Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth leading cancer in regards to incidence worldwide. The
epidemiologic data vary between different geographical regions.
Squamous cell carcinomas (SCC) accounts for more than 95% of all
HNSCC cases (1). Approximately
644,000 new cases of HNSCC occur every year (2). The tumorigenesis of SCC results from a
multistep accumulation of various genetic changes in squamous
cells. These different changes increasingly enhance the ability of
transformed cells to invade and proliferate (3). The heterogeneity of these alterations
elucidates why tumours with the same clinical stage and
localisation often show significant differences in their clinical
outcome and treatment response (4,5).
Multimodal therapy for HNSCC includes surgery, chemoradiation and
often the combination of these strategies (6). Chemotherapy is the primary treatment
for advanced tumours. Multimodal tumour treatment reduces the
quality of life of patients, and the psychosocial consequences are
greater than that of other neoplasms. Despite new modalities in
therapeutic strategies, the survival rate of SCC patients is still
poor, and the overall 5-year survival rate of HNSCC patients has
not improved over the past three decades (3,7,8). The
biological factors and pathways that cause locoregional and distant
spreading of the neoplasms are not completely understood. Many
biological markers have been characterized. Several proteins such
as epidermal growth factor receptor (EGFR), p53, and matrix
metalloproteinases (MMPs) have been described as potential markers
for survival prediction in SCC (3).
Targeting EGFR has been applied modestly to HNSCC tumours (9). Thus, new treatment modalities are
needed to increase the long-term survival of patients with
SCCs.
The main risk factors for the development of HNSCC
are alcohol and tobacco consumption. Clinical and pathological
evidence suggests that viral oncogenic human papillomavirus (HPV)
infection is another crucial etiologic factor (10). The epidemiological, genetic,
molecular and clinical profile of HPV-associated HNSCC cells
appears to differ from that of tobacco and alcohol-induced HNSCC
(non-HPV). HPV-positive oropharyngeal carcinomas are characterized
by young age at onset, male predominance, and strong association
with sexual behaviour (11,12). Kreimer and colleagues (13) analysed study locations and found
that HPV prevalence of oral SCCs was similar in Europe (16.0%) and
North America (16.1%) but significantly greater in Asia (33.0%). In
contrast, HPV prevalence of oropharyngeal SCCs was significantly
higher in North America (47%) compared with Europe (28%). Patients
with HPV-associated oropharyngeal cancer have a higher survival
rate, which is attributed to good performance status and the
presence of multiple polymorphisms form of the p53-related genes
(14). The viral aetiology is
linked to specific subtypes of HPV called high-risk HPV types, such
as HPV16 and HPV18, especially those that arise from SCC of the
tonsils and the tongue base (15).
The process of HPV-associated malignant transformation depends on
the presence of the viral oncogenes E6 and E7. The expression of E6
and E7 inhibits two tumour-suppressor proteins: p53 and
retinoblastoma protein (pRb). The loss of several key regulative
proteins activates dedifferentiation, proliferation and cell cycle
progression of HPV-infected epithelial cells and facilitates
induction of the transformed phenotype (12,16).
Loss of cell adhesion and transformation of epithelial cells to a
mesenchymal phenotype is described as epithelial-mesenchymal
transition (EMT). EMT is a fundamental step in cancer invasion and
progression. It is characterized by downregulation of
epithelial-specific adhesive proteins (e.g., tight and adherent
junction proteins such as E-cadherin), by induction of mesenchymal
proteins such as vimentin and development of migratory attributes
(17). In a model of breast cancer
cell lines, Tester and colleagues (18) described MMP-14-mediated MMP-2
activation to delineate the EMT in breast cancer progression. The
absence of MMP-9 led to the inability to activate MMP-2 and may
have been responsible for their decreased motility, invasiveness
and metastasis.
Matrix metalloproteinases (MMPs) represent a
subgroup of the metzincin superfamily of proteases that form one of
the several metalloendopeptidase families. Based on their
specificity of the extracellular matrix (ECM) proteins, MMPs could
be classified into collagenases, gelatinases, stromelysins and
matrilysins (19). As gelatinases,
MMPs play an important role in degrading the ECM. These enzymes are
also associated with morphogenesis, organization, bone development,
wound healing and cancer development (20–23).
They represent a key role in tumour cell invasion of the basement
membrane and stroma, blood vessel penetration, metastasis and
tumour promotion (24). Surface
expression of MMP-14 on ovarian cancer cells activates a
tumour-stromal signalling pathway that promotes angiogenesis and
tumour growth. Kaimal and colleagues (25) inhibited MMP-14-dependent invasion
and metastasis in ovarian cancer by intraperitoneal administration
of a monoclonal MMP-14 antibody. Bodnar and colleagues (2) suggested in laryngeal SCC that tumour
cells with low MMP-14 expression invade tumour stroma and form
metastases. MMP-2 has been evaluated in several types of cancer
cells and appears to be associated with low-grade differentiation
but also with promotion of tumour progression in, for example, head
and neck cancer (26,27).
Imatinib is a protein tyrosine-kinase inhibitor
(PTKI) (Glivec® or Gleevec®, STI-571;
Novartis, Basel, Switzerland) that is classified as a
2-phenylaminopyrimidine. Imatinib binds specifically at the
tyrosine kinase domain in Abl (the Abelson poto-oncogene), c-kit
and platelet-derived growth factor receptor (PDGFR). Bran and
colleagues (28) showed that PDGFR
plays an essential role in HNSCC growth. The activated protein
tyrosine-kinase (PTK) c-kit enables other proteins that are
necessary in the long-term survival of leukemic groups and
proliferation (29). Imatinib
impairs the immune system by inhibiting the T-cell pathway
(30). The protein tyrosine-kinase
features an essential element of the intracellular signalling
pathway of cell growth, apoptosis and metastasis. Imatinib is used
for its selectivity against BCR-ABL in the therapy of multiple
cancers, most notably against chronic myeloid leukemia (CML).
However, it is also appropriated for treatment against
gastrointestinal stromal tumours (GISTs) or neurofibromatosis
(31,32).
5-Fluorouracil (5-FU) is an analogue of uracil that
inhibits the nucleotide synthetic enzyme thymidylate synthase and
forestalls normal function by incorporating into RNA and DNA. This
antimetabolite drug is used to treat a wide range of cancers, such
as colorectal, breast, head and neck and other neoplasia of the
aerodigestive tract (33).
The aim of the present study was to evaluate the
expression pattern of MMP-14 and MMP-2 in p16-positive SCC and
HPV-negative HNSCC tumour cells. Furthermore, the chemosensitivity
of p16-positive SCC cells was compared to non-HPV tumour cell lines
after single drug treatment with 5-FU and imatinib as established
anticancer regimes for head and neck tumours.
Materials and methods
Cell lines and culture
The two different HNSCC cell lines 11A and 14C
(UMSCC11A/14C) were obtained from Dr T.E. Carey (University of
Michigan, Ann Arbor, MI, USA). They were derived from human HNSCC
of the larynx (HNSCC11A) and oral cavity (HNSCC14C). The
p16-positive cell line CERV196 (CLS Cell Lines Service, Eppelheim,
Germany) originated from a poorly differentiated xenotransplanted
cervical carcinoma MRI-H-196. Cell cultures were carried out at
37°C in a fully humidified atmosphere with 5% CO2 using
Dulbecco’s modified minimum essential medium (DMEM) (Fisher
Scientific Co., Pittsburgh, PA, USA) supplemented with 10% fetal
calf serum (FCS) and antibiotics (Life Technologies Inc.,
Gainthersburg, MD, USA). Imatinib was supplied by the manufacturer
(Novartis, Nürnberg, Germany). Imatinib and 5-FU were stored at 4°C
and dissolved in sterile water at the time of application. For
incubation with HNSCC cell lines, different concentrations of 5-FU
(5 μmol/ml) and imatinib (18 μmol/ml) were defined and used for
stimulation for 48 to 240 h. After performing the alamarBlue (AbD
Serotec, Oxfordshire, UK) cell proliferation assay, selection of
the different drug concentrations and times of stimulation were
defined. Following incubation, the supernatants were collected
together in sterile tubes and stored at −20°C until further
analysis.
Enzyme-linked immunosorbent assay
(ELISA)
MMP-14 and MMP-2 concentrations were determined by
the ELISA technique (DMP2F0; R&D Systems, Wiesbaden, Germany).
The system utilised a solid-phase monoclonal antibody and an
enzyme-linked polyclonal antibody against MMP-2 and MMP-14. The
specificity of the anti-human antibodies in the ELISA kit were
examined with sodium dodecyl sulphate polyacrylamide gel
electrophoresis (SDS-PAGE) followed by western blotting. In
accordance with the manufacturer’s instructions, each assay
measured 100 μl of supernatant. All analyses and calibrations were
performed in duplicate. Optical density was detected using a
microplate reader at a wavelength of 540 nm. Concentrations of
MMP-2 and MMP-14 were reported as ng/ml. After 48, 72, 120, 192 and
240 h of incubation with 18 μmol/ml imatinib and 5 μmol/ml 5-FU,
the expression of MMP-2 and MMP-14 was detected in the supernatants
of the incubated cell cultures and untreated cell cultures.
Immunohistochemistry
Immunohistochemical analysis was performed using a
monoclonal mouse anti-human antibody directed against MMP-2 and
MMP-14 (ab7032/ab73879; Abcam, Cambridge, UK). Immunostaining was
performed using the streptavidin horseradish method. Before
performing immunohistochemistry, HNSCC cells were cultured in
8-well chambers overnight. After growing to confluency, cells were
exposed to different concentrations of 5-FU (5 μmol/ml) and
imatinib (18 or 30 μmol/ml) for different incubation periods (0,
48, 72, 120 and 240 h). Subsequently, they underwent fixation with
acetone and alcohol (2:1) and were then washed with
phosphate-buffered saline solution (PBS). The following steps were
executed by an automated staining system, Dako TechMate 500 (Dako,
Hamburg, Germany). Cells were incubated with primary antibody
solution for 30 min at room temperature using a 1:300 working
solution of antibody to cells. Slices were washed three times with
PBS for 5 min each time (Buffer kit; Dako). An immunoreaction was
shown with the Dako ChemMate Detection kit according to the
guidelines of the manufacturer (APAAP, mouse, no. K5000; Dako).
Cells were incubated in sheep serum. An immunoreaction was
demonstrated with the monoclonal mouse anti-human antibody MMP-2
and MMP-14 (ab7032/ab73879; Abcam). Incubation was followed by the
addition of a specific biotinylated secondary antibody and
streptavidin-biotin horseradish peroxidase complex (Amersham,
Freiburg, Germany). To perform the peroxidase reaction,
aminoethylcarbazole as chromogen was used. Before washing cells
several times, endogenous peroxidase was blocked. For negative
controls, all reagents except for the primary antibody were used.
The sections received a counterstaining by Harris’ hematoxylin for
30 sec followed by dehydration in graded ethanol and coverslipping.
The immunohistochemistry demonstrated that rates of MMP-2 and
MMP-14 expression were determined semi-quantitatively. The staining
intensity was as follows: strong reactivity, >80% of the cells
stained positive; moderate reactivity, 50–80% of the cells stained
positive; and no positive cells.
Statistical analysis
Statistical analysis was performed in cooperation
with Dr C. Weiss, Institute of Biomathematics, Faculty of Medicine,
Mannheim, Germany. All data were expressed as the means. The
differences in MMP expression between incubated cultures and
control cultures were statistical analysed using Dunnett’s test as
part of generalised linear model (GLM) procedure. A p-value of
≤0.05 was considered to indicate a statistically significant
result.
Results
Immunohistochemistry
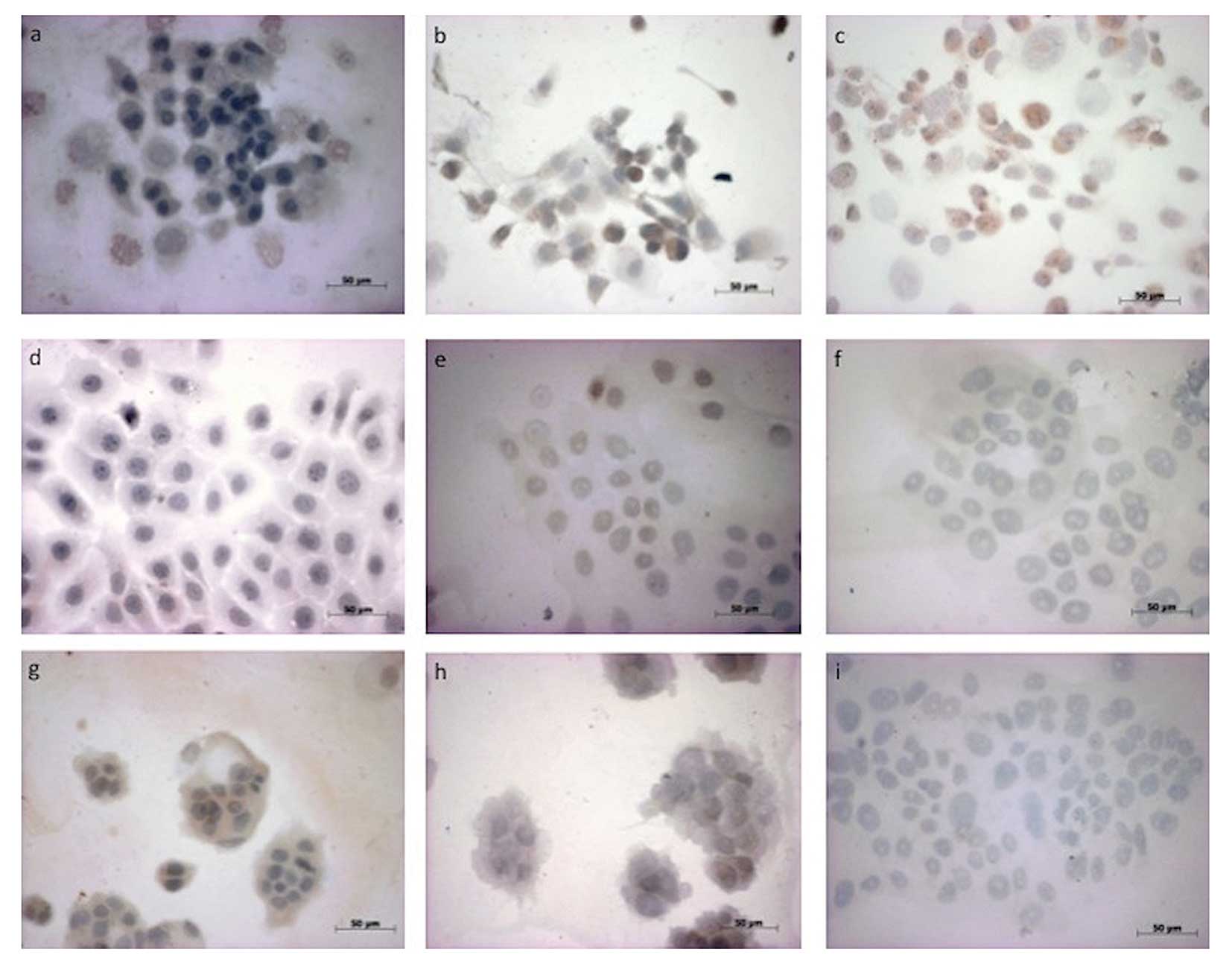
Immunhistochemical studies for MMP-14 illustrated
that all tumour cell lines, irrespective of HPV status, expressed
similarly moderate levels of MMP-14 compared to the chemonaive
controls. We detected decreased reactivity with rising
concentration of imatinib and with increasing time of incubation
from 48 to 240 h in the HNSCC14C, HNSCC11A and CERV196 cells.
Notably, we detected strong immunostaining for MMP-14 in the
p16-positive SCC line (CERV196) only after 48 h of imatinib (18
μmol/ml) treatment (Table I).
Compared to the differences in dose escalation, we showed stronger
immunostaining for MMP-14 in all incubated SCC cell lines after
incubation with imatinib at a lower concentration of 18 μmol/ml
(Table I). However, after treatment
with 5-FU in the CERV196 cells, we found slightly increased
reactivity for MMP-14 only after 72 h. In contrast, the
immunohistochemical studies against MMP-14 showed slightly
increased reactivity for MMP-14 only after 240 h of 5-FU treatment
in the HNSCC11A cells. In HNSCC14C, we detected no positive
reactivity for MMP-14 (Fig. 1;
Table I).
 | Table IImmunostaining score for MMP-14 in
HNSCC11A, HNSCC14C and CERV196 cells. |
Table I
Immunostaining score for MMP-14 in
HNSCC11A, HNSCC14C and CERV196 cells.
| Immunostaining | 48 h | 72 h | 120 h | 240 h |
|---|
| Control group |
| HNSCC11A | ++ | ++ | +++ | +++ |
| HNSCC14C | ++ | +++ | ++ | +++ |
| CERV196 | ++ | ++ | +++ | ++ |
| imatinib 18
μmol |
| HNSCC11A | ++ | ++ | ++ | ++ |
| HNSCC14C | ++ | + | + | + |
| CERV196 | +++ | ++ | ++ | |
| imatinib 30
μmol |
| HNSCC11A | + | + | + | + |
| HNSCC14C | ++ | 0 | 0 | 0 |
| CERV196 | ++ | ++ | ++ | + |
ELISA of total protein expression in
HNSCC11A, HNSCC14C and CERV196 cells
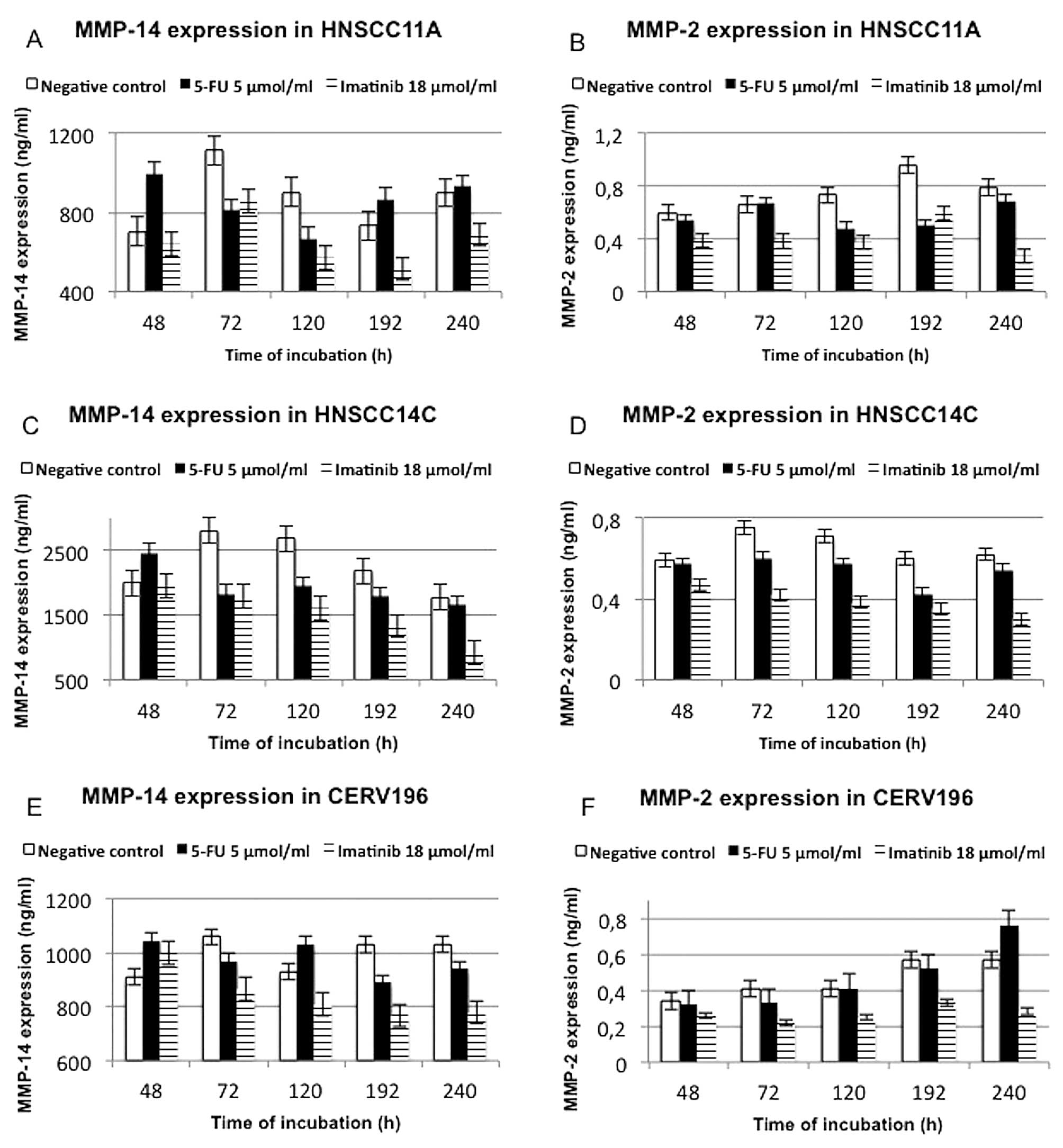
To analyze the effect of 5-FU and imatinib on HNSCC
cell lines and the HPV-positive cell line CERV196, we added
increasing concentrations of two chemotherapeutical agents,
imatinib (18 μmol/ml) and 5-FU (5 μmol/ml), to the cell cultures.
In order to determine MMP-2 as well as MMP-14 expression in the
supernatant of the cell lines, ELISA analysis was carried out 48,
72, 120, 192 and 240 h after the start of incubation.
Compared to the HPV-negative tumour cell lines, the
negative controls of the CERV196 cells exhibited higher total
protein expression levels. The HPV16-positive SCC cell line was
vulnerable to 5-FU and imatinib therapy. However, the CERV196 cells
showed only a less higher decreasing of total protein expression
after 240 h of treatment with 5-FU. In summary, the HPV-negative
cell lines showed no significant differences after treatment with
imatinib and 5-FU. We found a lower expression of total protein
after prolonged treatment time with imatinib in the HNSCC11A and
HNSCC14C cells compared to incubation with 5-FU. The drug
concentration had no significant influence on the expression of
total protein (Fig. 2).
ELISA of MMP-2 expression in HNSCC11A,
HNSCC14C and CERV196 cells
In summary, we detected weaker expression of MMP-2
in all three cell lines, particularly in the CERV196 cells. In the
p16-positive SCC cells, maximal reduction of MMP-2 was noted after
72 h of imatinib treatment (0.22 ng/ml; p<0.0075). We found a
distinct trend towards reduction in MMP-2 expression after
prolonged treatment time with imatinib in the HNSCC11A, HNSCC14C
and CERV196 cells. A significant time-dependent effect was noted in
the HNSCC11A cells when exposed to imatinib (18 μmol/ml) within the
48–240 h time frame (p<0.018). A statistically significant
impact on MMP-2 expression was found in the HNSCC14C and CERV196
cells after 3 days of incubation with imatinib (p<0.008).
Notably, compared to the HPV-negative cell lines, in the CERV196
cells, an increase in MMP-2 expression was noted after prolonged
treatment time with 5-FU (5 μmol/ml). We detected suppression of
MMP-2 only after a prolonged treatment time with 5-FU in the
HNSCC11A and HNSCC14C cells. However, a statistically significant
impact on MMP-2 suppression was noted after 192 h of treatment with
5-FU (p<0.0052) in the HNSCC11A cells. In the CERV196 and
HNSCC14C cells, no significant downregulation of MMP-2 was found
following treatment with 5-FU (5 μmol/ml). The concentration of
imatinib or 5-FU had no statistically significant impact on MMP-2
expression (Fig. 3; Table II).
 | Table IIEnzyme-linked immunosorbent assay
(ELISA) for MMP-2 expression in HNSCC11A, HNSCC14C and CERV196
cells after incubation with 5-FU and imatinib. |
Table II
Enzyme-linked immunosorbent assay
(ELISA) for MMP-2 expression in HNSCC11A, HNSCC14C and CERV196
cells after incubation with 5-FU and imatinib.
| MMP-2 expression
ng/ml (p-value) |
|---|
|
|
|---|
| Time of incubation
(h) | Control
Mean value | imatinib (18
μmol/ml)
Mean value (p-value) | 5-FU (5
μmol/ml)
Mean value (p-value) |
|---|
| HNSCC11A cells |
| 48 | 0.60 | 0.4
(0.0044) | 0.54 (0.5878) |
| 72 | 0.66 | 0.38
(0.0178) | 0.67 (0.9961) |
| 120 | 0.73 | 0.37
(0.0154) | 0.48 (0.1197) |
| 192 | 0.96 | 0.59
(0.0014) | 0.5
(0.0051) |
| 240 | 0.80 | 0.27
(0.0006) | 0.69 (0.4657) |
| HNSCC14C cells |
| 48 | 0.59 | 0.47 (0.3357) | 0.573 (0.6921) |
| 72 | 0.75 | 0.42
(0.0030) | 0.6 (0.2573) |
| 120 | 0.71 | 0.38
(0.0122) | 0.57 (0.2607) |
| 192 | 0.60 | 0.35 (0.0415) | 0.42 (0.3818) |
| 240 | 0.62 | 0.3
(0.0408) | 0.54 (0.6742) |
| CERV196 cells |
| 48 | 0.34 | 0.26 (0.9785) | 0.32 (0.9934) |
| 72 | 0.41 | 0.22
(0.0073) | 0.33 (0.7349) |
| 120 | 0.41 | 0.25 (0.2221) | 0.41 (0.9523) |
| 192 | 0.57 | 0.33 (0.0718) | 0.52 (0.9873) |
| 240 | 0.57 | 0.28 (0.0632) | 0.76 (0.7753) |
ELISA of MMP-14 expression in HNSCC11A,
HNSCC14C and CERV196 cells
In contrast to MMP-2, stronger expression of MMP-14
was exhibited in all three cell lines, particularly in the HNSCC14C
cells. CERV196 cells showed a consistent trend towards an
incubation time-dependent reduction in MMP-14, particularly after
treatment with imatinib (18 μmol/ml). We detected a statistically
significant impact on MMP-14 suppression in the p16-positive SCC
cells only after a 192-h treatment with imatinib (p<0.023).
However, the effect of the incubation with imatinib on CERV196
cells was less evident than on the HNSCC11A and HNSCC14C cell
lines. In the HNSCC11A cells, maximal reduction in MMP-14
expression was measured after 192 h of imatinib (18 μmol/ml)
treatment. Significant time-dependent suppression of MMP-14 was
detected in the HNSCC14C cells when exposed to imatinib (18
μmol/ml) for 72–240 h (p<0.0023). We showed a statistically
significant impact on MMP-14 expression in the HNSCC11A cells after
3 days (p<0.02).
In the CERV196 and HNSCC14C cells, we detected less
MMP-14 suppression after treatment with 5-FU (5 μmol/ml). Notably,
5-FU (5 μmol/ml) reduced MMP-14 expression in the HNSCC11A cells
between 72 and 192 h of treatment. A statistically significant
downregulation of MMP-14 was found in the HNSCC11A only after a
72-h incubation with imatinib (18 μmol/ml) (p<0.02).
Concentration of imatinib and 5-FU did not significantly influence
the effect of MMP-14 suppression in any of the cell lines (Fig. 3; Table
III).
 | Table IIIEnzyme-linked immunosorbent (ELISA)
assay for MMP-14 expression in HNSCC11A, HNSCC14C, and CERV196
cells after incubation with 5-FU and imatinib. |
Table III
Enzyme-linked immunosorbent (ELISA)
assay for MMP-14 expression in HNSCC11A, HNSCC14C, and CERV196
cells after incubation with 5-FU and imatinib.
| MMP-14 expression,
ng/ml (p-value) |
|---|
|
|
|---|
| Incubation time
(h) | Control
Mean value | imatinib (18
μmol/ml)
Mean value (p-value) | 5-FU (5
μmol/ml)
Mean value (p-value) |
|---|
| HNSCC11A cells |
| 48 | 703.33 | 638.33
(0.8027) | 997.33
(0.1162) |
| 72 | 1112.77 | 855.87
(0.0189) | 811.33
(0.1017) |
| 120 | 900.13 | 570 (0.2715) | 669 (0.6393) |
| 192 | 732.67 | 514.33
(0.5166) | 865.33
(0.5651) |
| 240 | 899.33 | 687.3 (0.1510) | 931 (0.9862) |
| HNSCC14C cells |
| 48 | 1989.1 | 1944 (0.9838) | 2462.53
(0.7141) |
| 72 | 2803.8 | 1788
(<0.0001) | 1827.03
(0.0019) |
| 120 | 2677.67 | 1605.33
(<0.0001) | 1939 (0.0135) |
| 192 | 2181 | 1331.67
(0.0022) | 1793.07
(0.5744) |
| 240 | 1775.67 | 917.33
(0.0001) | 1660.67
(0.8473) |
| CERV196 cells |
| 48 | 910.57 | 998.33
(0.7653) | 1045.53
(0.8791) |
| 72 | 1060 | 864.48
(0.2979) | 967.81
(0.3704) |
| 120 | 930 | 808.33
(0.5715) | 1031 (0.7483) |
| 192 | 1029.67 | 768.77
(0.0222) | 888.36
(0.7462) |
| 240 | 1031 | 778.67
(0.0569) | 940.04
(0.3486) |
Discussion
HNSCC is the sixth most common cancer in regards to
incidence worldwide. Known risk factors are tobacco use and alcohol
consumption, which appear to have a synergistic effect. In
addition, a subgroup of HNSCC is caused by infection with high-risk
types of HPV, particularly cancer of the oropharynx (34). The poor survival rate can be
attributed to local invasion, cervical lymph node dissemination,
distant metastasis and second primary cancers (35). To improve survival, it is important
to investigate phenotypic changes in HNSCC and examine how HNSCC
cells destroy the basement membrane, invade and metastasise. HNSCCs
show a high propensity for local recurrence after treatment
(36). A combination of
chemotherapy and radiotherapy improves the poor prognosis of
advanced HNSCC patients. Concomitant treatment of chemoradiotherapy
has less improved the overall and 5-year survival rates of patients
with advanced stage disease; however, the locoregional control rate
could be improved. Multimodal tumour therapy often reduces the
quality of life of patients, making the psychosocial consequences
of HNSCC greater than that of other neoplasms (5). To further improve the survival rate,
particularly for unresectable HNSCC, innovative strategies and
targeted therapies must be analysed. However, HNSCCs arise from the
accumulation of epigenetic and genetic factors, including the
ability to escape apoptosis, insensitivity to anti-growth signals,
abnormalities in cancer-associated signalling pathways, limitless
replicative potential, self-sufficiency in growth signals,
increased angiogenesis, metastasis and invasion (36). Degrading of the ECM by MMPs is one
of the most critical steps in tumour progression, including tumour
growth, invasion, metastasis and angiogenesis (24). The present study aimed to detect
altered expression of MMP-2 and MMP-14 in p16-negative (HNSCC11A,
HNSCC14C) and p16-positive SCC cell lines (CERV196). PTKs are
fundamental elements of the signalling pathway that may also be
targets for anticancer therapy. Imatinib is a specific protein
tyrosine-kinase inhibitor for c-Abl and BCR-Abl and was originally
designed to block the BCR-Abl tyrosine kinase in chronic myeloid
leukemia (37). Imatinib also
showed an inhibitory effect on the catalytic activity of PDGFR and
c-kit tyrosine kinases. It is used for the treatment of several
malignancies, such as colon adenocarcinoma and non-small cell lung
cancer (37–39). The aim of the present study was to
investigate the effects of imatinib and 5-FU on HPV16-associated
HNSCC compared to non-HPV-induced HNSCC and their impact on the
expression of MMP-2 and MMP-14 in order to examine the potency of
chemotherapeutic agents in HNSCC cell lines. The concentration of
imatinib and the time of stimulation used in our analyses were
defined after performing cell proliferating assays.
Compared to the HPV-negative tumour cell lines,
negative controls of CERV196 cells showed lower expression of
MMP-2. MMP-2 (gelatinase A) is known to play an important role in
the degradation of the basement membrane and ECM to promote tumour
cell invasion and metastasis. MMP-2 expression correlates with
local invasion, lymph node metastasis and poor prognosis. In human
colon cancer cells, imatinib was shown to be a powerful growth
inhibitor and an effective suppressor of stromal-induced
stimulation of cancer cell growth and activation of proMMP-2
(37). Recently, an experimental
study showed that HPV16-positive lung cancer cells exhibit
upregulation of pro-angiogenic MMP-2 and MMP-9 through induction of
IL-8 expression. Therefore, it may be concluded that the cytokines
induced by HPV infection may work together to confer malignant and
tumorigenic potential on the infected cells by promoting pathways
of growth, angiogenic and metastatic characteristics (40). Studies of uterine cervical neoplasms
also indicate that HPV oncoproteins promote MMP imbalance (41). Higher expression of MMP-14 and MMP-2
has been noted in HPV-positive cervical cancer-derived cells.
Notably, an associated downregulation of RECK, a membrane-bound
protein that has an inhibitory effect on the transcription,
synthesis and activation of MMPs, was shown in HPV16 E7-positive
cervical carcinoma cell lines (41). A decrease in RECK expression levels
was found in precancerous and cancerous lesions (41). We found a distinct trend towards
reduction in MMP-2 expression after prolonged treatment time with
imatinib. In p16-positive cells, we detected a maximal reduction in
MMP-2 after 72 h of imatinib treatment. Several growth factors,
such as transforming growth factor β (TGFβ1), stem cell factor
(SCF), hepatocyte growth factor (HGF) or insulin-like growth factor
(IGF), were found to be generated by fibroblasts and can activate
MMP-induced tumour growth stimulation by proteolytic cleavage. In
the HNSCC11A cells, a statistically significant time-dependent
downregulation of MMP-2 by imatinib was revealed. After treatment
with 5-FU, we detected a decrease in suppression of MMP-2 and
MMP-14 only after prolonged treatment time. Compared to the
HPV-negative cell lines, in the CERV196 cells, an increase in MMP-2
expression was noted after prolonged treatment time with 5-FU. MMP2
mRNA speaking is also well for the functional important role of
this proteinase in the process of carcinoma cell invasion and
epithelial-mesenchymal transition (EMT) in oral squamous cell
carcinoma cells (42). Qiao et
al suggested that the transcription factor Snail influences the
upregulation of MMP-2 expression initiating EMT in oral squamous
cell carcinoma (43). However,
relevant downregulation of MMP-2 such as by imatinib possibly could
inhibit promotion of EMT and invasion. Using 5-FU in the past, we
detected only a slight alteration in β-catenin expression in
p16-positive and p16-negative SCC cell lines (44). 5-FU is an established agent on which
all curative therapeutic approaches to different neoplasms are
based. However, little effect has been observed when 5-FU is used
as a single agent. Tumour cells are resistant to 5-FU due to
dynamic regulation of the pyrimidine network, adopting autonomous
strategies for primary and secondary resistance (37). In additional studies, data suggest
that the orotate phosphoribosyl-transferase (OPRT) gene enhances
the chemotherapeutic effect of 5-FU in HNSCC cells in vitro.
However, the level of OPRT expression could be used as a predictive
indicator of 5-FU efficacy against HNSCC (45). On the other hand, in different types
of tumours, dihydropyrimidine dehydrogenase (DPD), a natural enzyme
that influences pyrimidine degradation, was detected. This enzyme
is responsible for the rate-limiting step in the catabolism of 5-FU
that accounts for >80% of its elimination (46–49).
Thus, carcinomas with a high DPD level are more resistant to 5-FU
than carcinomas with low levels of DPD (46).
We detected higher expression of MMP-14 than that of
MMP-2 in all three SCC cell lines. MMP-14 is known to activate
gelatinase MMP-2 (50). However,
there may be an association between the fact that MMP-14 activates
MMP-2 and the higher expression of MMP-14 detected in all three
cell lines, particularly in the HPV-negative HNSCC cell lines. In
p16-positive and HNSCC14C cells, we found a consistent trend
towards an incubation time-dependent reduction in MMP-14 levels,
particularly after treatment with imatinib. MMP-14 (also called
membrane type 1 MMP, MT1-MMP) is thereby considered a key protein
in physiological and pathological processes from normal cell
development to cancer cell growth, promoting tissue remodelling,
invasion, and metastasis in malignant tumours [e.g., oral squamous
cell cancer (OSCC)] (51). Invasion
through collagen networks and collagenolysis is dependent on
MMP-14, not on secreted MMPs (52).
The interaction of MMP-14 with the membrane-associated glycoprotein
CD44 activates a migratory front (53). Recently, MMP-14-dependent invasion
and metastasis were effectively inhibited by intraperitoneal
administration of a monoclonal MMP-14 antibody in a model of
advanced peritoneal ovarian cancer (25).
Notably, CERV196 cells exhibited higher expression
of total protein. However, HPV-positive tumour cells have a
stronger basal metabolism in general. 5-FU had a stronger influence
on the alteration of the expression of total protein, particularly
in the p16-positive SCC cells.
The present study did not confirm the clinically
substantiated increase in chemosensitivity of p16-positive tumour
cells, but we did detect differences in the alteration of MMP-2 and
MMP-14 expression after treatment with the chemotherapeutic agents.
We found higher suppression of MMP-2 in CERV196 cells after
incubation with imatinib. However, for therapeutic treatment of
HNSCC, knowledge of the HPV-status is still an important factor. In
conclusion, we detected expression of MMP-2 and MMP-14 in the three
p16-positive and -negative SCC cell lines. We found that imatinib
exhibited a strong inhibitory effect on the downregulation of MMP
expression in the HNSCC cell lines via the blockage of signal
transduction of PTK receptors (37). Given these results and the low
toxicity of imatinib, further studies are needed to examine the
potency of imatinib in HNSCC treatment and to find innovative
strategies and targeted therapies to improve the clinical outcome
of patients with HNSCC, depending on HPV status.
Acknowledgements
The authors would like to thank Petra Prohaska for
her excellent technical assistance and Dr. C. Weiss for the
brilliant assistance in the statistical analysis.
References
|
1
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View
Article : Google Scholar
|
|
2
|
Bodnar M, Szylberg L, Kazmierczak W and
Marszalek A: Differentiated expression of membrane type
metalloproteinases (MMP-14, MMP-15) and pro-MMP2 in laryngeal
squamous cell carcinoma. A novel mechanism J Oral Pathol Med.
42:267–274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oliveira LR and Ribeiro-Silva A:
Prognostic significance of immunohistochemical biomarkers in oral
squamous cell carcinoma. Int J Oral Maxillofac Surg. 40:298–307.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Vicente JC, Lequerica-Fernandez P,
Santamaria J and Fresno MF: Expression of MMP-7 and MT1-MMP in oral
squamous cell carcinoma as predictive indicator for tumor invasion
and prognosis. J Oral Pathol Med. 36:415–424. 2007.PubMed/NCBI
|
|
5
|
Massano J, Regateiro FS, Januario G and
Ferreira A: Oral squamous cell carcinoma: review of prognostic and
predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 102:67–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schultz JD, Rotunno S, Riedel F, et al:
Synergistic effects of imatinib and carboplatin on VEGF, PDGF and
PDGF-Rα/β expression in squamous cell carcinoma of the head and
neck in vitro. Int J Oncol. 38:1001–1012. 2011.PubMed/NCBI
|
|
7
|
Myoung H, Kim MJ, Lee JH, Ok YJ, Paeng JY
and Yun PY: Correlation of proliferative markers (Ki-67 and PCNA)
with survival and lymph node metastasis in oral squamous cell
carcinoma: a clinical and histopathological analysis of 113
patients. Int J Oral Maxillofac Surg. 35:1005–1010. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sepiashvili L, Hui A, Ignatchenko V, et
al: Potentially novel candidate biomarkers for head and neck
squamous cell carcinoma identified using an integrated cell
line-based discovery strategy. Mol Cell Proteomics. 11:1404–1415.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonner JA, Harari PM, Giralt J, et al:
Radiotherapy plus cetuximab for locoregionally advanced head and
neck cancer: 5-year survival data from a phase 3 randomised trial,
and relation between cetuximab-induced rash and survival. Lancet
Oncol. 11:21–28. 2010.PubMed/NCBI
|
|
10
|
Nair S and Pillai MR: Human papillomavirus
and disease mechanisms: relevance to oral and cervical cancers.
Oral Dis. 11:350–359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sudhoff HH, Schwarze HP, Winder D, et al:
Evidence for a causal association for HPV in head and neck cancers.
Eur Arch Otorhinolaryngol. 268:1541–1547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schultz JD, Sommer JU, Hoedt S, et al:
Chemotherapeutic alteration of β-catenin and c-kit expression by
imatinib in p16-positive squamous cell carcinoma compared to
HPV-negative HNSCC cells in vitro. Oncol Rep. 27:270–280.
2012.
|
|
13
|
Kreimer AR, Clifford GM, Boyle P and
Franceschi S: Human papillomavirus types in head and neck squamous
cell carcinomas worldwide: a systematic review. Cancer Epidemiol
Biomarkers Prev. 14:467–475. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Z, Sturgis EM, Zhang Y, et al:
Combined p53-related genetic variants together with HPV infection
increase oral cancer risk. Int J Cancer. 131:E251–E258. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shiboski CH, Schmidt BL and Jordan RC:
Tongue and tonsil carcinoma: increasing trends in the U.S.
population ages 20–44 years. Cancer. 103:1843–1849. 2005.PubMed/NCBI
|
|
16
|
Tezal M, Scannapieco FA, Wactawski-Wende
J, et al: Local inflammation and human papillomavirus status of
head and neck cancers. Arch Otolaryngol Head Neck Surg.
138:669–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hay ED: The mesenchymal cell, its role in
the embryo, and the remarkable signaling mechanisms that create it.
Dev Dyn. 233:706–720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tester AM, Ruangpanit N, Anderson RL and
Thompson EW: MMP-9 secretion and MMP-2 activation distinguish
invasive and metastatic sublines of a mouse mammary carcinoma
system showing epithelial-mesenchymal transition traits. Clin Exp
Metastasis. 18:553–560. 2000. View Article : Google Scholar
|
|
19
|
Stamenkovic I: Extracellular matrix
remodelling: the role of matrix metalloproteinases. J Pathol.
200:448–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Al-Azri AR, Gibson RJ, Keefe DM and Logan
RM: Matrix metalloproteinases: do they play a role in mucosal
pathology of the oral cavity? Oral Dis. 19:347–359. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martins VL, Caley M and O’Toole EA: Matrix
metalloproteinases and epidermal wound repair. Cell Tissue Res.
351:255–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Andres MC, Kingham E, Imagawa K, et al:
Epigenetic regulation during fetal femur development: DNA
methylation matters. PLoS One. 8:e549572013.PubMed/NCBI
|
|
23
|
Tamamura R, Nagatsuka H, Siar CH, et al:
Comparative analysis of basal lamina type IV collagen α chains,
matrix metalloproteinases-2 and -9 expressions in oral dysplasia
and invasive carcinoma. Acta Histochem. 115:113–119. 2013.
|
|
24
|
Folgueras AR, Pendas AM, Sanchez LM and
Lopez-Otin C: Matrix metalloproteinases in cancer: from new
functions to improved inhibition strategies. Int J Dev Biol.
48:411–424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaimal R, Aljumaily R, Tressel SL, et al:
Selective blockade of matrix metalloprotease-14 with a monoclonal
antibody abrogates invasion, angiogenesis, and tumor growth in
ovarian cancer. Cancer Res. 73:2457–2467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mallis A, Teymoortash A, Mastronikolis NS,
Werner JA and Papadas TA: MMP-2 expression in 102 patients with
glottic laryngeal cancer. Eur Arch Otorhinolaryngol. 269:639–642.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gorogh T, Beier UH, Baumken J, et al:
Metalloproteinases and their inhibitors: influence on tumor
invasiveness and metastasis formation in head and neck squamous
cell carcinomas. Head Neck. 28:31–39. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bran B, Bran G, Hormann K and Riedel F:
The platelet-derived growth factor receptor as a target for
vascular endothelial growth factor-mediated anti-angiogenetic
therapy in head and neck cancer. Int J Oncol. 34:255–261.
2009.PubMed/NCBI
|
|
29
|
Heinrich MC, Griffith DJ, Druker BJ, Wait
CL, Ott KA and Zigler AJ: Inhibition of c-kit receptor tyrosine
kinase activity by STI 571, a selective tyrosine kinase inhibitor.
Blood. 96:925–932. 2000.PubMed/NCBI
|
|
30
|
Levitzki A: Tyrosine kinase inhibitors:
views of selectivity, sensitivity, and clinical performance. Annu
Rev Pharmacol Toxicol. 53:161–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Angelini S, Ravegnini G, Fletcher JA,
Maffei F and Hrelia P: Clinical relevance of pharmacogenetics in
gastrointestinal stromal tumor treatment in the era of personalized
therapy. Pharmacogenomics. 14:941–956. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rutkowski P and Gronchi A: Efficacy and
economic value of adjuvant imatinib for gastrointestinal stromal
tumors. Oncologist. 18:689–696. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Longley DB, Harkin DP and Johnston PG:
5-Fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Klussmann JP, Preuss SF and Speel EJ:
Human papillomavirus and cancer of the oropharynx. Molecular
interaction and clinical implications. HNO. 57:113–122. 2009.(In
German).
|
|
35
|
Scanlon CS, Van Tubergen EA, Inglehart RC
and D’Silva NJ: Biomarkers of epithelial-mesenchymal transition in
squamous cell carcinoma. J Dent Res. 92:114–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koontongkaew S: The tumor microenvironment
contribution to development, growth, invasion and metastasis of
head and neck squamous cell carcinomas. J Cancer. 4:66–83. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stahtea XN, Roussidis AE, Kanakis I, et
al: Imatinib inhibits colorectal cancer cell growth and suppresses
stromal-induced growth stimulation, MT1-MMP expression and pro-MMP2
activation. Int J Cancer. 121:2808–2814. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsao AS, Liu S, Fujimoto J, et al: Phase
II trials of imatinib mesylate and docetaxel in patients with
metastatic non-small cell lung cancer and head and neck squamous
cell carcinoma. J Thorac Oncol. 6:2104–2111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Popow-Wozniak A, Wozniakowska A, Kaczmarek
L, Malicka-Blaszkiewicz M and Nowak D: Apoptotic effect of imatinib
on human colon adenocarcinoma cells: influence on actin
cytoskeleton organization and cell migration. Eur J Pharmacol.
667:66–73. 2011. View Article : Google Scholar
|
|
40
|
Shiau MY, Fan LC, Yang SC, et al: Human
papillomavirus up-regulates MMP-2 and MMP-9 expression and activity
by inducing interleukin-8 in lung adenocarcinomas. PLoS One.
8:e544232013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cardeal LB, Boccardo E, Termini L, et al:
HPV16 oncoproteins induce MMPs/RECK-TIMP-2 imbalance in primary
keratinocytes: possible implications in cervical carcinogenesis.
PLoS One. 7:e335852012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Richter P, Umbreit C, Franz M, et al:
EGF/TGFbeta1 co-stimulation of oral squamous cell carcinoma cells
causes an epithelial-mesenchymal transition cell phenotype
expressing laminin 332. J Oral Pathol Med. 40:46–54. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qiao B, Johnson NW and Gao J:
Epithelial-mesenchymal transition in oral squamous cell carcinoma
triggered by transforming growth factor-beta1 is Snail
family-dependent and correlates with matrix metalloproteinase-2 and
-9 expressions. Int J Oncol. 37:663–668. 2010.
|
|
44
|
Umbreit C, Aderhold C, Faber A, et al:
Unexpected alteration of β-catenin and c-KIT expression by 5-FU and
docetaxel in p16-positive squamous cell carcinoma compared to
HPV-negative HNSCC cells in vitro. Anticancer Res.
33:2457–2465. 2013.
|
|
45
|
Yasumatsu R, Nakashima T and Komune S:
Overexpression of the orotate phosphoribosyl-transferase gene
enhances the effect of 5-fluorouracil in head and neck squamous
cell carcinoma in vitro. J Oncol. 2012:6496052012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Milano G and Etienne MC: Potential
importance of dihydropyrimidine dehydrogenase (DPD) in cancer
chemotherapy. Pharmacogenetics. 4:301–306. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jiang W, Lu Z, He Y and Diasio RB:
Dihydropyrimidine dehydrogenase activity in hepatocellular
carcinoma: implication in 5-fluorouracil-based chemotherapy. Clin
Cancer Res. 3:395–399. 1997.
|
|
48
|
Kawasaki G, Yoshitomi I, Yanamoto S,
Yamada S, Mizuno A and Umeda M: Expression of thymidylate synthase
and dihydropyrimidine dehydrogenase in primary oral squamous cell
carcinoma and corresponding metastases in cervical lymph nodes:
association with the metastasis suppressor CD82. Anticancer Res.
31:3521–3526. 2011.
|
|
49
|
Tamatani T, Ferdous T, Takamaru N, et al:
Antitumor efficacy of sequential treatment with docetaxel and
5-fluorouracil against human oral cancer cells. Int J Oncol.
41:1148–1156. 2012.PubMed/NCBI
|
|
50
|
Wang P, Nie J and Pei D: The hemopexin
domain of membrane-type matrix metalloproteinase-1 (MT1-MMP) is not
required for its activation of proMMP2 on cell surface but is
essential for MT1-MMP-mediated invasion in three-dimensional type I
collagen. J Biol Chem. 279:51148–51155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Weng CJ, Chen MK, Lin CW, Chung TT and
Yang SF: Single nucleotide polymorphisms and haplotypes of MMP-14
are associated with the risk and pathological development of oral
cancer. Ann Surg Oncol. 19(Suppl 3): S319–S327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sabeh F, Li XY, Saunders TL, Rowe RG and
Weiss SJ: Secreted versus membrane-anchored collagenases: relative
roles in fibroblast-dependent collagenolysis and invasion. J Biol
Chem. 284:23001–23011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zarrabi K, Dufour A, Li J, et al:
Inhibition of matrix metalloproteinase 14 (MMP-14)-mediated cancer
cell migration. J Biol Chem. 286:33167–33177. 2011. View Article : Google Scholar : PubMed/NCBI
|