Introduction
Tumor stage, postoperative residual tumor burden,
histological type and histological grade are the most important
clinical-pathological factors related to the prognosis of patients
with epithelial ovarian cancer (EOC) (1). However, patients with comparable risk
factors may have different prognoses underlying the need for
further prognostic factors (2).
Uncontrolled cellular proliferation is one of the
definitive characteristics of malignancies (3). Mitotic count is a traditional and
practical method to determine proliferative activity, but is
hampered by several conflicting factors (4). Alternatively, immunohistochemical
detection of proliferating cells may help to determine the
proliferative potential of a tumor. Ki-67 is expressed during all
active phases of the cell cycle, and the monoclonal Ki-67 antibody
MIB-1 binds to the nuclear Ki-67 antigen (5). Growing evidence underlines that high
expression of Ki-67 indicates poor prognosis in several types of
cancers (6–9). In EOC, however, Ki-67 has not yet been
fully established as a reliable prognostic factor thus showing the
need for further investigation.
Nulliparity and infertility as hormonal risk factors
have been identified in regards to the development of EOC in
epidemiological studies, while pregnancy and oral contraceptives
appear to protect from the disease (1). This has led to further investigation
of the role of the two steroid hormones estrogen and progesterone
and their receptors in EOC (10,11).
Even if growing evidence supports the theory that the progesterone
receptor (PR) has a favorable impact on the prognosis of EOC in
contrast to the estrogen receptor (ER), the available literature is
contradictory (11–15).
The invasion in surrounding tissue is another
definitive characteristic of malignancies (3). Urokinase-type plasminogen activator
(uPA) catalyzes the conversion of plasminogen to plasmin (16). Plasmin leads to the degradation of
the extracellular matrix and basement membrane (16). Plasminogen activator inhibitor-1
(PAI-1) inhibits the activation of uPA (17). However, PAI-1 modulates cell
migration and induces tumor proliferation by activation of
intracellular signal transduction (17). Thereby, uPA and PAI-1 are strongly
implicated as a promoter in various solid tumors (16,17).
Current literature concerning uPA and PAI-1 in EOC is still
controversial (18,19).
In this explorative, retrospective study, we
examined possible associations between Ki-67, PR, ER, uPA, PAI-1
and overall survival (OS) and disease-free survival (DFS) in an
unselected consecutive cohort of patients with EOC.
Materials and methods
Patients and tissue samples
We searched the archives for all patients with EOC
who underwent primary surgery during the time period of 1997 and
2004. Patients entered the present study when formalin-fixed
paraffin-embedded (FFPE) tissue or aliquots of fresh tissue were
available. Follow-up was conducted by writing letters to patients
or their physicians, phoning them, and by checking the patient
records until November of 2012. We documented i) death from EOC or
from other reasons unrelated to cancer and ii) recurrence of
disease, which included metastasis, local relapse and secondary
tumors. Patient charts were reviewed to collect data regarding age
at diagnosis, histological type, tumor stage of disease according
to the guidelines of the International Federation of Gynecology and
Obstetrics (FIGO) and lymph node status. We compared EOC with
high-grade serous carcinoma vs. other types in the analysis of
histological type. The amount of residual disease after primary
surgery was registered as R0, R1 and R2. Completed chemotherapy was
defined as 6 courses of platinum-based monotherapy in early EOC or
as platinum-based combination with paclitaxel for patients being
treated after 2000 or with cyclophosphamide for the rest of the
patients. The present study was approved by the Research Ethics
Committee of the University Medical Centre Mainz, Germany. Informed
consent was obtained from all patients. All specimens were handled
according to the ethical and legal standards.
Immunohistochemistry and evaluation of
staining of Ki-67, PR and ER
Serial sections of formalin-fixed slices were
stained with monoclonal MIB-1 antibodies (clone MIB-1; Dako,
Glostrup, Denmark) as previously described (20) (Fig.
1). In accordance with previous reports on Ki-67 staining,
overexpression was defined when >20% of the cancer cells were
stained (6,21).
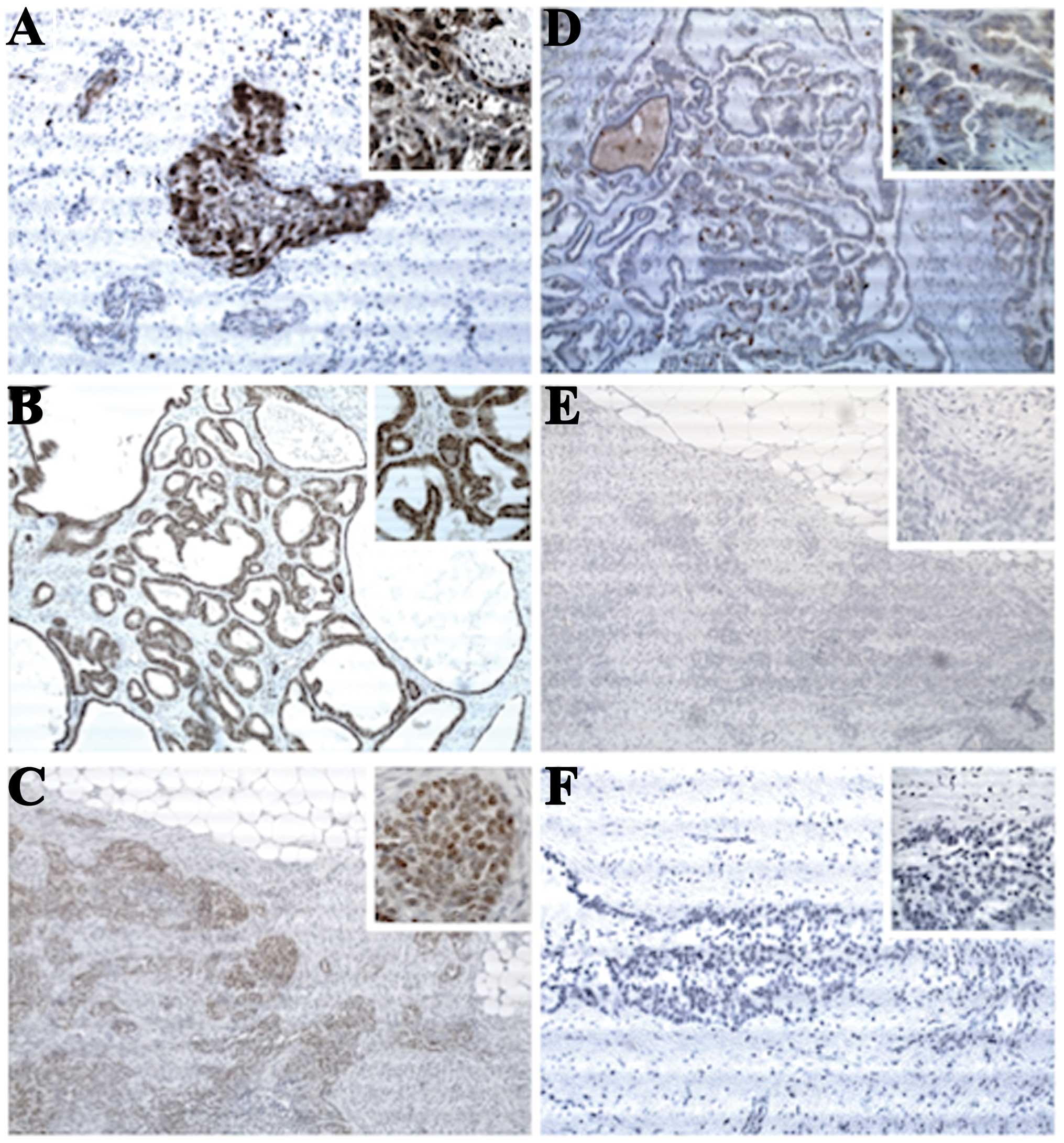 | Figure 1Representative images of
immunostaining against (A and D) Ki-67, (B and E) PR and (C and F)
ER. (A) Ki-67-positive infiltrate in >50% of the cancer cells in
a papillary serous EOC, histological grade 3 (original
magnification, ×100; inset, ×400). (B) Strong PR-positive
infiltrate in >80% of the cancer cells in an endometrioid EOC,
histological grade 1 (original magnification, ×100; inset, ×400).
(C) Strong ER-positive infiltrate in >80% of the cancer cells in
a papillary serous EOC, histological grade 2 (original
magnification, ×100; inset, ×400). (D) Ki-67-positive infiltrate in
<6% of the cancer cells in a papillary serous EOC, histological
grade 1 (original magnification, ×100; inset, ×400). (E) No
PR-positive infiltrate in a papillary serous EOC, histological
grade 2 (original magnification, ×100; inset, ×400). (F) No
ER-positive infiltrate in a papillary serous EOC, histological
grade 3 (original magnification, ×100; inset, ×400). PR,
progesterone receptor; ER, estrogen receptor; EOC, epithelial
ovarian carcinoma. |
Serial sections of formalin-fixed slices were
stained with either monoclonal ER antibodies (clone 1D5) or
monoclonal PR antibodies (clone PgR 636) (both from Dako), as
previously described (22)
(Fig. 1). Expression of ER and PR
was assessed using an immunoreactive score defined by the product
of a proportion (0, none; 1, <10%; 2, 10–50%; 3, 51–80%; 4,
81–100%) and an intensity score (0, no staining; 1, weak; 2,
moderate; 3, strong) (23).
Enzyme-linked immunosorbent assay (ELISA)
of uPA and PAI-1
The protein levels of uPA and PAI-1 were determined
as previously described by Steiner et al using commercially
available kits (Imibind tissue uPA ELISA kit product no. 894, and
Imibind tissue PAI-1 ELISA kit product no. 82; American Diagnostica
Inc., Greenwich, CT, USA) (24).
Statistical analyses
Statistical analyses were performed using the SPSS
statistical software program, version 21.0 (SPSS Inc., Chicago, IL,
USA). Patient characteristics were expressed as absolute and
relative numbers or as median with their quartiles. Cut-off
optimization was performed for PR and ER using univariable Cox
regression analyses. The Cox proportional hazard regression model
was used to evaluate the effect of explorative variables on DFS and
OS. First, univariable Cox regression analyses were performed for
every single variable. Secondly, variables with a p-value <0.05
entered the multivariable Cox regression analysis with a variable
selection via backward elimination. All associations were expressed
as hazard ratios (HR) with their 95% confidence intervals (CI) and
p-values. Kaplan-Meier estimations were performed to describe
survival rates. Correlations between the proliferation markers were
analyzed with the Spearman’s rank correlation coefficient. As this
was an explorative study, no adjustments for multiple testing were
carried out. The statistical tests were carried out for
illustrative purposes rather than hypothesis testing. p-values were
provided for descriptive reasons only and should be interpreted
with caution and in connection with effect estimates.
Results
A total of 162 patients were screened. Thirty-four
and 18 patients were excluded due to missing tissue samples and
inappropriate follow-up information, respectively. Two patients
suffered from a borderline tumor. Thereby, 108 patients entered the
present study. The median follow-up time was 43.3 (range 11.4–68.0)
months. Seventy-nine recurrences and 66 deaths occurred. The
characteristics of the patients are presented in Table I.
 | Table IPatient characteristics (n=108). |
Table I
Patient characteristics (n=108).
| Parameter | n (%) |
|---|
| Mean age ± SD
(years) | 61.7±11.4 |
| Tumor stage
(FIGO) |
| I | 27 (25.0) |
| II | 4 (3.7) |
| III | 66 (61.1) |
| IV | 11 (10.2) |
| Histological grade
(n=103) |
| G1 | 14 (13.6) |
| G2 | 44 (42.7) |
| G3 | 45 (43.1) |
| Histological type
(n=107) |
| Serous | 62 (57.9) |
| Mucinous | 5 (4.7) |
| Endometrioid | 14 (13.1) |
| Clear cell | 6 (5.6) |
|
Undifferentiated | 10 (9.3) |
| Mixed | 10 (9.3) |
| Postoperative
residual tumor burden (n=105) |
| R0 | 26 (24.1) |
| R1 | 21 (19.4) |
| R2 | 58 (53.7) |
| Chemotherapy
(n=97) |
| Complete | 70 (72.2) |
| Incomplete | 27 (27.8) |
| Prognostic
factors |
|
Ki-67+ | 76 (73.8) |
|
PR+ | 15 (14.3) |
|
ER+ | 20 (19.0) |
| uPA | 3 (1.0–6.0)a |
| PAI-1 | 21
(12.0–56.3)a |
Immunohistochemical analysis and ELISA were
performed on 103 and 87 EOC tissues, respectively. In this cohort
the optimal cut-off for PR was 6 (data not shown). For ER no
meaningful cut-off was detectable (data not shown). In analogy to
PR, patients with a score of 6 or higher for ER were considered
positive. Positive-staining of Ki-67, PR and ER was observed in
73.8, 14.3 and 19.0%, respectively. The median (quartiles) of uPA
and PAI-1 were 3.0 (1.0–6.0) and 21.0 (12.0–56.3), respectively
(Table I).
In univariable and mulitivariable Cox regression
analysis Ki-67 and PR were associated with DFS and OS (Table II). ER, uPA and PAI-1 were not
associated with prognosis (Table
II). Tumor stage, histological type, histological grade,
postoperative residual tumor burden and completeness of
chemotherapy were associated with DFS and OS in univariable Cox
regression analysis (Table II).
However, in multivariable Cox regression analysis only
postoperative residual tumor burden and completeness of
chemotherapy were associated with DFS and OS (Table II).
 | Table IIUnivariable and multivariable Cox
regression analyses for disease-free and overall survival. |
Table II
Univariable and multivariable Cox
regression analyses for disease-free and overall survival.
| A, Disease-free
survival |
|---|
|
|---|
| Univariable | Multivariable |
|---|
|
|
|
|---|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years) | 1.0
(0.99–1.03) | 0.536 | - | - |
| Tumor stage
(FIGO) | 2.7
(2.03–3.62) |
<0.001 | 1.3
(0.77–2.21) | 0.328 |
| Histological
grade | 1.8
(1.24–2.51) | 0.002 | 0.8
(0.48–1.20) | 0.239 |
| Histological
type | 0.5
(0.29–0.77) | 0.002 | 0.6
(0.32–1.18) | 0.146 |
| Postoperative
residual tumor burden | 5.1
(3.20–8.12) |
<0.001 | 5.5
(3.22–9.23) |
<0.001 |
| Completeness of
chemotherapy | 2.1
(1.28–3.54) | 0.004 | 1.8
(1.02–3.11) | 0.043 |
| Ki-67 | 2.2
(1.18–3.98) | 0.013 | 11.5
(2.64–49.7) | 0.001 |
| PR | 0.4
(0.15–0.81) | 0.015 | 0.15
(0.03–0.68) | 0.014 |
| ER | 0.9
(0.52–1.69) | 0.841 | - | - |
| uPA | 1.0
(0.95–1.06) | 0.850 | - | - |
| PAI-1 | 1.0
(0.99–1.01) | 0.889 | - | - |
|
| B, Overall
survival |
|
| Univariable | Multivariable |
|
|
|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value |
|
| Age (years) | 1.0
(0.99–1.04) | 0.223 | - | - |
| Tumor stage
(FIGO) | 2.7
(1.95–3.83) |
<0.001 | 1.2
(0.64–2.34) | 0.574 |
| Histological
grade | 1.8
(1.18–2.60) | 0.005 | 0.7
(0.44–1.23) | 0.245 |
| Histological
type | 0.6
(0.37–1.04) | 0.071 | - | - |
| Postoperative
residual tumor burden | 4.4
(2.70–7.26) |
<0.001 | 5.3
(2.85–9.85) |
<0.001 |
| Completeness of
chemotherapy | 2.7
(1.55–4.70) |
<0.001 | 2.4
(1.30–4.50) | 0.005 |
| Ki-67 | 2.8
(1.33–5.98) | 0.007 | 21.1
(9.9–113.1) |
<0.001 |
| PR | 0.4
(0.14–0.90) | 0.030 | 0.13
(0.03–0.68) | 0.016 |
| ER | 0.6
(0.28–1.26) | 0.176 | - | - |
| uPA | 0.9
(0.93–1.06) | 0.810 | - | - |
| PAI-1 | 1.0
(0.99–1.01) | 0.728 | - | - |
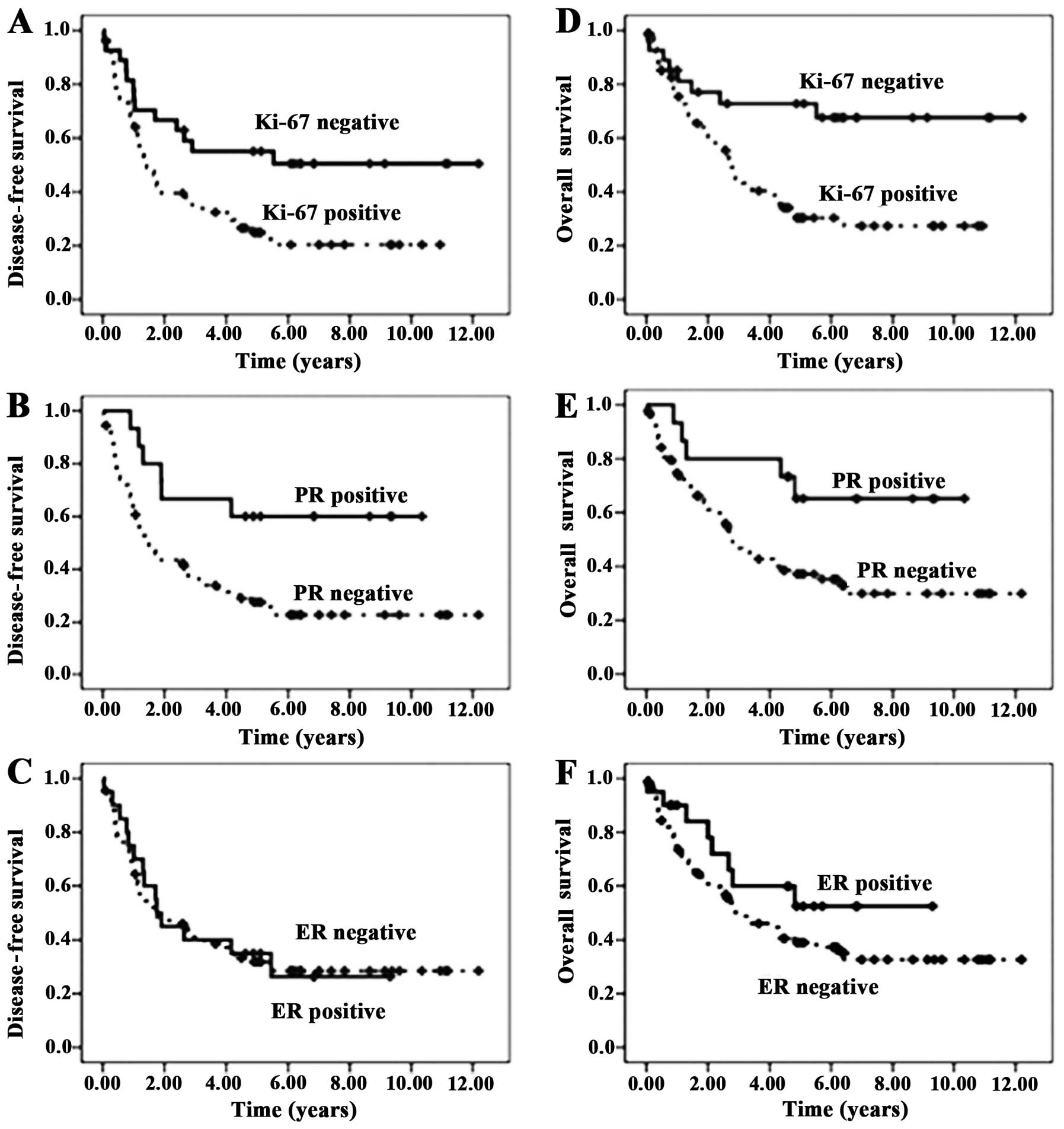
Kaplan-Meier plots demonstrated the influence of
Ki-67 and PR on the 5-year DFS rates (55.1 vs. 24.9%, p=0.011 and
60.0 vs. 27.6%, p=0.011, respectively) and on the 5-year OS rates
(72.8 vs. 30.3%, p=0.005 and 65.2 vs. 37.1%, p=0.023, respectively)
(Fig. 2).
Explorative correlation analyses revealed a
statistically relevant correlation between postoperative residual
tumor burden and tumor stage, between PR and ER, between
completeness of chemotherapy and age, between histological grade
and tumor stage and between histological grade and postoperative
tumor burden, respectively (all p-values <0.001) (Table III). Furthermore, correlation
analyses showed a strong correlation between postoperative residual
tumor burden and tumor stage and between PR and ER, respectively
(rho >0.4) (Table III).
 | Table IIIExplorative Spearman’s rank
coefficient analyses of all clinicopathological factors. |
Table III
Explorative Spearman’s rank
coefficient analyses of all clinicopathological factors.
| Age | Tumor stage
(FIGO) | Histological
grade | Histological
type | Post-operative
residual tumor burden | Completeness of
chemotherapy | Ki-67 | PR | ER | uPA |
|---|
| Tumor stage
(FIGO) | 0.028 | - | | | | | | | | |
| 0.777 | | | | | | | | | |
| Histological
grade | −0.051 | 0.379 | - | | | | | | | |
| 0.609 |
<0.001 | | | | | | | | |
| Histological
type | 0.026 | −0.275 | −0.261 | - | | | | | | |
| 0.792 | 0.004 | 0.004 | | | | | | | |
| Postoperative
residual tumor burden | 0.140 |
0.759 | 0.365 | −0.255 | - | | | | | |
| 0.154 |
<0.001 |
<0.001 | 0.009 | | | | | | |
| Completeness of
chemotherapy | 0.380 | 0.105 | −0.039 | 0.210 | 0.236 | - | | | | |
|
<0.001 | 0.304 | 0.714 | 0.041 | 0.021 | | | | | |
| Ki-67 | 0.087 | 0.201 | 0.194 | −0.142 | 0.228 | 0.121 | - | | | |
| 0.383 | 0.041 | 0.055 | 0.157 | 0.022 | 0.247 | | | | |
| PR | −0.154 | −0.303 | −0.229 | 0.205 | −0.325 | −0.130 | −0.004 | - | | |
| 0.116 | 0.002 | 0.022 | 0.038 | 0.001 | 0.208 | 0.996 | | | |
| ER | −0.046 | −0.057 | −0.165 | 0.162 | −0.036 | 0.053 | −0.098 |
0.426 | - | |
| 0.644 | 0.564 | 0.101 | 0.103 | 0.718 | 0.612 | 0.324 |
<0.001 | | |
| uPA | 0.161 | 0.187 | 0.124 | −0.226 | 0.189 | −0.071 | −0.043 | −0.067 | −0.088 | - |
| 0.137 | 0.083 | 0.269 | 0.037 | 0.085 | 0.539 | 0.699 | 0.545 | 0.424 | |
| PAI-1 | 0.018 | 0.172 | 0.140 | −0.040 | 0.117 | −0.019 | 0.114 | −0.111 | −0.181 | 0.151 |
| 0.867 | 0.109 | 0.207 | 0.715 | 0.286 | 0.869 | 0.302 | 0.308 | 0.095 | 0.163 |
Discussion
The present study examined the influence of the
expression of Ki-67, PR and ER as well as uPA/PAI-1 on the
prognosis of an unselected cohort of 108 patients with EOC
utilizing Kaplan-Meier estimations as well as univariable and
multivariable Cox regression analyses.
There is a growing body of evidence for a prognostic
role of Ki-67 in EOC. In several reports, patients with high Ki-67
were found to have a less favorable 5-year survival (8,25).
These patients were also more likely to have other poor prognostic
factors, including advanced tumor stage (21,26),
higher tumor grade (21,27) and postoperative residual tumor
burden (27). However, other
studies were not able to show significant associations between
Ki-67 and tumor stage (6,28), histological type (21,25,27)
and tumor grade (6,28). In the present study, patients with
high Ki-67 showed a much shorter OS and DFS compared to EOC cases
with low Ki-67 in the univariable and multivariable analyses. The
5-year overall survival rate for patients with a low Ki-67 was more
than two times higher when compared to Ki-67-positive tumors. This
relation is similar to the findings of other studies (6,21,27,28).
However, the high HR of Ki-67 on DFS and OS and their broad 95% CI
can be regarded as a result of the small number of events in this
relatively small cohort with 108 patients and should thereby be
interpreted with caution. In the present study, Ki-67 correlated
only weakly with tumor stage and with postoperative residual tumor
burden. Surprisingly, no association was detected between Ki-67 and
histological grade or other clinicopathological factors. This may
be due to the fact, that the predictive value of histological grade
was hampered by several conflicting factors such as interobserver
variability and the co-existence of different definitions (29). On the other hand, this supports our
finding that Ki-67 behaves as an independent prognostic marker in
patients with EOC.
The available literature concerning the prognostic
impact of PR and ER on the prognosis of EOC is contradictory.
Several studies report no association between the expression of PR
and the survival of EOC patients (12,13),
while other studies report that a higher PR status indicates a
favorable prognosis (14,15). This may rely on different analytical
methods, cut-off and scoring systems (12,14,15,30).
Furthermore, the inclusion criteria of several steroid receptor
expression studies for EOC are broad so that even patients with
certain histological subtypes, histological grade or tumor stage
are included (30,31). However, a meta-analysis conducted by
Zhao et al (11) found that
higher levels of PR predicted favorable prognosis whereas
expression of ER did not influence prognosis. The results from our
study are in concordance with these results. In the present study,
PR and ER showed a moderate correlation. This finding is in line
with in vitro results and clinical results (30,32,33).
Furthermore, the expression of PR correlated only weakly with tumor
stage or histological grade or histological type or postoperative
residual tumor burden supporting the theory that PR represents an
independent prognostic marker.
Notably, expression of uPA and PAI-1 was not
associated with prognosis or other clinicopathological factors in
this cohort of patients. These findings confirm the results of a
study by van der Burg et al although uPA and PAI-1 levels
were higher in ovarian cancer patients when compared to the levels
in benign lesions (18). On the
contrary, other studies showed an independent association of the
overexpression of uPA and PAI-1 with impaired prognosis in EOC
(19,34). However, to the best of our knowledge
these results have not yet been validated.
Unfortunately, to date, no meaningful therapeutic
benefit results out of the growing, but partially conflicting body
of evidence on endocrine regulation and proliferation of EOC.
Endocrine therapy, e.g. with the aromatase inhibitor letrozole or
with tamoxifen, has shown small clinical benefit only in subgroups
of patients with EOC (35,36). Even if it is self-evident that
antiproliferative chemotherapy is more effective in tumors which
are highly proliferative, possibly due to an increased
chemosensitivity, the treatment decision for adjuvant chemotherapy
mostly relies on the tumor stage and not on proliferation (37). The decision regarding chemotherapy
is influenced by the histological grade only in the early stages.
However, to date, no single validated prognostic biomarker is
available for patients with EOC. This may depend on the
multifarious demands for biomarkers being regarded as valuable
prognostic factors. The proposed biomarker should provide
specificity, sensitivity and should be prognostic, reproducible,
independent and validated. Thereby, the introduction of a new
biomarker may facilitate a simpler and more consistent method with
which to stratify patients. Possibly, this may improve our
understanding of carcinogenesis, tailor adjuvant therapy and
improve prognosis. At present, the significant and already
established prognostic factors are clinicopathological variables
such as postoperative residual tumor burden, tumor stage,
histological type, histological grade, completeness of chemotherapy
and age.
In conclusion, we demonstrated in an explorative,
retrospective study that Ki-67 is an independent prognostic marker
for patients with EOC. After cut-off optimization, PR also
functioned as an independent prognostic marker. ER, uPA and PAI-1
were not associated with prognosis. Clearly, these findings should
be validated in additional prospective studies.
Acknowledgements
Sections of the results presented in the present
study derived from the doctoral thesis of Ms. Nina Mantai.
References
|
1
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gadducci A, Cosio S, Tana R and Genazzani
AR: Serum and tissue biomarkers as predictive and prognostic
variables in epithelial ovarian cancer. Crit Rev Oncol Hematol.
69:12–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Linden MD, Torres FX, Kubus J and Zarbo
RJ: Clinical application of morphologic and immunocytochemical
assessments of cell proliferation. Am J Clin Pathol. 97(Suppl 1):
S4–S13. 1992.PubMed/NCBI
|
|
5
|
Cattoretti G, Becker MH, Key G, et al:
Monoclonal antibodies against recombinant parts of the Ki-67
antigen (MIB 1 and MIB 3) detect proliferating cells in
microwave-processed formalin-fixed paraffin sections. J Pathol.
168:357–363. 1992. View Article : Google Scholar
|
|
6
|
Garzetti GG, Ciavattini A, Goteri G, et
al: Ki67 antigen immunostaining (MIB 1 monoclonal antibody) in
serous ovarian tumors: index of proliferative activity with
prognostic significance. Gynecol Oncol. 56:169–174. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Viale G, Giobbie-Hurder A, Regan MM, et
al: Prognostic and predictive value of centrally reviewed Ki-67
labeling index in postmenopausal women with endocrine-responsive
breast cancer: results from Breast International Group Trial 1–98
comparing adjuvant tamoxifen with letrozole. J Clin Oncol.
26:5569–5575. 2008.PubMed/NCBI
|
|
8
|
Kritpracha K, Hanprasertpong J, Chandeying
V, Dechsukhum C and Geater A: Survival analysis in advanced
epithelial ovarian carcinoma in relation to proliferative index of
MIB-1 immunostaining. J Obstet Gynaecol Res. 31:268–276. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Margulis V, Lotan Y, Karakiewicz PI, et
al: Multi-institutional validation of the predictive value of Ki-67
labeling index in patients with urinary bladder cancer. J Natl
Cancer Inst. 101:114–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scambia G, Ferrandina G, Agostino GD, et
al: Oestrogen and progesterone receptors in ovarian carcinoma.
Endocr Relat Cancer. 3:293–301. 1998. View Article : Google Scholar
|
|
11
|
Zhao D, Zhang F, Zhang W, He J, Zhao Y and
Sun J: Prognostic role of hormone receptors in ovarian cancer: a
systematic review and meta-analysis. Int J Gynecol Cancer.
23:25–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bizzi A, Codegoni AM, Landoni F, et al:
Steroid receptors in epithelial ovarian carcinoma: relation to
clinical parameters and survival. Cancer Res. 48:6222–6226.
1988.PubMed/NCBI
|
|
13
|
Geisler JP, Wiemann MC, Miller GA and
Geisler HE: Estrogen and progesterone receptor status as prognostic
indicators in patients with optimally cytoreduced stage IIIc serous
cystadenocarcinoma of the ovary. Gynecol Oncol. 60:424–427. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee P, Rosen DG, Zhu C, Silva EG and Liu
J: Expression of progesterone receptor is a favorable prognostic
marker in ovarian cancer. Gynecol Oncol. 96:671–677. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sinn BV, Darb-Esfahani S, Wirtz RM, et al:
Evaluation of a hormone receptor-positive ovarian carcinoma subtype
with a favourable prognosis by determination of progesterone
receptor and oestrogen receptor 1 mRNA expression in formalin-fixed
paraffin-embedded tissue. Histopathology. 59:918–927. 2011.
View Article : Google Scholar
|
|
16
|
Andreasen PA, Kjøller L, Christensen L and
Duffy MJ: The urokinase-type plasminogen activator system in cancer
metastasis: a review. Int J Cancer. 72:1–22. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nykjaer A, Conese M, Christensen EI, et
al: Recycling of the urokinase receptor upon internalization of the
uPA:serpin complexes. EMBO J. 16:2610–2620. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van der Burg ME, Henzen-Logmans SC, Berns
EM, van Putten WL, Klijn JG and Foekens JA: Expression of
urokinase-type plasminogen activator (uPA) and its inhibitor PAI-1
in benign, borderline, malignant primary and metastatic ovarian
tumors. Int J Cancer. 69:475–479. 1996.PubMed/NCBI
|
|
19
|
Konecny G, Untch M, Pihan A, et al:
Association of urokinase-type plasminogen activator and its
inhibitor with disease progression and prognosis in ovarian cancer.
Clin Cancer Res. 7:1743–1749. 2001.PubMed/NCBI
|
|
20
|
Chen Z, Gerhold-Ay A, Gebhard S, et al:
Immunoglobulin kappa C predicts overall survival in node-negative
breast cancer. PloS One. 7:e447412012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anttila BM, Kosma V, Ji H, et al: Clinical
significance of alpha-catenin, collagen IV, and Ki-67 expression in
epithelial ovarian cancer. J Clin Oncol. 16:2591–2600.
1998.PubMed/NCBI
|
|
22
|
Schmidt M, Bremer E, Hasenclever D, et al:
Role of the progesterone receptor for paclitaxel resistance in
primary breast cancer. Br J Cancer. 96:241–247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Remmele W and Stegner H: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German).
|
|
24
|
Steiner E, Pollow K, Hasenclever D, et al:
Role of urokinase-type plasminogen activator (uPA) and plasminogen
activator inhibitor type 1 (PAI-1) for prognosis in endometrial
cancer. Gynecol Oncol. 108:569–576. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aune G, Stunes AK, Tingulstad S, Salvesen
O, Syversen U and Torp SH: The proliferation markers Ki-67/MIB-1,
phosphohistone H3, and survivin may contribute in the
identification of aggressive ovarian carcinomas. Int J Clin Exp
Pathol. 4:444–453. 2011.PubMed/NCBI
|
|
26
|
Harlozińska A, Bar JK, Sedlaczek P and
Gerber J: Expression of p53 protein and Ki-67 reactivity in ovarian
neoplasms. Correlation with histopathology. Am J Clin Pathol.
105:334–340. 1996.PubMed/NCBI
|
|
27
|
Korkolopoulou P, Vassilopoulos I,
Konstantinidou AE, et al: The combined evaluation of
p27Kip1 and Ki-67 expression provides independent
information on overall survival of ovarian carcinoma patients.
Gynecol Oncol. 85:404–414. 2002.
|
|
28
|
Kerns BJ, Jordan PA, Faerman LL, Berchuck
A, Bast RC Jr and Layfield LJ: Determination of proliferation index
with MIB-1 in advanced ovarian cancer using quantitative image
analysis. Am J Clin Pathol. 101:192–197. 1994.PubMed/NCBI
|
|
29
|
Silverberg SG: Histopathologic grading of
ovarian carcinoma: a review and proposal. Int J Gynecol Pathol.
19:7–15. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lenhard M, Tereza L, Heublein S, et al:
Steroid hormone receptor expression in ovarian cancer: progesterone
receptor B as prognostic marker for patient survival. BMC Cancer.
12:5532012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kommoss F, Pfisterer J, Thome M, Schäfer
W, Sauerbrei W and Pfleiderer A: Steroid receptors in ovarian
carcinoma: immunohistochemical determination may lead to new
aspects. Gynecol Oncol. 47:317–322. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Langdon SP, Hirst GL, Miller EP, et al:
The regulation of growth and protein expression by estrogen in
vitro: a study of 8 human ovarian carcinoma cell lines. J Steroid
Biochem Mol Biol. 50:131–135. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akahira J, Inoue T, Suzuki T, et al:
Progesterone receptor isoforms A and B in human epithelial ovarian
carcinoma: immunohistochemical and RT-PCR studies. Br J Cancer.
83:1488–1494. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuhn W, Pache L, Schmalfeldt B, et al:
Urokinase (uPA) and PAI-1 predict survival in advanced ovarian
cancer patients (FIGO III) after radical surgery and platinum-based
chemotherapy. Gynecol Oncol. 55:401–409. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ahlgren JD, Ellison NM, Gottlieb RJ, et
al: Hormonal palliation of chemoresistant ovarian cancer: three
consecutive phase II trials of the Mid-Atlantic Oncology Program. J
Clin Oncol. 11:1957–1968. 1993.PubMed/NCBI
|
|
36
|
Papadimitriou CA, Markaki S, Siapkaras J,
et al: Hormonal therapy with letrozole for relapsed epithelial
ovarian cancer. Long-term results of a phase II study. Oncology.
66:112–117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kommoss S, du Bois A, Schmidt D,
Parwaresch R, Pfisterer J and Kommoss F: Chemotherapy may be more
effective in highly proliferative ovarian carcinomas - a
translational research subprotocol of a prospective randomized
phase III study (AGO-OVAR 3 protocol). Gynecol Oncol. 103:67–71.
2006. View Article : Google Scholar
|