
streptomycin (100 mg/l), and gentamycin (40 mg/l) at 37˚C in a 5%
CO2 environment. For routine passage, cultures were
spilt 1:10 when they reached 90% confluence, generally every 3
days. Cells at the fifth to ninth passage were used for liver
metastasis experiments, which were performed with
exponentially-growing cells.
Chemotherapeutic drugs
5-Fluorouracil (5-FU) and irinotecan (CPT-11) were
purchased from Sigma-Aldrich, Inc. The stock solutions of these
drugs were made in dimethylsulfoxide (DMSO) and then were dissolved
in appropriate concentrations with distilled water for in
vivo study. To image the response to 5-FU or CPT-11 for liver
metastatic xenografts, 5-FU (50 mg/kg) or CPT-11 (20 mg/kg) was
administered intraperitoneally three times a week for three
weeks.
Murine liver metastasis model
RFP-HT29 cells were inoculated into the spleens of
GFP nude mice, as a colorectal liver metastatic xenograft model.
RFP-HT29 cells at the fifth to ninth passage were harvested with
trypsin/EDTA, and washed in serum-containing RPMI-1640 medium to
inactivate any remaining trypsin. The cells were centrifuged and
resuspended in phosphate-buffered saline (PBS). Finally, the cells
were adjusted to 2×107 cells/ml for single cell
suspensions. GFP nude mice were initially anesthetized by
intraperitoneal injection of chloral hydrate (Sigma). Under direct
vision, 2×106 cells were injected into the spleen using
a 30-gauge needle through a small incision in the left lateral
abdomen of anesthetized GFP nude mice.
Liver stabilization for intravital
TPLSM
After inoculation, GFP nude mice were initially
anaesthetized using an anaesthetic mask with 4 l/min of isofluorane
(4%; Forane, Abbott, Japan). Anaesthetic maintenance was achieved
using 1.5–2% isoflurane and 4 l/min of O2. Body
temperature was kept at 37˚C throughout the experiments using a
heating pad. Normal saline (200 μl) was administered
intraperitoneally at 1–2 h intervals for hydration during
anesthesia. The upper midline laparotomy was made as short as
possible (<15 mm). The left lateral lobe of the liver was
identified and exteriorized through the laparotomy. The liver lobe
was then put onto an organ-stabilizing system (Japanese patent
application number, P2007-129723) using a solder l μg terminal with
an instant adhesive agent (KO-10-p20, Daiso, Japan). The organ
stabilizer minimized the microvibration of the observed area caused
by heart beat and respiratory movements.
Stabilization and fixation of the liver lobe
represented a critical but technically difficult part of the
intravital TPLSM procedure. After the application of PBS to the
observed area, a thin cover glass was placed gently on the liver
surface. After intravital TPLSM, the exteriorized liver lobe was
gently removed from the organ-stabilizing system using a release
agent (KO-10-p8, Daiso), to prevent liver injury. The liver surface
were extensively washed by PBS to remove a release agent and blood
coagulation mass. A sodium hyaluronate and carboxymethylcellulose
membrane (Seprafilm Adhesion Barrier, Genzyme Corporation,
Cambridge, MA) was placed between the liver and the abdominal wall
to prevent postoperative dense adhesion.
TPLSM setup
The procedures for TPLSM setup were performed as
previously described (16).
Experiments were performed using an upright microscope (BX61WI;
Olympus, Tokyo, Japan) and an FV1000-2P laser-scanning microscope
system (Fluoview FV1000MPE, Olympus). The use of special stage
risers enabled the unit to have an exceptionally wide working
distance. This permitted the stereotactically immobilized,
anesthetized mouse to be placed on the microscope stage. The
microscope was fitted with several lenses with high numeric
apertures to provide the long working distances required for in
vivo work, and with water-immersion optics. The excitation
source in TPLSM mode was Mai Tai Ti:sapphire lasers (Spectra
Physics, Mountain View, CA), tuned and mode-locked at 910 nm. The
Mai Tai produces light pulses of ~100 fs width (repetition rate 80
MHz). Laser light reached the sample through the microscope
objectives, connected to an upright microscope (BX61WI; Olympus). A
mean laser power at the sample was between 10 and 40 mW, depending
on the depth of imaging. Microscope objective lens used in this
study were 4xUPlanSApo (numerical aperture of 0.16), 10xUPlanSApo
(numerical aperture of 0.4), and 60xLUMPlanFI/IR (water dipping,
numerical aperture of 0.9, working distance 2 mm),
respectively.
Data were analyzed using the FV10-ASW system
(Olympus). TPLSM images were acquired with 512×512 pixels spatial
resolution, from 210 μm field of view dimension, using a pixel
dwelling time 4 μsec. Two-photon fluorescence signals were
collected by an internal detector (non-descanned detection method)
at an excitation wavelength, to enable the simultaneous acquisition
of EGFP signal and RFP (DsRed2) signal. An excitation wavelength of
1050 nm is optimum for DsRed2 as reported by Kawano et al
(18), although an excitation
wavelength of 910 nm is optimum for EGFP. Therefore, it is
difficult to amply excite DsRed2 at 910-nm. To overcome this
difficulty, we have selected and used the tumor cells in which
expression level of DsRed2 is so high that we can identify the
DsRed2 labeled tumor cells clearly even with 910-nm excitation.
Color-coded green and red images were taken at the same time, and
subsequently merged to produce single images.
Imaging methods using TPLSM
The surface of the liver lobe was initially screened
at lower magnifications by setting out the X/Y plane and adjusting
the Z axis manually to detect the optimal observation area
containing RFP-expressing cancer cells (at least five areas). Each
area of interest was subsequently scanned at a higher magnification
(water-immersion objective ×60 with or without ×2 zoom) by manually
setting the X/Y plane and adjusting the Z axis (either
automatically or manually) to obtain high-resolution, clear TPLSM
images. The scanning areas were 200×200 μm (x600) or 100×100 μm
(x600 with ×2 zoom) respectively. The imaging depth or imaging
stack was determined arbitrarily to allow real-time
three-dimensional visualization of colorectal liver metastasis
in vivo. The laser power was adjusted according to the
imaging depth. When imaging at larger depths, we increase the laser
power level (≤100%) manually using laser power level controller. To
image the optimal simultaneous imaging of EGFP and RFP (DsRed2),
detection sensitivity (brightness by HV) was adjusted manually for
EGFP (450–500) or RFP (550–600), respectively.
Time-series imaging using intravital
TPLSM
Intravital TPLSM for imaging of liver metastatic
xenografts can be performed in the same mice until the formation of
non-dissecting adhesions between the liver and the abdominal wall.
A sodium hyaluronate and carboxymethylcellulose membrane (Seprafilm
Adhesion Barrier, Genzyme Corporation) was useful to prevent the
formation of postoperative adhesions between the liver and the
abdominal wall. However, our preliminary experiments demonstrated
that the intravital TPLSM images at four or more time points were
not clear enough to observe liver metastatic xenografts because of
dense fibrous adhesions. To keep the TPLSM images clear, intravital
TPLSM using the same mice was performed up to three time points for
time-series imaging of liver metastatic xenografts in the same
mice. Precautions were also taken during the entire surgical
procedure of time-series intravital TPLSM imaging to prevent
postoperative intraperitoneal infection.
The timing of intravital TPLSM imaging
(Fig. 1)
After inoculation of RFP-HT29 cells into the spleens
of GFP nude mice, macroscopic liver metastases were observed by 8
weeks in our model. The 1st intravital TPLSM was performed at 2 h
after inoculation to confirm the presence of RFP-HT29 cells in
hepatic sinusoids, which indicate the successful inoculation. The
2nd inrtavital TPLSM was performed in the same mice to observe the
liver metastatic formation and their tumor microenvironment at 6–8
weeks after inoculation. 5-FU (50 mg/kg) or CPT-11 (20 mg/kg) was
administered intraperitoneally three times a week for three weeks
after the confirmation of macroscopic liver metastases. The 3rd
intravital TPLSM was also performed after the above drug
administration to observe in vivo real-time chemotherapy
response on the tumor microenvironment of liver metastatic
xenografts in the same living mice. Thus, all mice with macroscopic
liver metastases were treated with either 5-FU or CPT-11
chemotherapy. These mice were imaged at three time points by
time-series intravital TPLSM. At the end of the experiments (after
the 3rd intravital TPLSM), whole livers were harvested and
subjected to histopathological analysis.
Immunohistochemistry for cytokeratin
20
The lack of immunological cross-reactivity with
other cytokeratins means that cytokeratin 20 (CK20) has become an
important tool for delineating the origin of metastatic human
adenocarcinomas arising from an unknown primary source. Mouse
livers were removed and fixed in 4% formaldehyde in PBS (pH 7.4)
for 24 h, processed, and embedded in paraffin wax according to
standard procedures. Formalin-fixed, paraffin-embedded tissue was
sliced at a thickness of 3 μm, and the sections were placed on
silane-coated slides. After deparaffinization and dehydration, the
sections were autoclaved for 10 min in 10 mM sodium citrate buffer
for antigen retrieval. They were blocked and incubated with primary
antibody overnight at 4˚C. Primary monoclonal anti-human CK20
antibody (Clone KBsB20.8; DakoCytomation, Denmark) was used at a
dilution of 1:50 for implementation of the labeled
streptavidin-biotin method (LASB2 kit/HRP, DakoCytomation). CK20
was detected using Envision reagents (Envision kit/HRP,
DakoCytomation). The sections were counterstained using
hematoxylin. Negative controls were run simultaneously with
pre-immune immunoglobulin.
Results
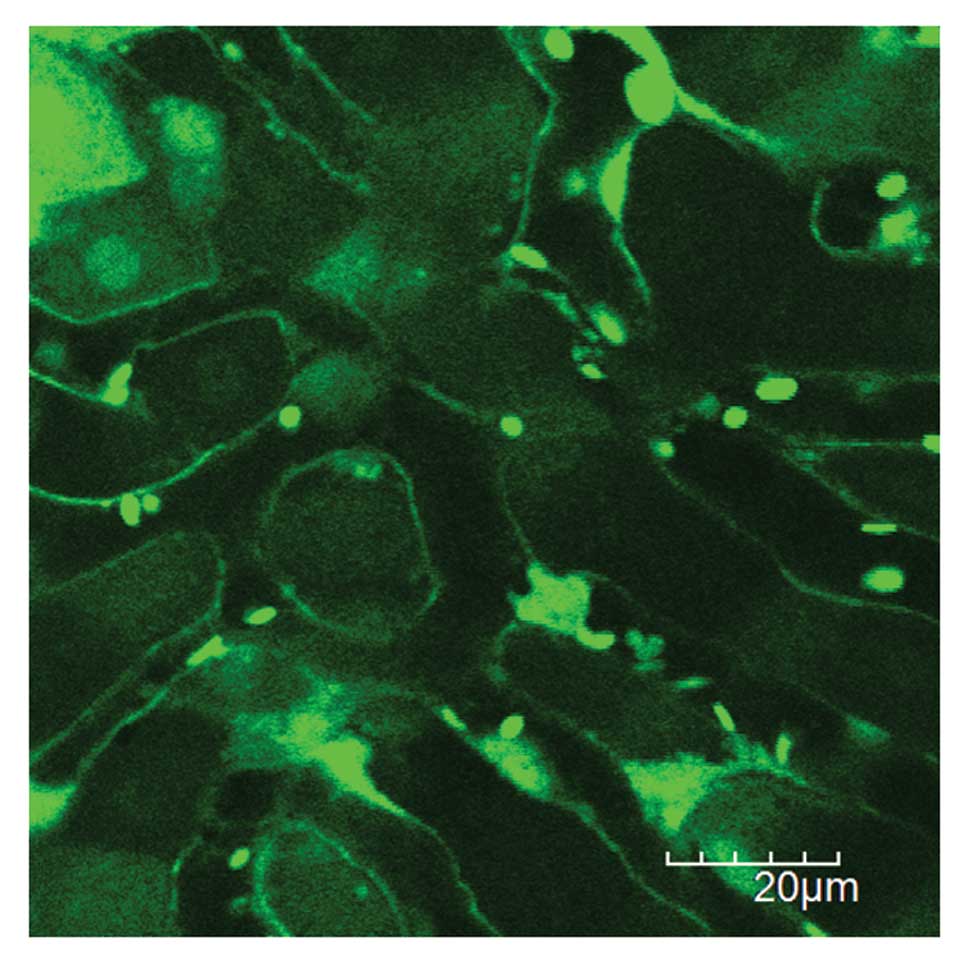
A single cancer cell in hepatic sinusoids by 1st
intravital TPLSM. Intravital TPLSM imaging was represented as a
time-lapse two-dimensional film, and also as a z-stack
three-dimensional movie from the liver surface to ~100–200 μm
depth. The optimal imaging depth was determined arbitrarily and
depended in part on the positioning of the liver lobe using the
organ-stabilizing system, or on the laser power. Liver structures
such as hepatocytes, hepatic sinusoids, hepatic endothelium, and
blood cells such as leukocytes and platelets were clearly imaged
with acceptable motion artifacts (Fig.
2). Since erythrocytes were not visualized in GFP mice
(19), leukocytes were recognized
as larger round cells, and platelets were recognized as smaller
ones within hepatic vessels.
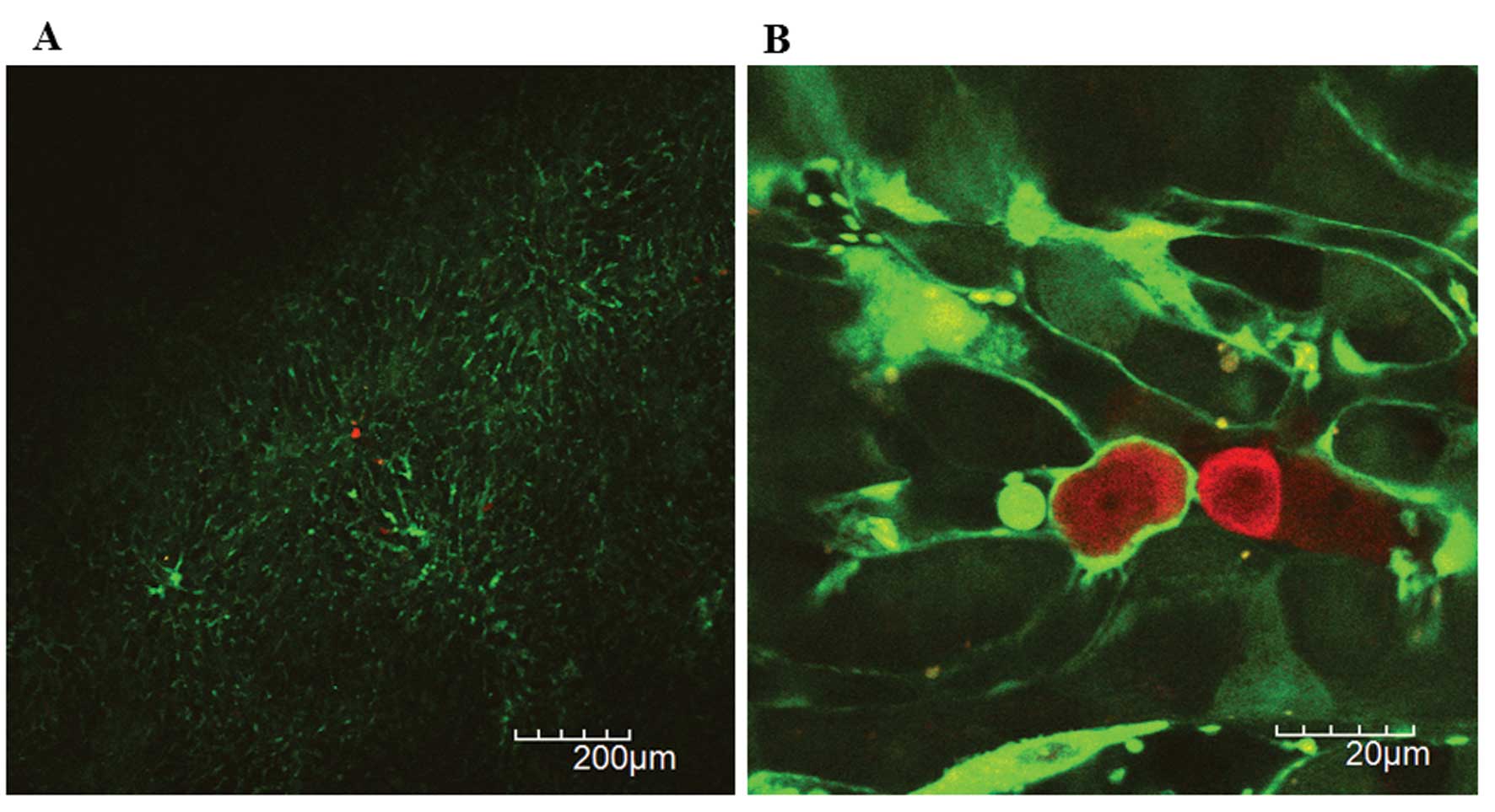
The 1st intravital TPLSM was performed at 2 h after
inoculation. Initially, we identified at least five areas probably
existing RFP-HT29 cells at lower magnification (x40-x100) (Fig. 3A). Next, we imaged each area of
interest at higher magnification (x600) to confirm the presence of
RFP-HT29 cells in hepatic sinusoids, which indicate the successful
inoculation. Two hours after the inoculation of 2×106
RFP-HT29 cells to the spleen of the GFP nude mice, ~5–10 RFP-HT29
cells were identified in the voxel of 210×210×100 μm (x1200)
(Fig. 3B).
Liver metastatic formation by 2nd
intravital TPLSM
The preliminary experiments demonstrated that
macroscopic liver metastases could be identified by 8 weeks after
the inoculation of 2×106 RFP-HT29 cells to the spleen of
GFP nude mice. Approximately 70% of all mice inoculated with
RFP-HT29 cells had macroscopic liver metastatic nodules on the
surface of the liver. Among them, ~70 % of the mice with
macroscopic liver metastases could be imaged by 2nd inrtavital
TPLSM. The remaining mice with macroscopic liver metastases could
not be imaged because their location is too difficult to observe in
our system. The 2nd inrtavital TPLSM was performed in the same mice
to observe the liver metastatic formation and their tumor
microenvironment at 6–8 weeks after inoculation. There is no mouse
which could not be imaged by 2nd intravital TPLSM for dense fibrous
adhesions between the liver and abdominal wall. Macroscopic liver
metastatic nodules were composed of tumor cell clusters (red) and
the surrounding reactive stroma with dilated/tortuous tumor vessels
(green) (Fig. 4).
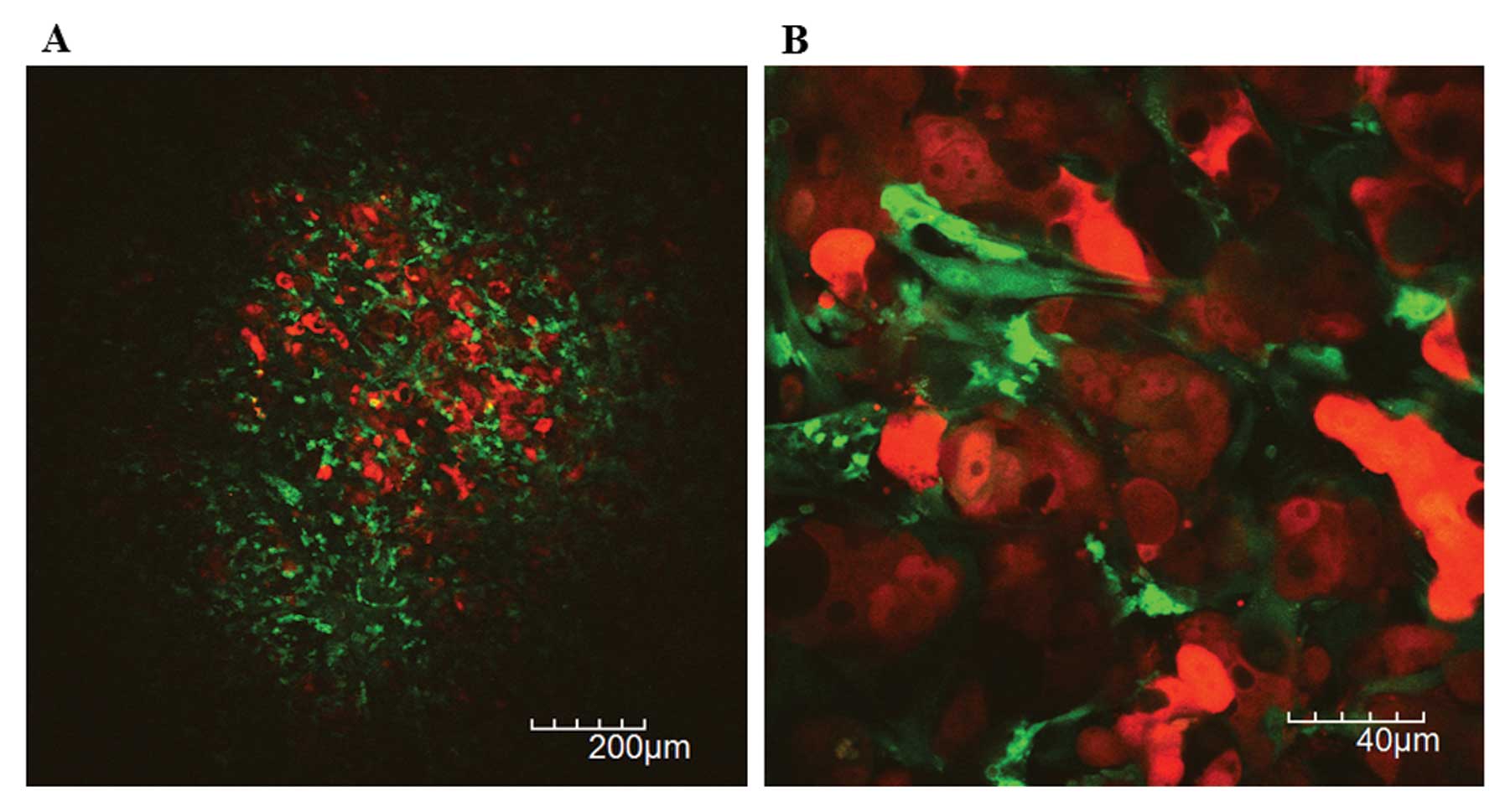
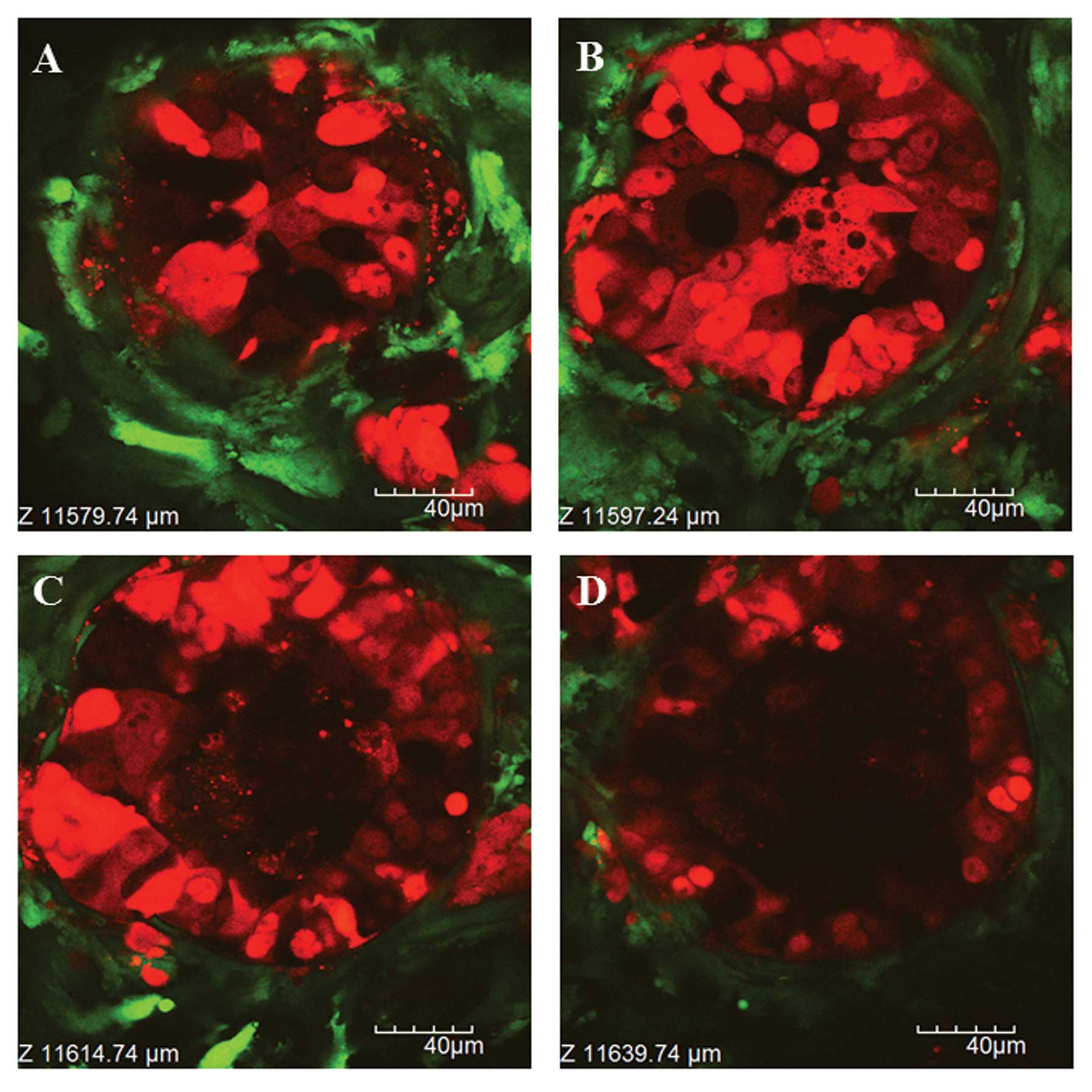
Tumor angiogenesis in liver metastases by
2nd intravital TPLSM
Abnormally dilated or tortuous tumor vessels were
observed in liver metastatic xenografts of RFP-HT29 cells at lower
magnification (Fig. 5A). Blood
cells except erythrocytes were clearly visualized within tumor
vessels at higher magnification over ×600 (Fig. 5B). The blood flow by the movement of
platelet was heterogeneous and frequently non-directional in tumor
vessels of metastatic xenografts. The aggregated platelets were
frequently flowing, which may indicate tumor vessel damage or
intratumoral coagulation abnormality.
In vivo chemotherapy response on liver
metastatic xenografts by 3rd intravital TPLSM
After the 2nd itravital TPLSM followed by 5-FU or
CPT-11 administration, the 3rd inrtavital TPLSM was performed to
observe in vivo real-time chemotherapy response on the tumor
microenvironment of liver metastatic xenografts in the same living
mice. We imaged at least three or more mice for each treatment
group which were subject to the time-series intravital TPLSM in the
same mice.
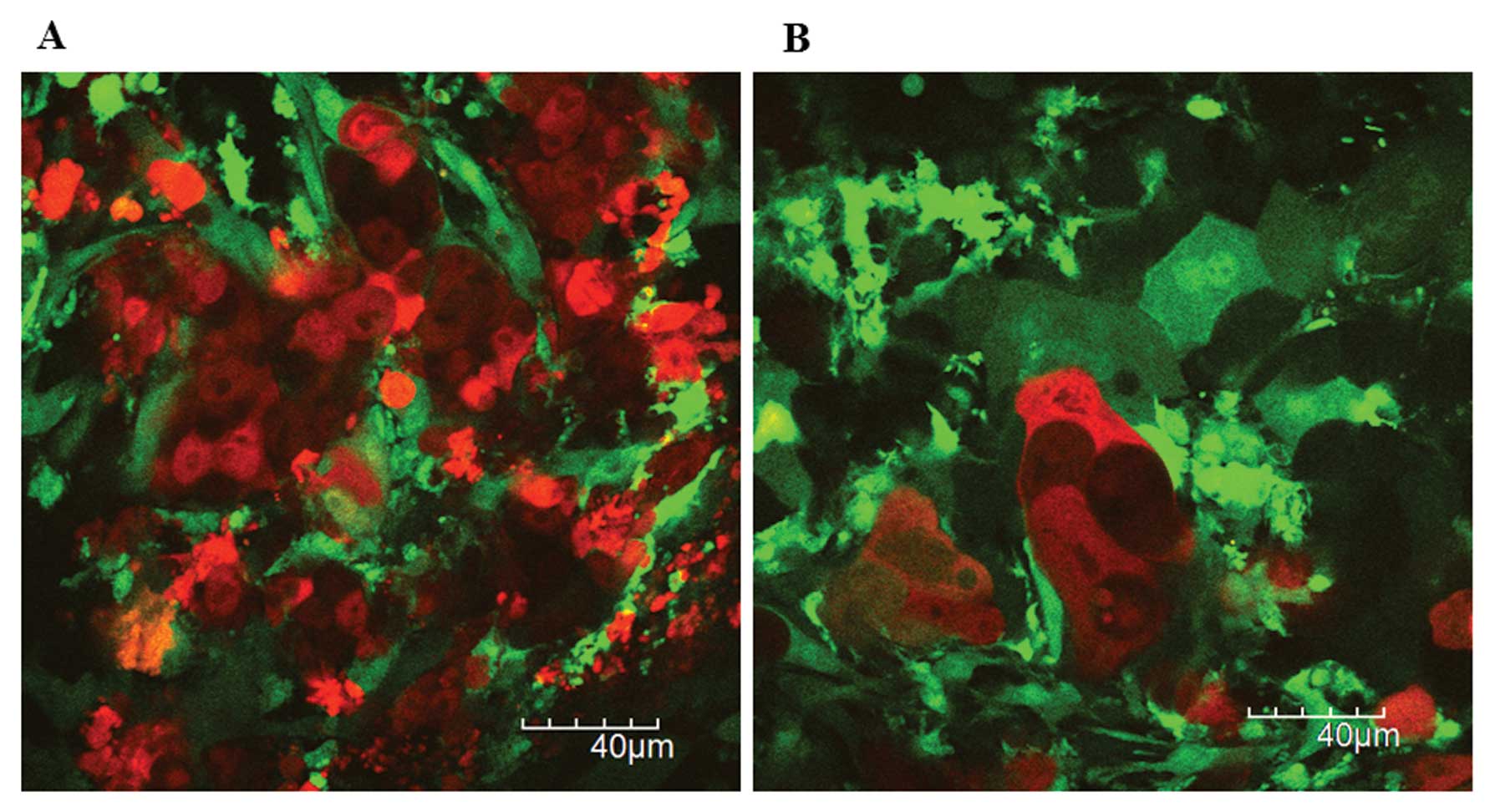
With regard to the in vivo real-time
chemotherapy response of metastatic tumor cells, tumor cell
fragmentation, condensation, swelling and intracellular vacuoles
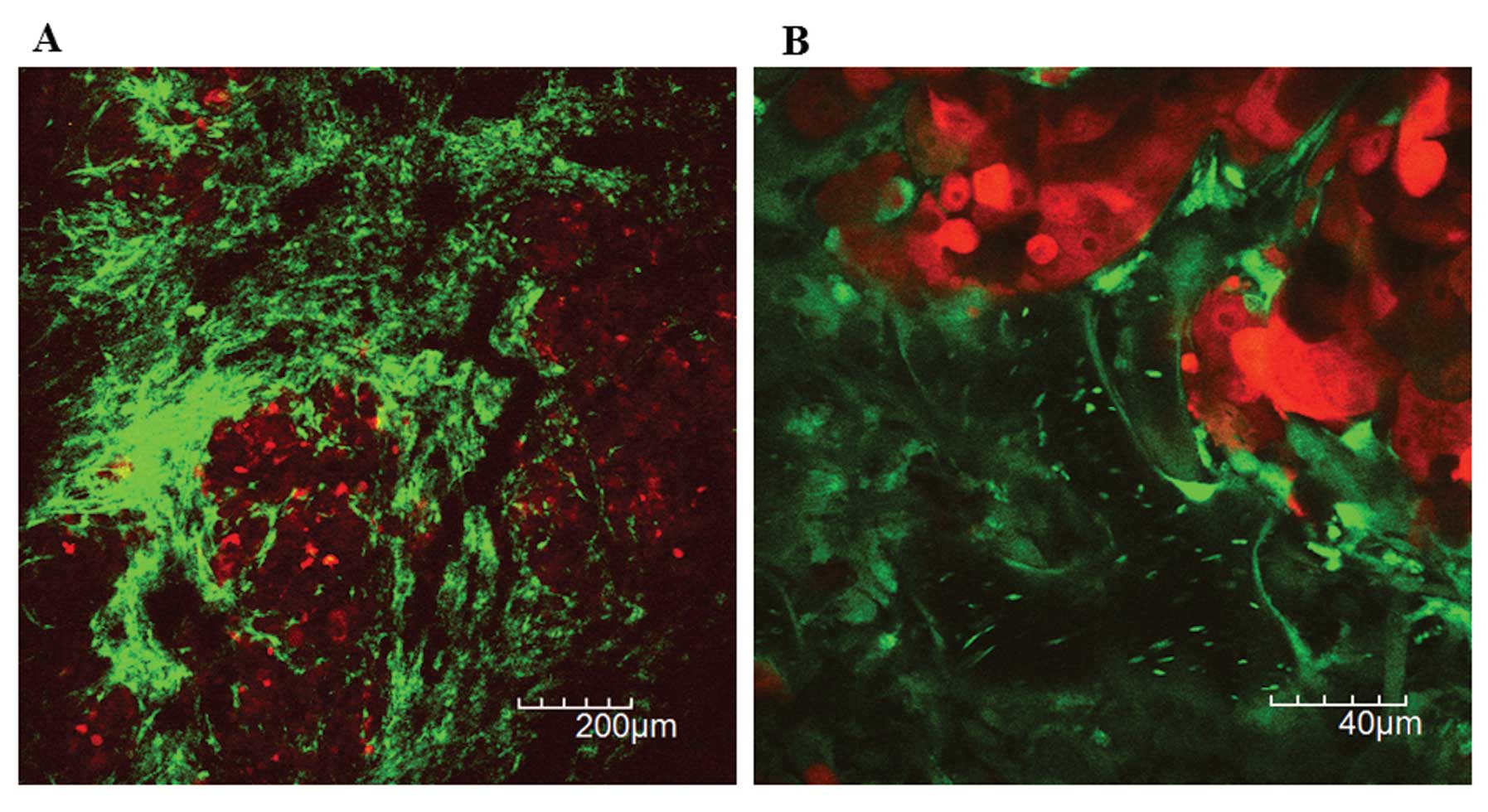
were observed under the 3rd intravital TPLSM (Fig. 6). The smaller micrometastatic nodule
was observed which showed the viable tumor cell in the central area
of the nodule, the RFP-expressing fragments in the peripheral area,
and the surrounding GFP-expressing host stromal cells (Fig. 7). The peripheral tumor cells may
induce apoptosis by chemotherapy, resulting in the peripheral tumor
cell fragmentation.
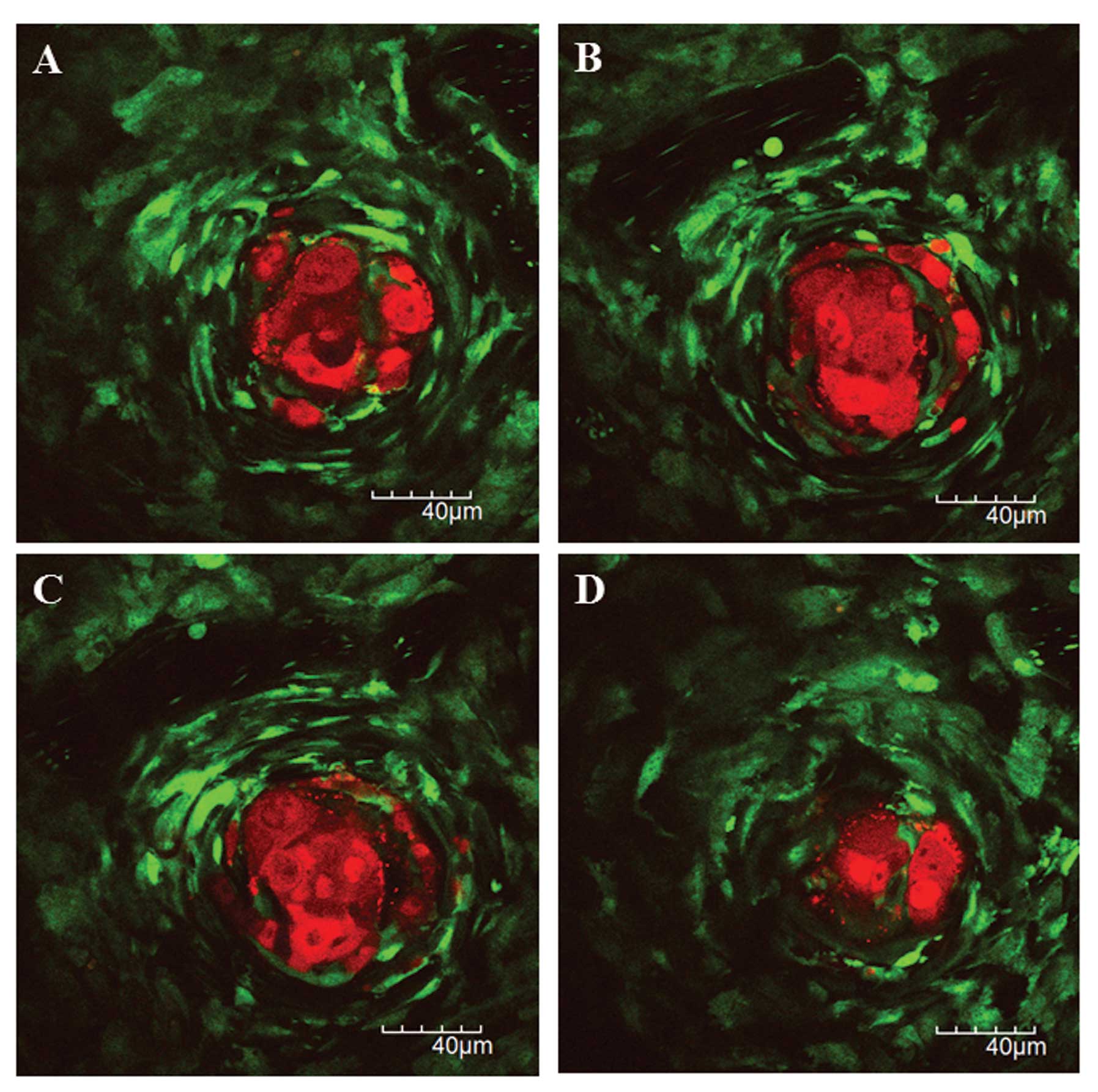
The other larger one was observed which showed the
acellular central area with RFP-expressing fragments, the viable
tumor cell in the peripheral area, and the surrounding
GFP-expressing host stromal cells (Fig.
8). The central tumor cells may induce central tumor necrosis
or apoptosis due to tumor hypoxia or chemotherapy. There was no
obvious morphological difference in tumor response on liver
metastatic xenografts between 5-FU and CPT-11.
Tumor vessel abnormality on liver
metastatic xenografts by 3rd intravital TPLSM
With regard to the in vivo real-time
chemotherapy response of host cells, the flowing of the aggregated
platelets and the adhesion of them to tumor vessels were frequently
observed under the 3rd intravital TPLSM. The blood flow by the
platelet movement in the liver metastatic xenografts was slower
than that in normal liver of the same mouse (data not shown). These
results suggest that the endothelial damage or coagulation
abnormality may occur within the tumor vessels after chemotherapy.
The phenomena of leukocyte arrest or rolling to the endothelium was
not observed within the tumor vessels of metastatic xenografts
either before or after chemotherapy, as those found in the
inflammatory bowel disease model.
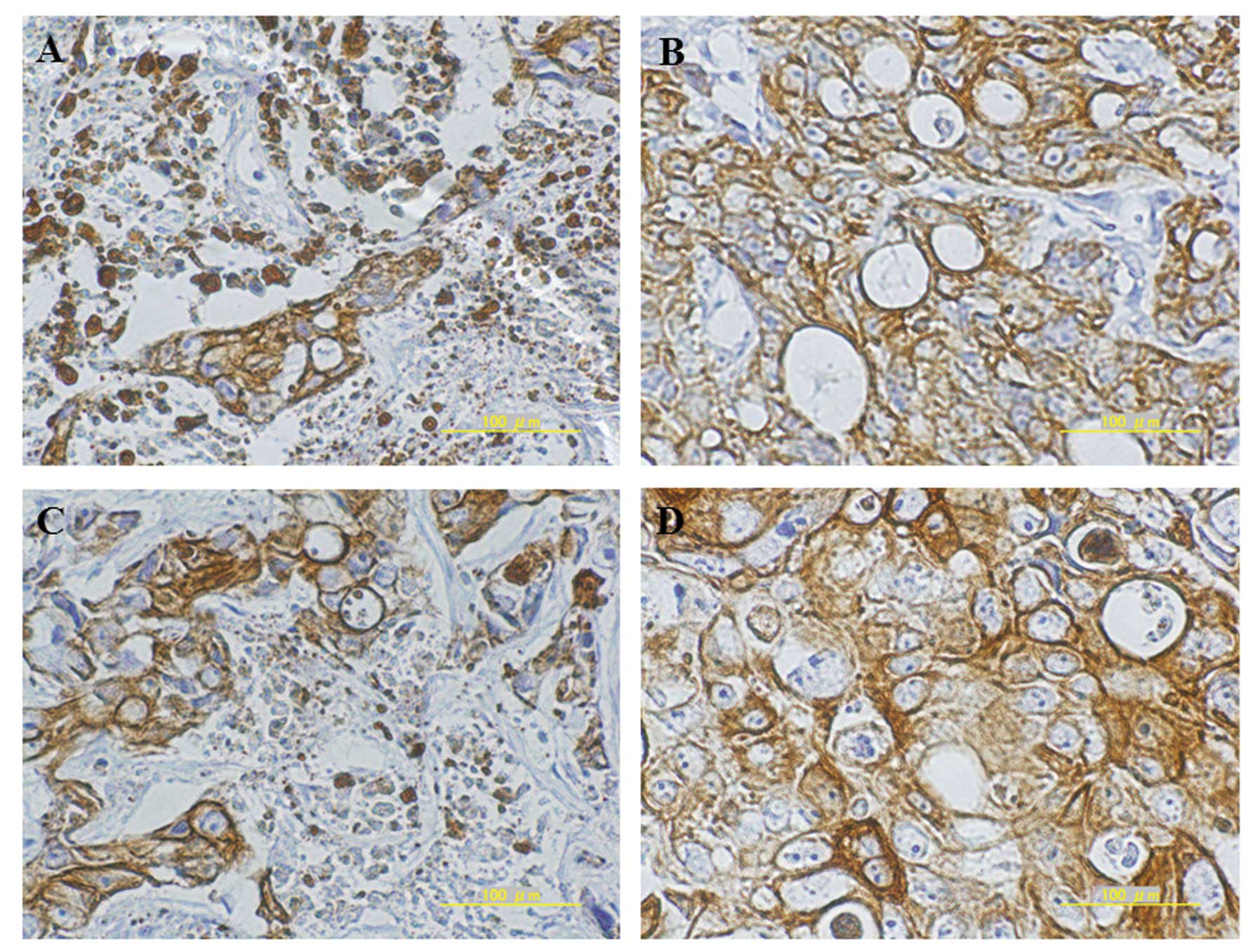
Human CK20 immunostaining of liver
metastatic xenografts after chemotherapy
Anti-human CK20 antibody was used for the detection
of RFP-HT29 cells in the xenogeneic liver metastasis model.
Basically, histopathological findings supported in vivo
real-time observation of anticancer drug efficacy for liver
metastatic xenografts by intravital TPLSM (Fig. 9A and B).
Discussion
In patients with mCRC, the control of metastatic
disease directly reflects their prognosis. Although we have now
many types of treatment options for mCRC patients, metastatic
disease control depends in part on whether or how metastatic
diseases respond to chemotherapy (20). Thus, it remains particularly
important to understand the mechanisms how metastatic tumor cells
(and/or host stromal cells) respond or resist to chemotherapy on
the metastatic organs.
Previously, we established time-series intravital
TPLSM imaging of colorectal liver metastasis model using RFP
expressing cancer cells and GFP expressing nude mice (16). We have also reported the in
vivo real-time development of liver metastatic formation and
tumor angiogenesis at the cellular level in the metastatic organs
of the same living mice (17). In
this study, we visualized the response of metastatic tumor cells to
5-FU or CPT-11 in the three-dimensional microenvironment in
vivo real-time using a z-stacks imaging. We also visualized the
dynamics of host response to chemotherapy, especially
platelet-endothelial interactions, in the tumor vessels of
metastatic liver xenografts using a time-lapse imaging.
Chemotherapy responses such as tumor cell
fragmentation, condensation, swelling and intracellular vacuoles
have been observed by conventional histopathological examinations
including hematoxylin-eosin or immunohistochemistry (21–23).
Morphological 3D analysis can be performed by reconstructing the
serial sections of the excised sample. However, we can easily image
and analyze the chemotherapy response consisting of ‘viable tumor
cells’ and ‘viable stromal cells’ in either two-dimensionally or
three-dimensionally on metastatic tumor xenografts of the living
mice using our model. Although there was no obvious morphological
difference in tumor response between 5-FU and CPT-11, the phenomena
of peripheral and central fragmentation in the micrometastatic
nodules observed in this study seem to be important on the research
of cancer cell death. They may be associated with drug delivery,
tumor hypoxia, and the both in the metastatic xenografts.
The platelet aggregation and the relatively
decreased blood flow in the tumor vessels of colorectal liver
metastases are also particular important findings. The platelet
aggregation has been considered the surrogate of endothelial damage
or hypercoagulable condition (24,25).
In this study, we did not demonstrate the direct evidence of
endothelial damage such as the identification of the discontinuity
of endothelium by isolectin staining. However, these finings
indicate that chemotherapy may induce tumor vessel damage or
intratumoral coagulation abnormality. The endothelial damage or
coagulation abnormality by chemotherapy may be associated with the
mechanisms of metronomic chemotherapy (26,27).
Preclinically, murine xenograft models are still
used for the development of new anticancer therapeutics although
they are not ideal based on the criticisms voiced. There is a
significant site-specific variation in response to chemotherapy for
a number of human malignant tumors (28). Wilmanns et al have reported
that the tumor xenografts of human colon cancer cell line, KM12L4a,
at different anatomical locations showed different tumor responses
to doxorubicin whose growth inhibition rate were 80% in
subcutaneous xenograft, 40% in orthotopic xenograft, and 10% in
liver metastatic xenograft, respectively (29,30).
In the preclinical model of anticancer therapeutics development, it
remains an unanswered question whether the chemotherapy response of
the subcutaneous (non-metastatic) tumor xenografts is
representative of clinically metastatic disease which is a
substantial target of chemotherapy. We believe that the metastatic
tumor xenografts may be good and practical for the preclinical
murine models of the evaluation of new anticancer
chemotherapeutics. In this regard, time-series intravital TPLSM
imaging in the same animals may be a useful tool for screening and
evaluating new chemotherapeutics with less interindividual
variability.
In conclusion, intravital TPLSM imaging can directly
visualize the cellular morphology in tumor microenvironment in the
living organs of the living animals. Time-series intravital TPLSM
imaging can provide the dynamic (in vivo real-time)
pathology in the same living organs of the same living animals at
the indicated time points, resulting in the less interindividual
variability between animals.
Acknowledgements
This study was supported by grants from the Ministry
of Education, Culture, Sports, Science and Technology of Japan
(KAKENHI 22591484 to K.T., 21591723 to Y.I. and 21390377 to M.K.).
This work was also supported by a grant-in-aid of The Public Trust
Fund for the Promotion of Surgery, Tokyo, Japan. K.T. was supported
by the Okasan-Kato Foundation with regard to this study.
References
|
1
|
Gallagher DJ and Kemeny N: Metastatic
colorectal cancer: from improved survival to potential cure.
Oncology. 78:237–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cunningham D, Atkin W, Lenz HJ, et al:
Colorectal cancer. Lancet. 375:1030–1047. 2010. View Article : Google Scholar
|
|
3
|
Folprecht G, Gruenberger T, Bechstein WO,
et al: Tumour response and secondary resectability of colorectal
liver metastases following neoadjuvant chemotherapy with cetuximab:
the CELIM randomised phase 2 trial. Lancet Oncol. 11:38–47. 2010.
View Article : Google Scholar
|
|
4
|
Brouquet A, Abdalla EK, Kopetz S, et al:
High survival rate after two-stage resection of advanced colorectal
liver metastases: response-based selection and complete resection
define outcome. J Clin Oncol. 29:1083–1090. 2011. View Article : Google Scholar
|
|
5
|
Tanaka K, Inoue Y and Kusunoki M: The role
of cytoreduction as a multidisciplinary treatment modality for
metastatic colorectal cancer. Colorectal Cancer: Risk, Diagnosis
and Treatments. Jenkins JE: Nova Science Publishers; New York, NY:
pp. 161–176. 2010
|
|
6
|
Longley DB, Harkin DP and Johnston PG:
5-Fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu Y and Villalona-Calero MA: Irinotecan:
mechanisms of tumor resistance and novel strategies for modulating
its activity. Ann Oncol. 13:1841–1851. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raymond E, Faivre S, Chaney S, Woynarowski
J and Cvitkovic E: Cellular and molecular pharmacology of
oxaliplatin. Mol Cancer Ther. 1:227–235. 2002.
|
|
9
|
Le Dévédec SE, van Roosmalen W, Pont C, et
al: Two-photon intravital multicolour imaging to study metastatic
behaviour of cancer cells in vivo. Methods Mol Biol. 769:331–349.
2011.PubMed/NCBI
|
|
10
|
Beerling E, Ritsma L, Vrisekoop N, Derksen
PW and van Rheenen J: Intravital microscopy: new insights into
metastasis of tumors. J Cell Sci. 124:299–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ustione A and Piston DW: A simple
introduction to multiphoton microscopy. J Microsc. 243:221–226.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang BG, König K and Halbhuber KJ:
Two-photon microscopy of deep intravital tissues and its merits in
clinical research. J Microsc. 238:1–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Toiyama Y, Mizoguchi A, Okugawa Y, et al:
Intravital imaging of DSS-induced cecal mucosal damage in
GFP-transgenic mice using two-photon microscopy. J Gastroenterol.
45:544–553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koike Y, Tanaka K, Okugawa Y, et al: In
vivo real-time two-photon microscopic imaging of platelet
aggregation induced by selective laser irradiation to the
endothelium created in the beta-actin-green fluorescent protein
transgenic mice. J Thromb Thrombolysis. 32:138–145. 2011.
View Article : Google Scholar
|
|
15
|
Morimoto Y, Tanaka K, Toiyama Y, et al:
Intravital three-dimensional dynamic pathology of experimental
colitis in living mice using two-photon laser scanning microscopy.
J Gastrointest Surg. 15:1842–1850. 2011. View Article : Google Scholar
|
|
16
|
Tanaka K, Morimoto Y, Toiyama Y, et al:
Intravital dual-colored visualization of colorectal liver
metastasis in living mice using two photon laser scanning
microscopy. Microsc Res Tech. 75:307–315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanaka K, Morimoto Y, Toiyama Y, et al: In
vivo time-course imaging of tumor angiogenesis in colorectal liver
metastases in the same living mice using two-photon laser scanning
microscopy. J Oncol. 2012:2654872012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawano H, Kogure T, Abe Y, Mizuno H and
Miyawaki A: Two-photon dual-color imaging using fluorescent
proteins. Nat Methods. 5:373–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okabe M, Ikawa M, Kominami K, Nakanishi T
and Nishimune Y: ‘Green mice’ as a source of ubiquitous green
cells. FEBS Lett. 407:313–319. 1997.
|
|
20
|
Small RM, Lubezky N, Shmueli E, et al:
Response to chemotherapy predicts survival following resection of
hepatic colo-rectal metastases in patients treated with neoadjuvant
therapy. J Surg Oncol. 99:93–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mandard AM, Dalibard F, Mandard JC, et al:
Pathologic assessment of tumor regression after preoperative
chemoradiotherapy of esophageal carcinoma. Clinicopathologic
correlations Cancer. 73:2680–2686. 1994.PubMed/NCBI
|
|
22
|
Dworak O, Keilholz L and Hoffmann A:
Pathological features of rectal cancer after preoperative
radiochemotherapy. Int J Colorectal Dis. 12:19–23. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Becker K, Mueller JD, Schulmacher C, et
al: Histomorphology and grading of regression in gastric carcinoma
treated with neoadjuvant chemotherapy. Cancer. 98:1521–1530. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Furie B and Furie BC: Mechanisms of
thrombus formation. N Engl J Med. 359:938–949. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ruggeri ZM: Platelet adhesion under flow.
Microcirculation. 16:58–83. 2009. View Article : Google Scholar
|
|
26
|
Pasquier E, Kavallaris M and André N:
Metronomic chemotherapy: new rationale for new directions. Nat Rev
Clin Oncol. 7:455–465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kerbel RS and Kamen BA: The
anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer.
4:423–436. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Talmadge JE, Singh RK, Fidler IJ and Raz
A: Murine models to evaluate novel and conventional therapeutic
strategies for cancer. Am J Pathol. 170:793–804. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wilmanns C, Fan D, Obrian C, et al:
Modulation of doxorubicin sensitivity and level of P-glycoprotein
expression in human colon-carcinoma cells by ectopic and orthotopic
environments in nude-mice. Int J Oncol. 3:413–422. 1993.PubMed/NCBI
|
|
30
|
Wilmanns C, Fan D, O’Brian CA, Bucana CD
and Fidler IJ: Orthotopic and ectopic organ environments
differentially influence the sensitivity of murine colon carcinoma
cells to doxorubicin and 5-fluorouracil. Int J Cancer. 52:98–104.
1992. View Article : Google Scholar
|