Introduction
Tumors harbor different cell types consisting of
complex, phenotypically/genotypically heterogeneous cell
populations. Within the tumor mass there resides a rare group of
cancer cells known as cancer-initiating or cancer stem cells (CSCs)
(1,2). Similar to normal stem cells, CSCs
undergo asymmetrical cell division, giving rise to one daughter
cell that becomes a committed progenitor. As a result, hierarchies
of actively proliferating as well as progressively differentiating
cancer cells are formed, contributing to the cellular heterogeneity
of human cancers (3). These cells,
which are able to self-renew and differentiate, are not only the
potential origin of the tumor, but also the possible source of
recurrence and chemoresistance (4).
Recent studies support this proposal and suggest the utility of
several factors to induce the differentiation of CSCs (5).
It has been demonstrated that cells at metastatic
sites possess differentiation properties unique to CSCs and have
heterogeneous histological features suggesting ability to
self-renew (6). Established cancer
cell lines contain CSCs, which can be propagated in vitro
using defined conditions to form 3D tumor spheroids (7). The in vivo cellular
microenvironment plays a critical role in the growth and
development of both normal and cancer tissues. It is regulated by a
complex interplay of soluble factors and signaling molecules
secreted by cells. Accumulating data suggest that in vitro
three dimensional tumor cell cultures reflect the complex in
vitro microenvironment more accurately than simple
two-dimensional monolayers, especially with respect to gene
expression profiles, signaling pathway activity and drug
sensitivity (8,9). Also, CSCs spontaneously tend to exist
in spheroid formation, as seen in the embroid body formation during
development (10). In light of
these reports, it may be assumed that spheroids grown in serum
contained medium in vitro, may reflect the differentiation
properties of CSCs better when compared to other in vitro
models. Spheroids have been used as a metastasis model in several
studies (11–13).
We hypothesized that the differentiation process in
CSCs, when compared to non-CSCs, could be followed by the adhesion
molecules that are expressed on the cells. These molecules could
provide the means for the future establishment of therapeutic
strategies in cancer. In the present study, we described approaches
to image and analyze the differentiation properties of human
prostate CSCs within 3D spheroids. We believe our approach may
serve as a model for defining expression and protein profiles of
CSCs in a 3D environment.
Materials and methods
Cell culture conditions and reagents
The DU145 human prostate cancer cell line was
supplied by the American Type Culture Collection (ATCC; Rockville,
MD, USA) and was grown in monolayer culture in Dulbecco’s modified
Eagle’s medium-F12 (DMEM-F12; Biological Industries, Israel)
supplemented with 10% heat-inactivated fetal calf serum, 100 U/ml
penicillin and 100 μg/ml streptomycin (Sigma Chemical Co., St.
Louis, MO, USA). Cells in semi-confluent flasks were harvested
using 0.05% trypsin (Sigma Chemical Co.), centrifuged after
addition of DMEM-F12 for trypsin inactivation, and then resuspended
in culture medium. Antibodies used for immunohistochemistry and
western blot analysis were TGFβ1, versican, CDH1 (Abcam), ICAM1,
Col7A1, ITGβ3, MMP1, MMP14, MMP16 (Bioss), NCAM1 (Abcam), SPP1
(Bioss), THBS1 (Abcam).
Fluorescence activated cell sorting and
experimental groups
For fluorescence activated cell sorting (FACS),
cells were detached using non-enzymatic cell dissociation solution
(Sigma). Approximately 56,100 cells were incubated with antibody,
diluted 1:100 in FACS wash (0.5% bovine serum albumin; 2 mM
NaN3; 5 mM EDTA), for 15 min at 4°C. For controls, an
isotype and concentration matched PE labeled control antibody was
used as well as samples labeled with PE attached CD133/1 (clone
AC133/1) (both from Miltenyi Biotec, UK) and FITC labeled CD44
(clone G44-26, BD Pharmingen). After three 5 min washes (with FACS
wash), the cells were re-suspended and sorted for a
CD133high/CD44high population. Both the
sorted cell population and its remaining non-sorted counterpart
were collected for further analyses. The two separate cell
populations were cultured in two different settings; either as
monolayer 2D culture or as 3D multicellular tumor spheroids.
Construction of spheroids and sphere
formation assay
The clonogenic potential of phenotypically different
populations was analyzed in 3D non-adherent cell culture
conditions. Tumor cells grown as a monolayer were re-suspended,
with trypsin, counted, seeded 103 cells/well and
cultured over 3% Noble agar-coated 6-well culture plates (Difco,
USA). Two weeks after initiation, plates were inspected for colony
(sphere) growth. The number of colonies within each well was
counted under the microscope and representative fields were
photographed. First passage floating spheres were removed, gently
disaggregated and transferred to a new 3% Noble agar-coated
well.
PCR array assay
Total RNA was extracted from sorted cells and
non-sorted counterparts (miRNeasy kit; Qiagen, Germany). Synthesis
of cDNA was carried out using C-03 RT2 First Strand kit (SA
Biosciences, Frederick, MD, USA). Stem cell-specific gene
expression profiles were analyzed by PCR Array Assay (Custom Panel
384; Roche) in accordance with the manufacturer’s recommendations.
Briefly, total RNA was isolated from monolayer cell populations or
whole floating spheroids. Up to 1 μg of total RNA was treated with
DNase and cDNA was prepared using RT2 First Strand kit. Pairs of
the test and control cDNA samples were added to RT2 qPCR master mix
and distributed across the 96-well PCR array plates. Each plate
contained 84 adhesion molecule-related probes and control
housekeeping genes. Triplicate assays were performed. Following
real-time PCR [LightCycler 480 (LC 480); Roche Molecular Systems],
amplification data (fold-changes in Ct values of all the genes) was
analyzed by software and ≥1.5 fold-change was used as filtering
criteria. The list of genes analyzed for differential expression is
as follows: ADAMTS1 (ADAM metallopeptidase with
thrombospondin type 1 motif, 1); COL11A1 (collagen type XI,
α1); COL4A2 (collagen type IV, α2); VCAN (versican);
ECM1 (extracellular matrix protein 1); ITGA3
(integrin α3); ITGAL (integrin αL); ITGβ4 (integrin
β4); LAMB1 (laminin β1); MMP11 (matrix
metallopeptidase 11); MMP16 (matrix metallopeptidase 16);
MMP9 (matrix metallopeptidase 9); SELP (selectin P);
TGFβ1 (transforming growth factor β1); TIMP2 (TIMP
metallopeptidase inhibitor 2); VTN (Vitronectin);
ADAMTS13 (ADAM metallopeptidase with thrombospondin type 13
motif, 1); COL12A1 (collagen 12 α1); COL5A1 (collagen
12 α1); CTGF (connective tissue growth factor); FN1
(fibronectin 1); ITGA4 (integrin α4); ITGAM (integrin
αM); ITGβ5 (integrin β5); LAMB3 (laminin β3);
MMP12 (matrix metallopeptidase 12); MMP2 (matrix
metallopeptidase 2); NCAM1 (neural cell adhesion molecule
1); SGCE (sarcoglycan-ɛ); THBS1 (thrombospondin-1);
TIMP3 (TIMP metallopeptidase inhibitor 3); GAPDH
(glyceraldehyde-3-phosphate dehydrogenase); ADAMTS8 (ADAM
metallopeptidase with thrombospondin type 1 motif, 8);
COL14A1 (collagen XIV, α1); COL6A1 (collagen VI, α1);
CTNNA1 (catenin α1); HAS1 (hyaluronan synthase 1);
ITGA5 (integrin α5); ITGAV (integrin αV); KAL1
(Kallmann syndrome 1); LAMC1 (laminin γ1); MMP13
(matrix metallopeptidase 13); MMP3 (matrix metallopeptidase
3); PECAM1 (platelet endothelial cell adhesion molecule 1);
SPARC (secreted protein acidic and rich in cysteine);
THBS2 (thrombospondin-2); CLEC3B (C-type lectin
domain family 3, member B); ACTB (β-actin); CD44
(cell surface glycoprotein CD44); COL15A1 (collagen XV α1);
COL6A1 (collagen VI α1); CTNNB1 (β catenin);
ICAM1 (intercellular adhesion molecule 1); ITGA6
(integrin α6); ITGβ1 (integrin β1); LAMA1 (laminin,
α1); MMP1 (matrix metallopeptidase 1); MMP14 (matrix
metallopeptidase 14); MMP7 (matrix metallopeptidase 7); SELE
(E-selectin); SPG7 (spastic paraplegia 7); THBS3
(thrombospondin 3); TNC (tenascin C); RPL13A
(ribosomal protein L13a); CDH1 (cadherin 1, type 1);
COL16A1 (collagen XVI α1); COL7A1 (collagen VII α1);
CTNND1 (catenin δ1); ITGA1 (integrin α1);
ITGA7 (integrin α7); ITGβ32 (integrin β2);
LAMA2 (laminin, α2); MMP10 (matrix metallopeptidase
10); MMP15 (matrix metallopeptidase 15); MMP8 (matrix
metallopeptidase 8); SELL (selectin L); SPP1
(secreted phosphoprotein 1); TIMP1 (TIMP metallopeptidase
inhibitor 1); VCAM1 (vascular cell adhesion molecule 1);
B2M (β-2-microglobulin); CNTN1 (contactin 1);
COL1A1 (collagen I α1); COL8A1 (collagen VIII α1);
CTNND2 (catenin δ2); ITGA2 (integrin α2);
ITGA8 (integrin α8); ITGβ3 (integrin β3);
LAMA3 (laminin, α3); HPRT1 (hypoxanthine
phosphoribosyltransferase 1).
Western blot analysis
Cell pellets were lysed in Mammalian Protein
Extraction Reagent (M-PER; Thermo Fisher Scientific, Rockford, IL,
USA). Following centrifugation at 14,000 × g for 15 min, protein
concentrations were quantified (in duplicate) by the Bradford
method (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of
protein were run on SDS polyacrylamide gel electrophoresis (PAGE)
and transferred to nitrocellulose membranes (Bio-Rad). Membranes
were blocked with 5% non-fat dry milk prepared in Tris-buffered
saline containing 0.1% Tween-20 (TBST) at room temperature for 1 h.
The membrane was then incubated with primary antibodies at 4°C
overnight. Antibodies (Cdh1 and Thbs1) were obtained from Abcam
(Abcam Ltd., UK) and prepared according to the manufacturer’s
instructions. Following several washes in TBST, membranes were
incubated with appropriate secondary antibodies (1:100 dilutions;
Millipore Upstate USA, Charlottesville, VA, USA) at room
temperature for 1 h. Protein bands were visualized by the Kodak Gel
Logic 1500 Imaging System.
Immunohistochemical analysis
Our immunohistochemistry protocols were published
previously (14). Briefly,
monolayer cells were maintained in 24-well plates and fixed with
paraformaldehyde; spheroids were processed by routine histological
processing and embedded in paraffin wax. Cells were initially
incubated with primary antibodies overnight at 40°C in a humidity
chamber, followed by a modified streptavidin-peroxidase treatment.
After incubation with DAB (Invitrogen Ltd., UK), sections were
counterstained with Mayer’s hematoxylin (Sigma Chemical Co., St.
Louis, MO, USA). Immunoreactivity of molecules was assessed by
light microscopy using an Olympus BX-51 with an Olympus C-5050
digital camera. Staining was graded independently by two
investigator-blind specialists. Evaluation was carried out
semi-quantitatively on the following scale: mild, moderate and
strong.
Transmission electron microscopy
Harvested monolayers and spheroids were fixed with
2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer and
post-fixed in 1% osmium tetroxide/0.1 M sodium cacodylate buffer
for 1 h at 4°C. Cells were incubated in 1% uranyl acetate for 1 h
at 4°C, dehydrated in graded acetone series, and embedded in Epon
812. Samples were cut using a rotating-blade microtome (Leica,
Heerbrugg, Switzerland). Sections (70 nm) were mounted on copper
grids. Sections were subsequently stained with 5% uranyl acetate
and counterstained with Reynold’s lead citrate. Sections were
examined using a JEOL JEM 1011 transmission electron
microscope.
Results
The purity of
CD133high/CD44high sorted and non-sorted
subpopulations and sorting rates
DU145 human prostate cancer cells were separated
with FACS as CD133high/CD44high population
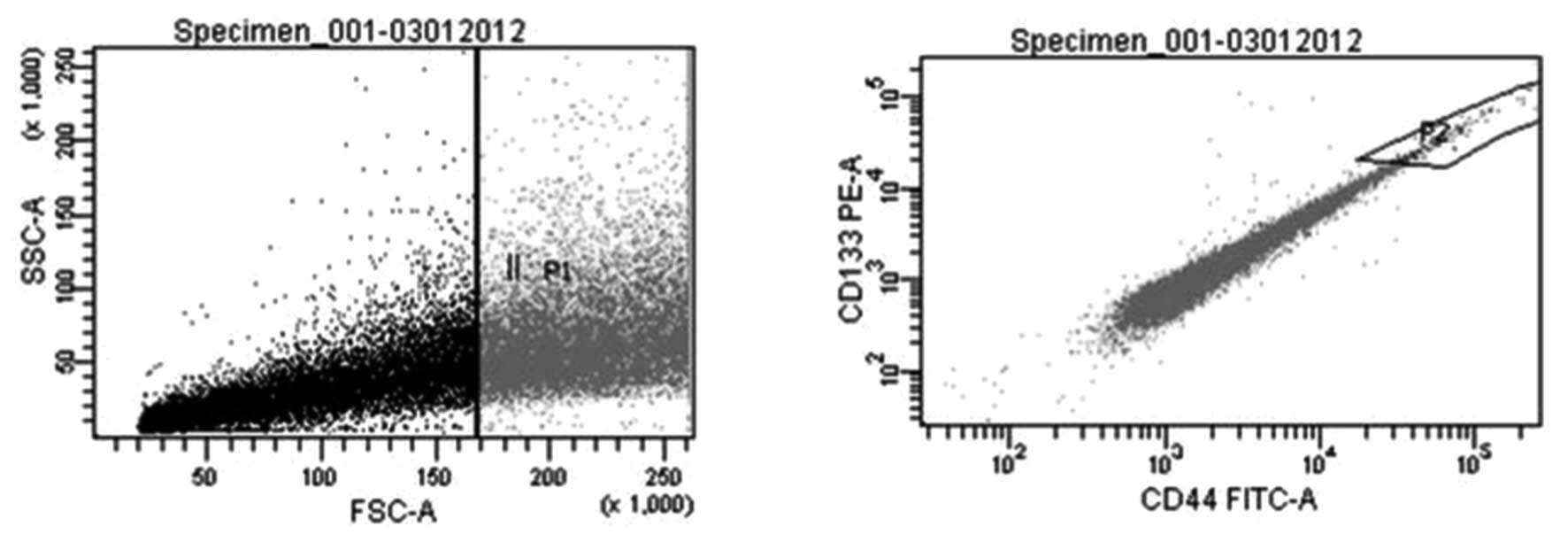
(sorted cells) and non-sorted counterparts (Fig. 1). In order to assess the degree to
which genetic imbalances were observed in CSCs vs. non-CSCs, we
compared array profiles of both cell types and the two culture
conditions that they were grown in (monolayer and spheroids).
Prior to the PCR expression microarray, purity of
CSC and non-CSC samples was tested with CD133 and CD44 antibodies.
Sorting rate analysis and purity of cells were evaluated
sequentially. Rates were 96,7±5,4% for sorted cells and 90,33±5,4
for non-sorted cells. In order to confirm the flow cytometry
analyses, cells were re-evaluated after sorting and the analyses
were repeated after one passage. Results showed the cell purity
after sorting was 85%. Immunofluorescence staining yielded a cell
purity of >85% in all samples.
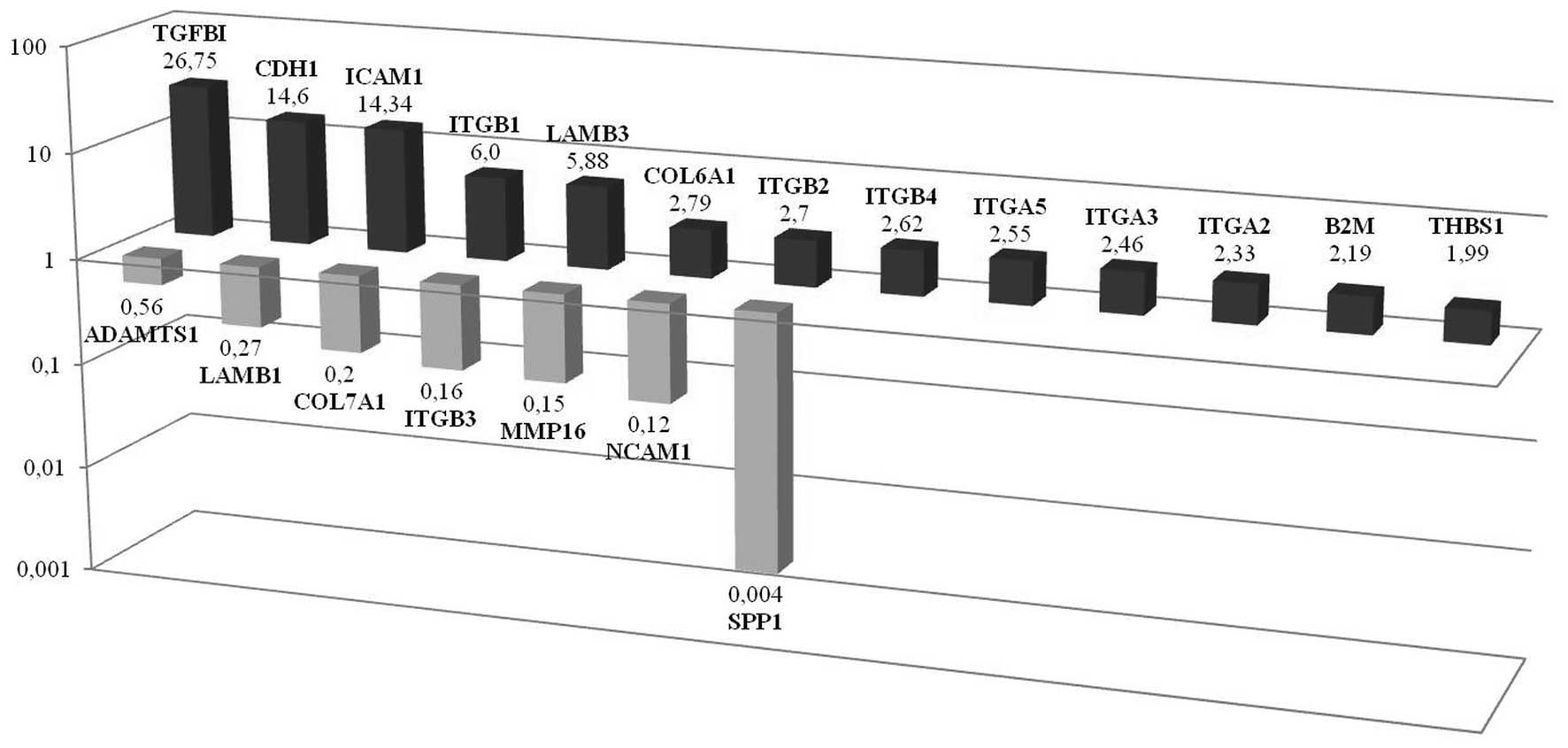
TGFβ1 triggers molecules in monolayer
CD133+/CD44+ prostate cells when cells
arrange for 3D composition
TGFβ1, CDH1 and ICAM1 gene
expressions were significantly upregulated in
CD133+/CD44+ CSCs grown as a monolayer when
compared to their CD133−/CD44− counterpart.
From the latter candidates’ gene, we validated the ten top-ranked
genes and ITGβ1, LAMB3, ITBG2, ITGA2,
COL6A1, ITB4, ITGA3, THBS, B2M
and ITGA5 were observed to be markedly upregulated.
TGFβ1 is emphasized since it is the most upregulated gene in
monolayer cells. Secreted phosphoprotein 1 (SSP1) is observed to be
the most downregulated gene in monolayer CSCs. Additionally
NCAM1, MMP16, ITGβ3, COL7A1,
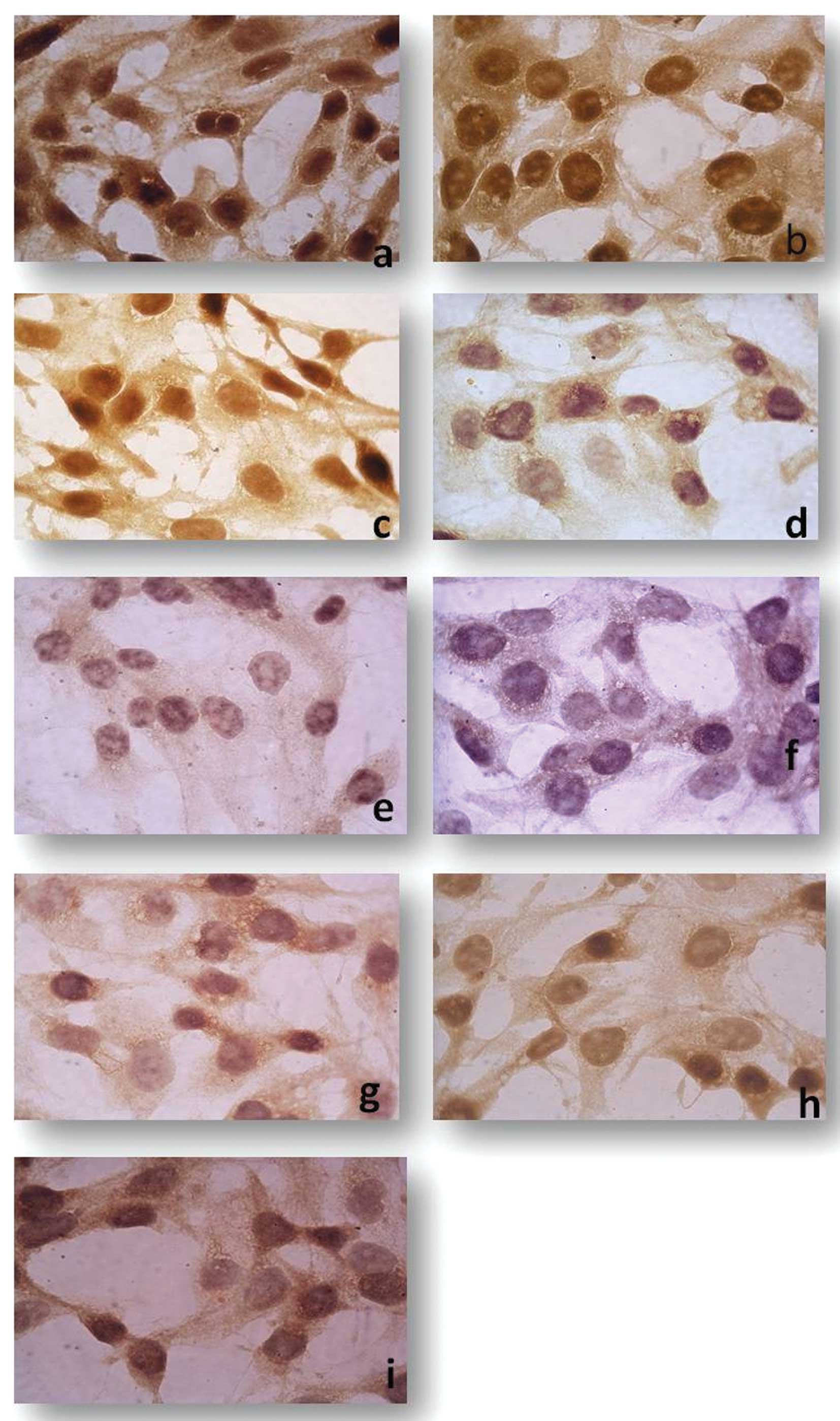
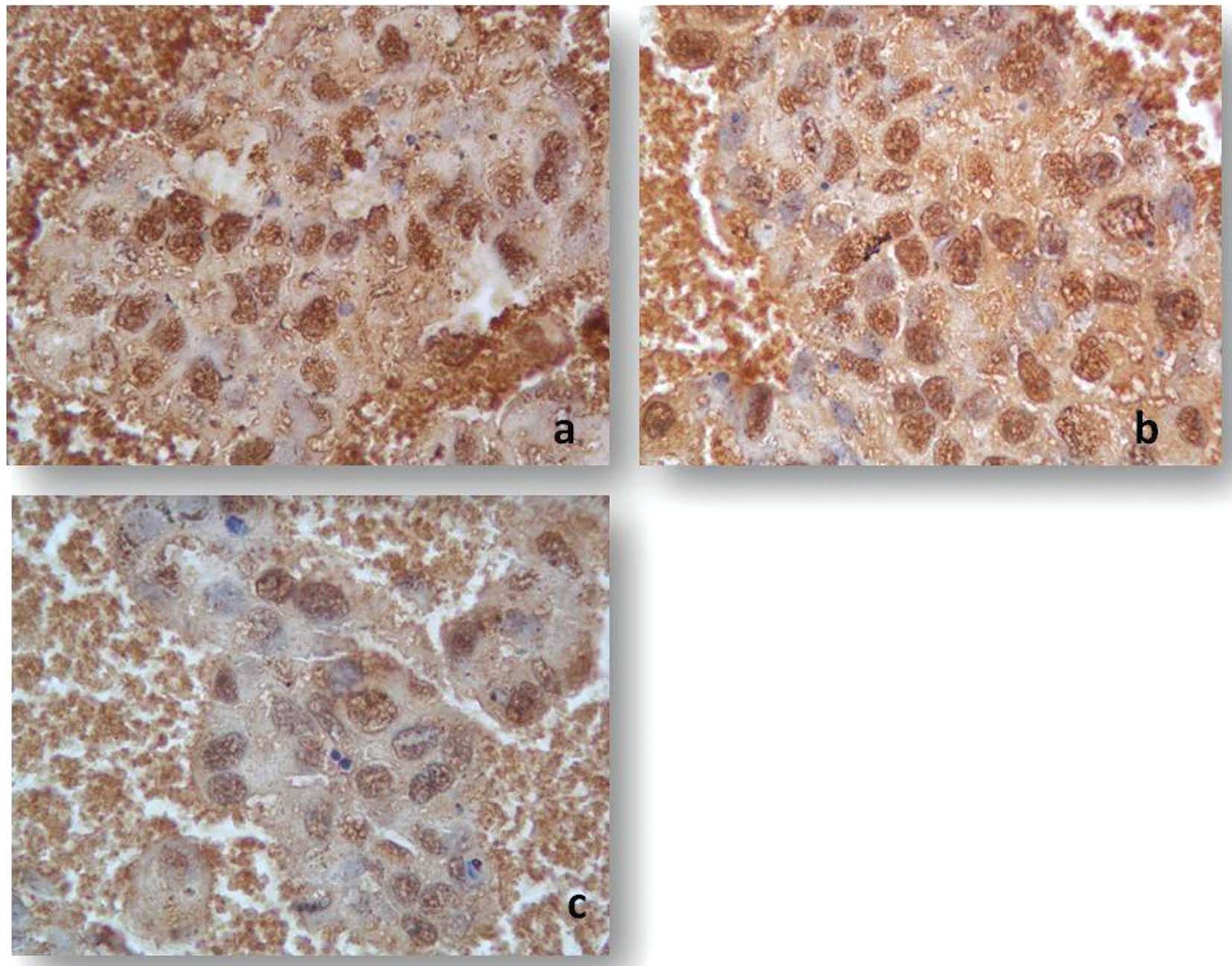
LAMB1, ADAMTS1 were also downregulated (Fig. 2). Immunohistochemistry of monolayer
CD133+/CD44+ cells also showed the strong
immunoreactive staining of TGFβ1, CDH1, ICAM1 and THBS1 when
compared to the bulk counterpart. In addition, staining intensity
was significantly decreased for SPP1, NCAM1, MMP16, ITGβ3 and
COL7A1 (Fig. 3).
ITGβ3 upregulation and PECAM1
downregulation are characteristic of CSCs with respect to non-CSCs
in spheroids
Significant differences were observed mainly in this
group according to adhesion molecules between CSC and non-CSC
populations grown as spheroids. Array results showed that
ITGβ3, ITGA2, ITGA5 and SPG7 were
upregulated, while PECAM1, ADAMTS1, VCAN,
CTNND2, TNC, ACTB, COL12A1,
MMP14, TGFβ1, SPP1 and ITGA6 were
downregulated in CSCs. ITGβ3 was the third upregulated gene
in CSC spheroids when compared with the differential expression in
monolayer cells. A significant increase was observed in CSC
spheroids when we compared with the non-CSC population. It is of
note that VCAN was significantly high (46-fold change) in CSC
spheroids when compared to its monolayer counterpart. However, VCAN
is downregulated in CSC spheroids when compared to its non-CSC
spheroid counterpart (Fig. 4).
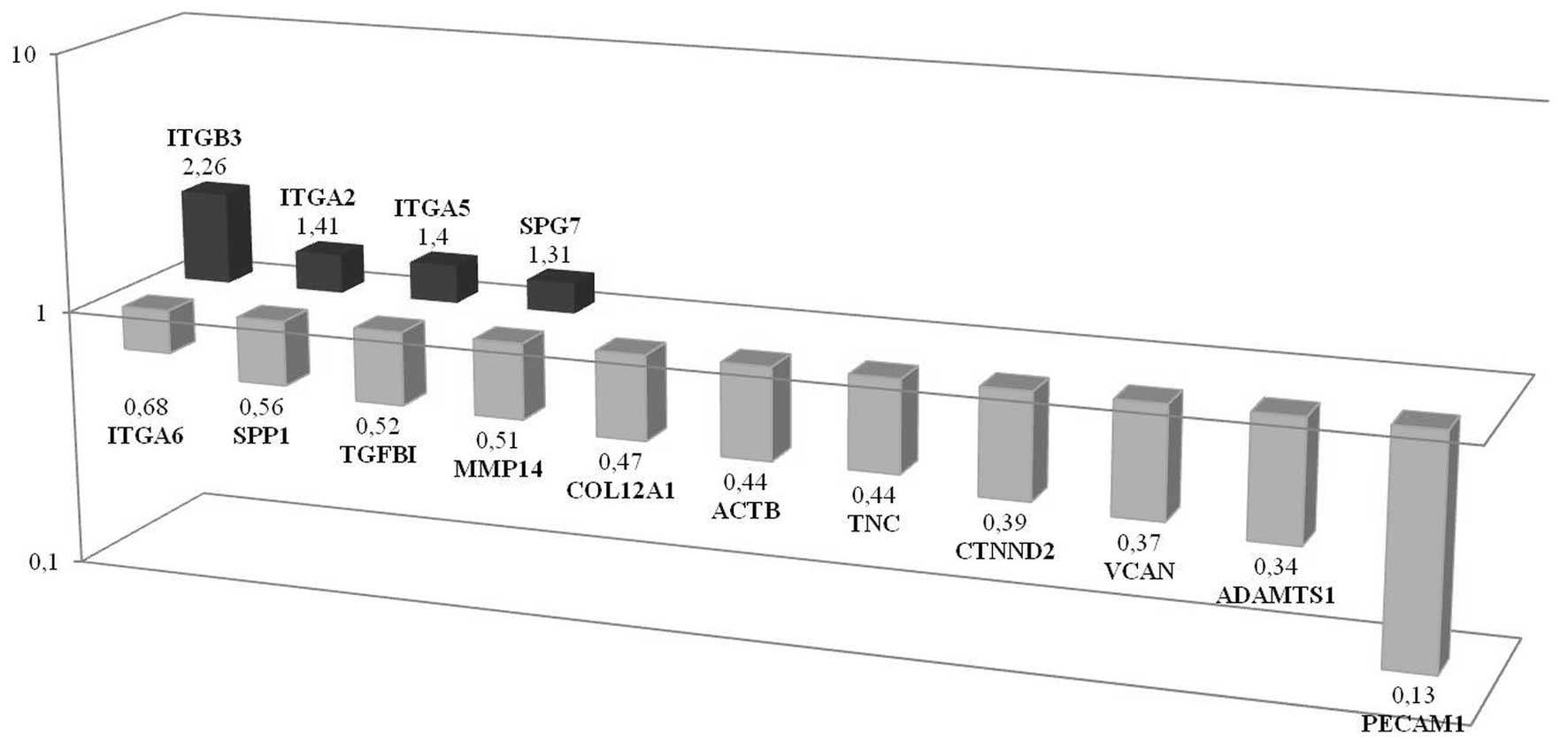 | Figure 4Differences of genetic profile
between CSC and non-CSC populations investigated in spheroids in
adhesion molecules. Results of array showed that ITGβ3,
ITGA2, ITGA5 and SPG7 were upregulated while
PECAM1, ADAMTS1, VCAN, CTNND2,
TNC, ACTB, COL12A1, MMP14,
TGFβ1, SPP1 and ITGA6 were downregulated,
respectively, in CSCs according to non-CSCs. |
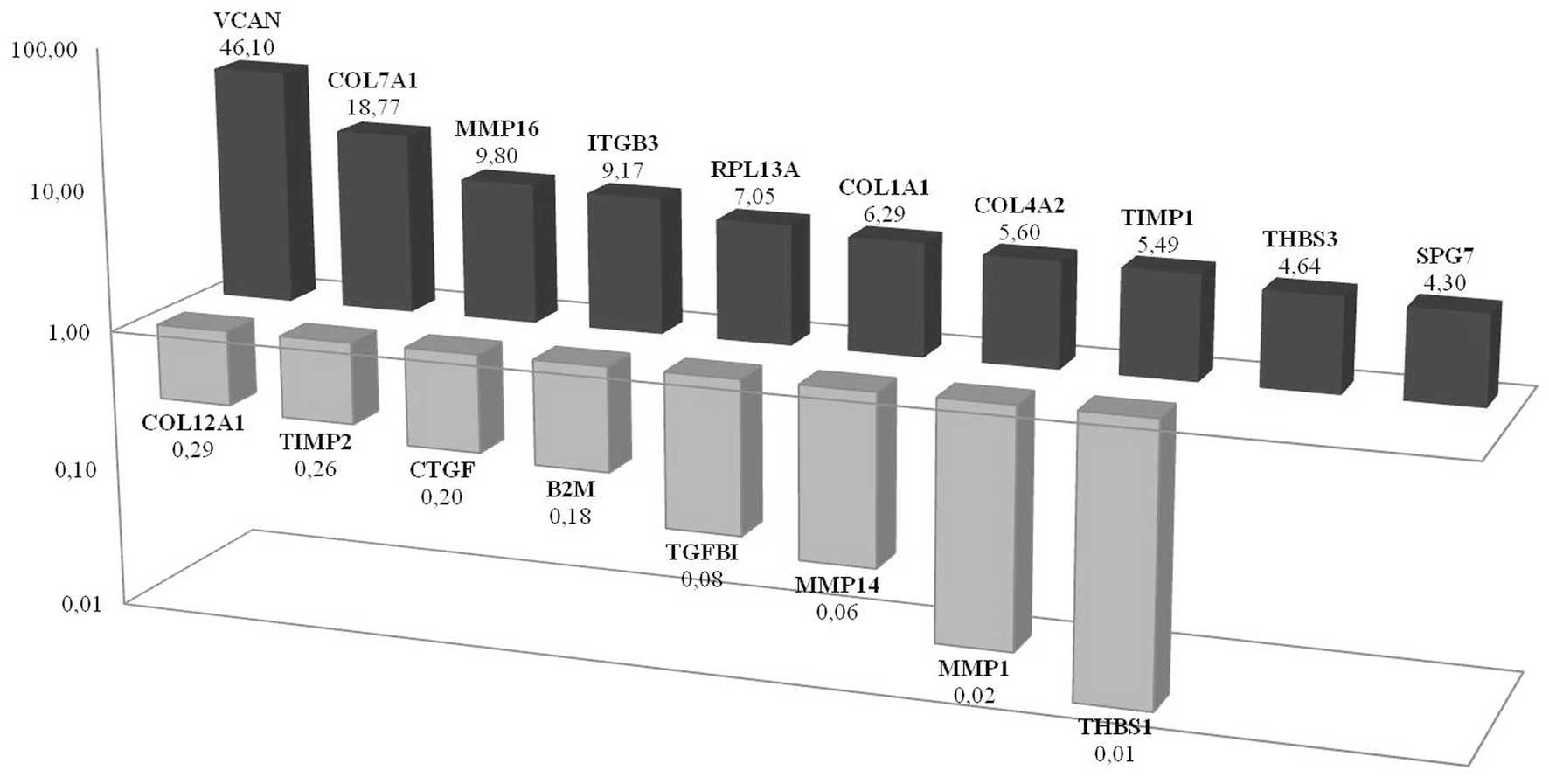
VCAN expression significantly increases
in CD133+/CD44+ prostate cancer cells
propagated as tumor spheroids
DU145 tumor spheroids were cultured in low-adherence
culture conditions as described above and maintained in culture for
10–14 days. Spheroid forming CD133+/CD44+
cells showed elevated expression of VCAN, COL7A1,
ITGβ3, MMP16, RPL13A, COL4A2 and
TIMP1 when compared with the
CD133+/CD44+ monolayer cells they originated
from. The most significant change was demonstrated in VCAN
expression where a 46-fold change was observed (Fig. 5). Increased VCAN expression
was also demonstrated by immunohistochemistry in CSC spheroids when
compared to other cell groups. On the other hand, THBS1 is
the most downregulated gene in CSC spheroids when compared with CSC
monolayers. PCR expression array results showed that MMP1,
MMP14, TGFβ1, B2M, CTGF, TIMP2 and
COL12A1 were all downregulated in prostate CSC spheroids.
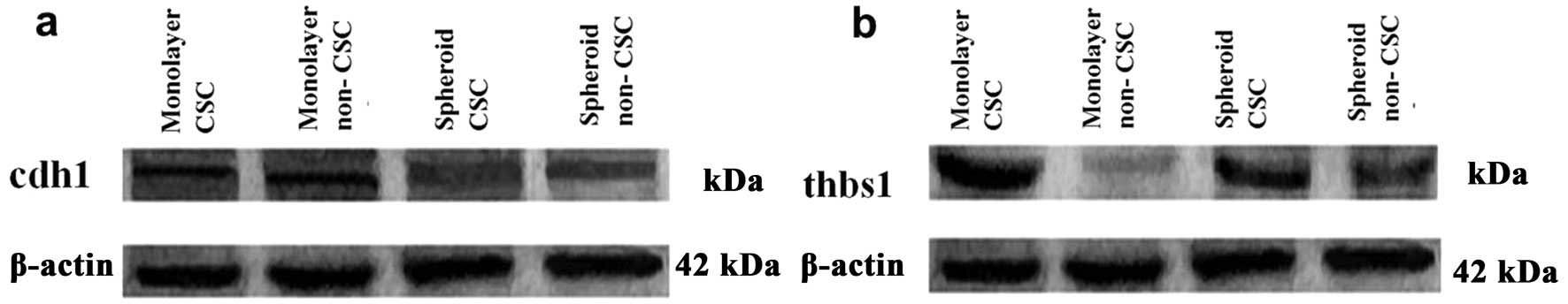
Western blot analysis of Thbs1 showed an increased protein level in
monolayer CSCs when compared with spheroid CSCs. However, the most
significant decrease for THBS1 was observed in monolayer forming
non-CSCs (Fig. 7b).
Immunohistochemistry supported the increased expression in CSC
spheroids for VCAN, COL7A1 and MMP16 molecules (Fig. 6).
CDH1 expression is significantly
increased in non-CSCs propagated as tumor spheroids
PCR-array analyses performed in non-CSCs that were
grown in culture as spheroids showed that CDH1,
PECAM1, COL4A2, GAPDH, FN1,
ITGβ4, COL1A1, TNC, RPL13A,
CTNND2, MMP15, ICAM1, MMP9,
ITGβ1 and TGFβ1 expressions were significantly
upregulated while MMP1, THBS1, SPP1,
LAMB1, MMP14, TIMP2, B2M and
ITGA6 expressions were downregulated. Among these molecules,
the most upregulated gene was observed to be CDH1 and the most
downregulated MMP1. It should be noted that in the previous
experiments where we compared the spheroid and monolayer CSCs
groups, VCAN was the most upregulated and THBS1 was the most
downregulated gene. At this point, our results suggest that these
differences may be an important lead to fully characterize the
mechanisms of cell organization (Fig.
8). Western blotting also showed increased CDH1 protein levels
in non-CSC spheroids when compared to its monolayer counterpart
(Fig. 7a).
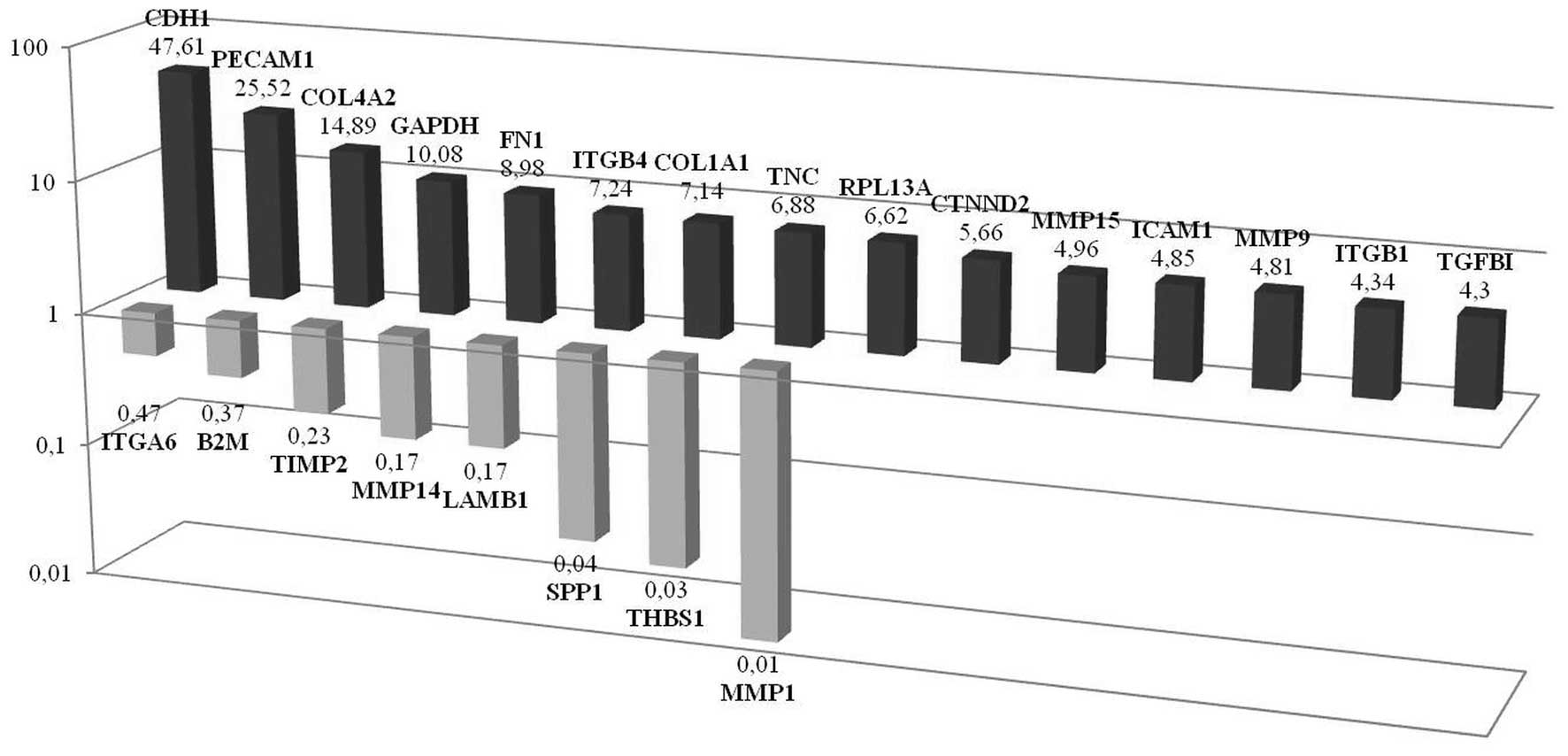 | Figure 8PCR-array analysis performed in the
cells which sorted to be non-CSCs and maintained to be spheroids.
Results showed that CDH1, PECAM1, COL4A2,
GAPDH, FN1, ITGB4, COL1A1, TNC,
RPL13A, CTNND2, MMP15, ICAM1,
MMP9, ITGB1 and TGFβ1 were significantly
upregulated while MMP1, THBS1, SPP1,
LAMB1, MMP14, TIMP2, B2M and
ITGA6 were downregulated, respectively. |
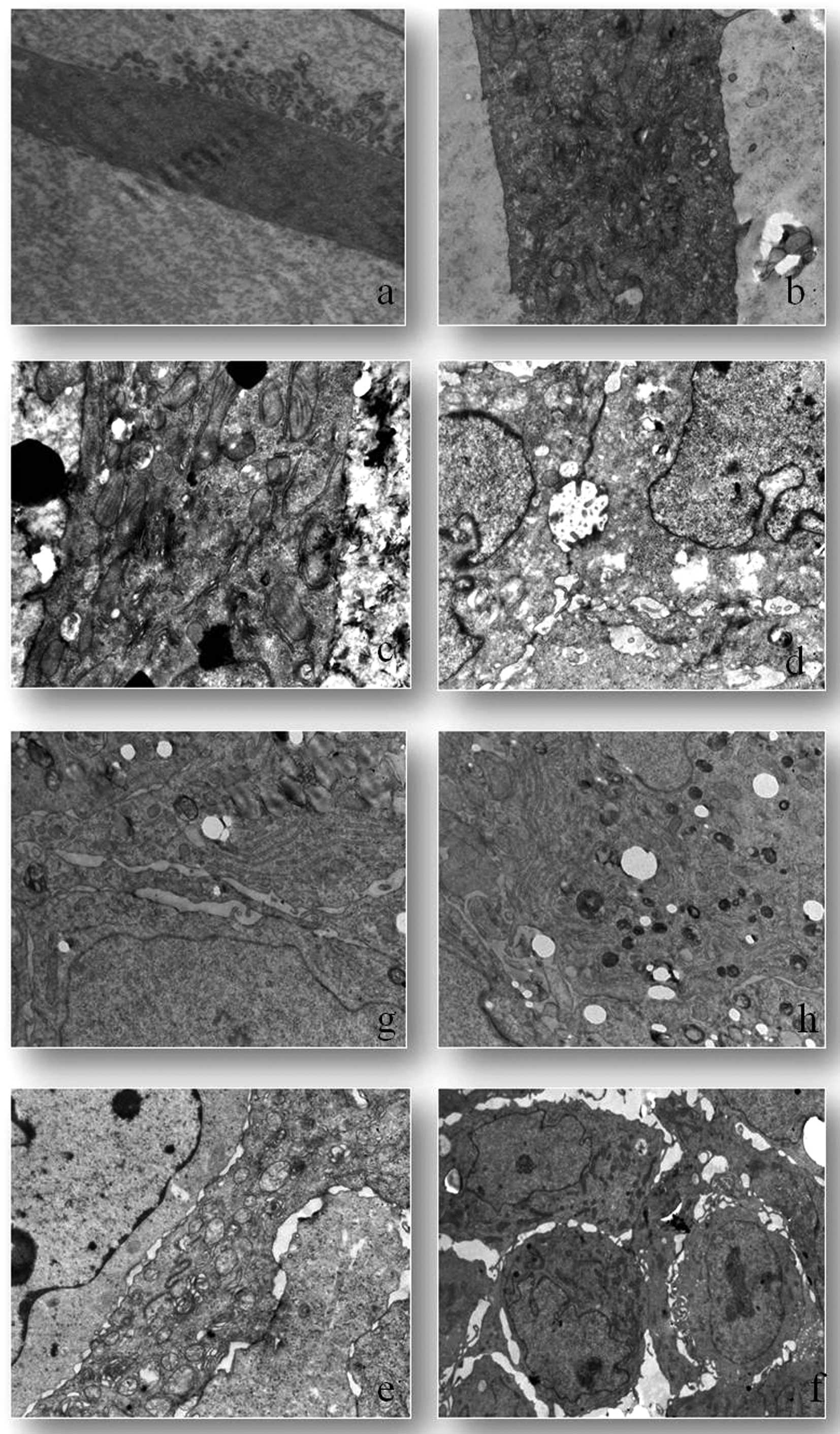
Three dimensional composition changes
cell ultrastructure
CSCs grown as a monolayer had intact cell and
nuclear membranes. These cells have villous-like protrusions over
the surface. These villi-like structures are observed abundantly in
nucleus adjacent cell membrane domains (Fig. 9a). Non-CSC populations were observed
to have an increased size and number of their organelles such as
golgi, mitochondria and granular endoplasmic reticulum. The
villous-like structures observed in CSCs were not detected in this
group (Fig. 9b). Vacuolization and
mitophagy increased in non-CSCs (Fig.
9c). Electron microscopic investigations were performed on
cells maintained as spheroids. These tight intercellular
connections were observed to be intermittently interrupted along
membranes and resulted in cellular gaps. The villous-like
protrusions observed in monolayer CSCs were also observed in CSC
spheroids. However, as a distinctive feature, these villous-like
structures were observed in intercellular lacunae (Fig. 9d). In CSC spheroids, the organelle
most increased in size was the golgi (Fig. 9e). However, the cellular organelles
were observed to be clusters in the cytoplasm (Fig. 9f). Increased lipid depositions were
observed in cytoplasm and among organelles. Non-CSC spheroids
indicated euchromatic nucleus and smooth nuclear membrane. As for
spheroid non-CSCs, the most increased organelle was observed to be
the granular endoplasmic reticulum (Fig. 9g). Another important observation of
the electron microscopic analyses was the significant increase of
autophagic vacuoles in non-CSCs (Fig.
9h).
Discussion
Our data suggest that
CD133+/CD44+ prostate cancer stem cells
(CSCs) affect their microenvironment and the cellular signaling in
surrounding tissue resulting in changes in their behavior reflected
as different expression profiles. When CSCs constitute a complex
and organized formation, versican is the highest upregulated gene
among adhesion molecules. We believe it is important that versican
upregulation is only observed in CD133+/CD44+
prostate CSCs and that this upregulation may be specific for CSCs.
Versican is a hyalectan, a specific proteoglycan that affects cell
signaling, motility, adhesion, growth and apoptosis. Changes in the
differential expression of proteoglycans in a specific tissue may
promote or inhibit tumor progression (15). In prostate cancer, versican produced
by prostatic fibroblasts inhibited the attachment of the tumor
cells to fibronectin. This inhibition provides increased tumor cell
motility and facilitates local invasion and possibly metastasis
(16,17). CD44 is a hyaluronan receptor and it
displays increased expression in the CSC populations of several
types of cancer. Formation of the hyaluronan-CD44-versican complex
plays an effective role in the assembly of a polarized pericellular
sheath to promote tumor cell motility (18,19).
We demonstrated increased gene expression in prostate CSC
spheroids, which could have resulted from tumor cells increasing
the transcription of this molecule during the cellular
organization. This observation correlates with other data in our
study such as the upregulation of transforming growth factor β1
(TGFβ1) in monolayer CSCs. Our study suggests the tumor cells
themselves are the source of versican, and it has previously been
reported that TGFβ1 can induce versican production in
prostate cancer cells (20). It is
possible to assume that during tumor organization, TGFβ1 is
upregulated initially creating an environment favoring the
organization of the hyaluronan-CD44-versican complex in prostate
CSCs. In addition, decrease in ADAMTS (a disintegrin and
metalloproteinase with trombosondin motifs) is found to accompany
the enhanced expression of versican by TGFβ1 (21). Our data reveals a decreased ADAMTS
expression in CSC spheroids, where versican accumulation may be a
secondary response to ADAMTS degradation. Knaup et al
demonstrated that activated TGFβ signaling (either in response to
wounding or to simulate type VII collagen expression) could
facilitate cancer development and progression (22). In the present study, we demonstrated
significant upregulation in COL7A1 expression in CSC spheroids and
this is probably related to the positive stimuli of TGFβ1
signaling. On the other hand, COL7A1 functions as an anchoring
fibril between the external epithelia and the underlying stroma.
Mutations in this gene are associated with all forms of dystrophic
epidermolysis bullosa (23). In the
present study, increased COL7A1 in sorted spheroids suggest that
proliferated CSCs used this molecule for anchoring to the
extracellular matrix.
Integrins are transmembrane adhesion molecules that
mediate cell-cell and cell-extracellular matrix attachment
(24). Integrins can influence cell
migration and invasion by directly mediating adhesion to the
extracellular matrix or regulating intracellular signaling pathways
that control cytoskeletal organization, force dependent effects and
survival (25). These glycoproteins
regulate cell growth, proliferation, migration and apoptosis and,
as a consequence, can have a potential role in tumor progression
and metastasis with the aberrant expression of its genes (26,27).
The ex vivo expansion of stem cells using extracellular
proteins such as fibronectin and laminin enhances the homing and
differentiation of these cells and also promotes the upregulation
of β1 integrins (28,29). In addition, vitronectin is a main
receptor for integrin α5 β3 and is directly related to
differentiation of CSCs (6). In the
present study, we observed a differential integrin expression in
which there was a predominant overexpression in CSC spheroids.
ITGβ3 (CD61) was found to be significantly high in this cell
population. Lo et al recently deciphered the link in the
integrin β3-TGFβ signaling mode and the mechanisms underlying
increased activation of TGFβ signaling in tumor initiating cells
(30). Similar to our findings,
TGFβ could induce integrin β3 expression when cells form complex
spheric organizations.
Involvement between cells and the ECM plays an
important role in normal development and differentiation. However,
remodeling of the ECM occurs in many pathological states. Changes
in the ECM are regulated by a system of proteolytic enzymes that
are responsible for the proteolysis of a large quantity of ECM
components (31). Previous studies
demonstrated that CSCs require these for the maintenance of the
extracellular matrix and CSCs express different molecules for
detachment from the niche (32–34).
In the present study, notably, we determined a significant increase
in MMP16 whereas here was a decrease in MMP1 and 14. Astarci et
al showed that cell differentiation in the Caco-2 cell line is
accompanied by decreased MMP-16 mRNA and protein expression. This
group demonstrated that the forced expression of miR-146a in HT29
colon cancer cells resulted in decreased expression of MMP16
(35). Nevertheless, Inoue et
al demonstrated in glioma cells that MMP13 was specifically
expressed in tumor sphere-forming cells and that it is related to
the invasive potential of CSCs (32). On the other hand, it has been shown
in breast cancer cells that cancer progression inhibitors suppress
the mRNA expression of some matrix metalloproteinases (MMPs), but
stimulate that of others.
Our electron microscopic data support the theory
that the predominant cell death mechanism in non-CSCs is autophagy.
In spheroids, CSCs lose their villous protrusions and cellular
organelles are observed to be clusters on the side of the
cytoplasm. During our literature review, we did not find any
definite data regarding these electron microscopic results.
In conclusion, the present study demonstrated first
that isolated CSCs were found to possess multipotential
differentiation capabilities regulated through the upregulation
and/or downregulation of their markers, particularly versican,
TGFβ1, Col7A1 and ITGβ3. The most striking observation is the
upregulation of versican that was only determined in CSCs. We
assume that one must engage CSCs or more signaling cascades to
differentiate and initiate tumor formation. This mechanism occurs
with specific intracellular and extracellular signals. It is
possible that CSCs themselves may be a source of extracellular
signaling molecules. This may be a triggering mechanism for the
construction of required extracellular matrix compositions. These
molecules that are effective during tumor progression
differentiation could be targeted for future therapeutic
interventions. Developing new therapeutic strategies that will
effectively target this critically important population of cancer
cells may be a cornerstone in cancer therapy.
Acknowledgements
This study was funded by the Ege University
Scientific Research Project Fund.
References
|
1
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
2
|
Yu Z, Pestell TG, Lisanti MP and Pestell
RG: Cancer stem cells. Int J Biochem Cell Biol. 44:2144–2151. 2012.
View Article : Google Scholar
|
|
3
|
Neumüller RA and Knoblich JA: Dividing
cellular asymmetry: asymmetric cell division and its implications
for stem cells and cancer. Genes Dev. 23:2675–2699. 2009.PubMed/NCBI
|
|
4
|
La Porta CA: Mechanism of drug sensitivity
and resistance in melanoma. Curr Cancer Drug Targets. 9:391–397.
2009.PubMed/NCBI
|
|
5
|
Yin G, Alvero AB, Craveiro V, Holmberg JC,
Fu HH, Montagna MK, Yang Y, Chefetz-Menaker I, Nuti S, Rossi M,
Silasi DA, Rutherford T and Mor G: Constitutive proteasomal
degradation of TWIST-1 in epithelial-ovarian cancer stem cells
impacts differentiation and metastatic potential. Oncogene.
32:39–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hurt EM, Chan K, Serrat MA, Thomas SB,
Veenstra TD and Farrar WL: Identification of vitronectin as an
extrinsic inducer of cancer stem cell differentiation and tumor
formation. Stem Cells. 28:390–398. 2010.PubMed/NCBI
|
|
7
|
Robertson FM, Ogasawara MA, Ye Z, Chu K,
Pickei R, Debeb BG, Woodward WA, Hittelman WN, Cristofanilli M and
Barsky SH: Imaging and analysis of 3D tumor spheroids enriched for
a cancer stem cell phenotype. J Biomol Screen. 15:820–829. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vinci M, Gowan S, Boxall F, Patterson L,
Zimmermann M, Court W, Lomas C, Mendiola M, Hardisson D and Eccles
SA: Advances in establishment and analysis of three-dimensional
tumor spheroid-based functional assays for target validation and
drug evaluation. BMC Biol. 10:292012. View Article : Google Scholar
|
|
9
|
Ayla S, Bilir A, Soner BC, Yilmaz-Dilsiz
O, Ergüven M and Oktem G: Notch signaling-related therapeutic
strategies with novel drugs in neuroblastoma spheroids. J Pediatr
Hematol Oncol. 36:37–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Diaz JA and Murillo MF: Phenotype
characterization of embryoid body structures generated by a crystal
comet effect tail in an intercellular cancer collision scenario.
Cancer Manag Res. 4:9–21. 2012. View Article : Google Scholar
|
|
11
|
Gaedtke L, Thoenes L, Culmsee C, Mayer B
and Wagner E: Proteomic analysis reveals differences in protein
expression in spheroid versus monolayer cultures of low-passage
colon carcinoma cells. J Proteome Res. 6:4111–4118. 2007.
View Article : Google Scholar
|
|
12
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24−/low/CD44+ breast
cancer-initiating cells to radiation. J Natl Cancer Inst.
98:1777–1785. 2006.PubMed/NCBI
|
|
13
|
Oktem G, Bilir A, Ayla S, Yavasoglu A,
Goksel G, Saydam G and Uysal A: Role of intercellular
communications in breast cancer multicellular tumor spheroids after
chemotherapy. Oncol Res. 16:225–233. 2006.PubMed/NCBI
|
|
14
|
Oktem G, Bilir A, Selvi N, Yurtseven ME,
Vatansever S, Ates U, Uysal A and Omay SB: Chemotherapy influences
inducible nitric oxide synthase (iNOS) and endothelial nitric oxide
synthase (eNOS) activity on 3D breast cancer cell line. Oncol Res.
16:195–203. 2006.PubMed/NCBI
|
|
15
|
Edwards IJ: Proteoglycans in prostate
cancer. Nat Rev Urol. 9:196–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ricciardelli C, Sakko AJ, Ween MP, Russell
DL and Horsfall DJ: The biological role and regulation of versican
levels in cancer. Cancer Metastasis Rev. 28:233–245. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakko AJ, Ricciardelli C, Mayne K, Suwiwat
S, LeBaron RG, Marshall VR, Tilley WD and Horsfall DJ: Modulation
of prostate cancer cell attachment to matrix by versican. Cancer
Res. 63:4786–4791. 2003.PubMed/NCBI
|
|
18
|
Ricciardelli C, Russell DL, Ween MP, Mayne
K, Suwiwat S, Byers S, Marshall VR, Tilley WD and Horsfall DJ:
Formation of hyaluronan- and versican-rich pericellular matrix by
prostate cancer cells promotes cell motility. J Biol Chem.
282:10814–10825. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ween MP, Hummitzsch K, Rodgers RJ, Oehler
MK and Ricciardelli C: Versican induces a pro-metastatic ovarian
cancer cell behavior which can be inhibited by small hyaluronan
oligosaccharides. Clin Exp Metastasis. 28:113–125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakko AJ, Ricciardelli C, Mayne K, Tilley
WD, Lebaron RG and Horsfall DJ: Versican accumulation in human
prostatic fibroblast cultures is enhanced by prostate cancer
cell-derived transforming growth factor β1. Cancer Res. 61:926–930.
2001.PubMed/NCBI
|
|
21
|
Cross NA, Chandrasekharan S, Jokonya N,
Fowles A, Hamdy FC, Buttle DJ and Eaton CL: The expression and
regulation of ADAMTS-1, -4, -5, -9, and -15, and TIMP-3 by TGFβ1 in
prostate cells: relevance to the accumulation of versican.
Prostate. 63:269–275. 2005.PubMed/NCBI
|
|
22
|
Knaup J, Gruber C, Krammer B, Ziegler V,
Bauer J and Verwanger T: TGFβ-signaling in squamous cell carcinoma
occurring in recessive dystrophic epidermolysis bullosa. Anal Cell
Pathol. 34:339–353. 2011.
|
|
23
|
Ryynänen M, Knowlton RG, Parente MG, Chung
LC, Chu ML and Uitto J: Human type VII collagen: genetic linkage of
the gene (COL7A1) on chromosome 3 to dominant dystrophic
epidermolysis bullosa. Am J Hum Genet. 49:797–803. 1991.PubMed/NCBI
|
|
24
|
Switala-Jelen K, Dabrowska K, Opolski A,
Lipinska L, Nowaczyk M and Gorski A: The biological functions of β3
integrins. Folia Biol. 50:143–152. 2004.
|
|
25
|
Hood JD and Cheresh DA: Role of integrins
in cell invasion and migration. Nat Rev Cancer. 2:91–100. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hieken TJ, Farolan M, Ronan SG, Shilkaitis
A, Wild L and Das Gupta TK: β3 integrin expression in melanoma
predicts subsequent metastasis. J Surg Res. 63:169–173. 1996.
|
|
27
|
Vacca A, Ria R, Presta M, Ribatti D,
Iurlaro M, Merchionne F, Tanghetti E and Dammacco F:
αvβ3 integrin engagement modulates cell
adhesion, proliferation, and protease secretion in human lymphoid
tumor cells. Exp Hematol. 29:993–1003. 2001.
|
|
28
|
Rentala S, Yalavarthy PD and Mangamoori
LN: α1 and β1 integrins enhance the homing and differentiation of
cultured prostate cancer stem cells. Asian J Androl. 12:548–555.
2010.
|
|
29
|
Sagar BM, Rentala S, Gopal PN, Sharma S
and Mukhopadhyay A: Fibronectin and laminin enhance engraftibility
of cultured hematopoietic stem cells. Biochem Biophys Res Commun.
350:1000–1005. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lo PK, Kanojia D, Liu X, Singh UP, Berger
FG, Wang Q and Chen H: CD49f and CD61 identify Her2/neu-induced
mammary tumor-initiating cells that are potentially derived from
luminal progenitors and maintained by the integrin-TGFβ signaling.
Oncogene. 31:2614–2626. 2012.PubMed/NCBI
|
|
31
|
Pytliak M, Vargová V and Mechírová V:
Matrix metalloproteinases and their role in oncogenesis: a review.
Onkologie. 35:49–53. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Inoue A, Takahashi H, Harada H, Kohno S,
Ohue S, Kobayashi K, Yano H, Tanaka J and Ohnishi T: Cancer
stem-like cells of glioblastoma characteristically express MMP-13
and display highly invasive activity. Int J Oncol. 37:1121–1131.
2010.PubMed/NCBI
|
|
33
|
Barami K: Relationship of neural stem
cells with their vascular niche: implications in the malignant
progression of gliomas. J Clin Neurosci. 15:1193–1197. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Calabrese C, Poppleton H, Kocak M, Hogg
TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M,
Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A and
Gilbertson RJ: A perivascular niche for brain tumor stem cells.
Cancer Cell. 11:69–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Astarci E, Erson-Bensan AE and Banerjee S:
Matrix metalloprotease 16 expression is downregulated by
microRNA-146a in spontaneously differentiating Caco-2 cells.
Dev Growth Differ. 54:216–226. 2012.PubMed/NCBI
|