Introduction
Breast cancer accounts for 25% of all cancers in
women worldwide. In the US 296,000 women were diagnosed with breast
cancer and over 39,000 died of the disease in 2013 (1). Numerous treated breast cancer patients
will develop incurable metastatic disease, with a median survival
of 3 years. Standard adjuvant therapies have a small impact on
breast cancer survival, yet more than $16 billion were spent on
breast cancer clinical care in 2010 (2). Most breast cancer risk factors cannot
be modified, and include age, family and reproductive history,
BRCA1 status and breast density.
Mammary stem cells (MSCs) are the progenitor
population for human breast epithelia (3–5). MSCs
give rise during mammary gland development to estrogen receptor
(ER)-negative basal cells and the ER− luminal progenitor
(LP) population which maintains ER+ and ER−
luminal cells (6–8). MSCs have been isolated from humans and
mice using cell surface markers (9–11). The
MSC population is expanded and tumorigenic in some mouse mammary
cancer models (12,13), and these tumor-initiating cells have
been isolated from human breast cancers (14–18).
MSC expansion is associated with aggressive biological behavior in
human breast cancer (19,20). The LP population is tumorigenic in
some mouse mammary cancer models (21), and is the progenitor population of
basal breast cancer in humans (22).
The enhancer of zeste homolog 2 (EZH2) is a
methyltransferase which catalyzes methylation of lysine 27 in
histone H3 resulting in suppression of target gene expression
(23). The histone demethylase
JMJD3 opposes the activity of EZH2 by demethylating histone H3
lysine 27. EZH2 is a member of the polycomb group of proteins which
regulates cell type identity (24).
EZH2 overexpression in the mouse mammary gland was found to produce
intraductal hyperplasia and to delay involution. EZH2 expression
was increased in histologically normal human breast tissue among
women at high breast cancer risk (25), and was elevated in ductal
hyperplasia and ductal carcinoma in situ (26). EZH2 overexpression is associated
with poorly differentiated and aggressive breast cancer in humans
(27,28). However, the mechanisms by which EZH2
results in increased breast cancer risk and aggressive tumors are
not completely characterized. Using in vivo transplantation
of mammary cancer stem cells transduced with EZH2 or JMJD3 shRNAs,
we demonstrated that EZH2 promotes mammary stem and LP cell
expansion, metastasis and inhibits ER-positive cellular
differentiation.
Materials and methods
Mouse breeding and procedures
We bred MMTV-Wnt1 mice obtained from The Jackson
Laboratories (Bar Harbor, ME, USA). Mammary tumorigenesis is driven
by Wnt1 oncogene expression which is a model of basal subtype
breast cancer. All mice were genotyped using PCR amplification of
extracted tail DNA according to The Jackson Laboratories protocols.
Twenty tumors were obtained for analysis. Tumors were trypsin
dissociated for cryopreservation in liquid nitrogen.
Fluorescence-activated cell sorting
Dissociated tumor cells were incubated with
phycoerythrin-conjugated anti-CD24 and AlexaFluor 488 conjugated
anti-CD49f antibodies (Stem Cell Technologies, Vancouver, BC,
Canada), washed in phosphate-buffered saline (PBS), and the
CD24+/CD49fhi MSC fraction was sorted by flow
cytometry (MoFlo Astrios, Becton Dickinson, Franklin Lakes, NJ).
The CD24+/CD49flo/CD61+ LP
fractions were sorted in separate experiments. Dissociated tumor
cells were incubated with fluorescein-conjugated estradiol to
identify ER-positive cells prior to sorting.
Cell culture, lentiviral transduction and
transplantation
Sorted MSCs (104) from MMTV-Wnt1 tumors
were cultured in 3:1 Dulbecco’s modified Eagle’s medium:F12 medium
containing 1X B27 supplement, 10 ng/ml epidermal growth factor, 25
ng/ml basic fibroblast growth factor, 0.2% heparin, 40 µg/ml
gentamicin, 2.5 µg/ml amphotericin B (MSC medium) at 37°C in
a humidified atmosphere of 5% CO2 and separately
transduced with lentiviruses containing control, EZH2 or JMJD3
shRNAs with 5 µg/ml Polybrene overnight according to the
manufacturer’s protocol (Thermo Scientific, Waltham, MA, USA) at
37°C in a humidified atmosphere of 5% CO2. MSC medium
was replaced and cells were cultured for 24 h. Puromycin (2
µg/ml) was added and the cells were incubated for 48 h. MSC
medium was replaced and the cells were injected into the fat pads
of 2-month-old immunocompromised NU/J mice. In separate
experiments, fat pads were transplanted with 104 sorted
MSCs with either ER+ or ER− luminal cells
from the MMTV-Wnt1 mammary tumors. Mice were examined weekly for
tumor formation for up to 6 months. The latency and volume of
tumors were recorded for each mouse. Complete necropsy was
performed on each mouse. Portions of each tumor were fixed in 10%
formalin, flash frozen for storage at −80°C and trypsin dissociated
for cryopreservation in liquid nitrogen.
qRT-PCR
RNA was extracted from sorted MSCs and reverse
transcribed according to the manufacturer’s instructions
(Invitrogen, Carlsbad, CA, USA). PCR reactions without cDNA
template were used as the negative control. cDNA was amplified
using EZH2, JMJD3 and β-actin primers. PCR was performed using
thermal cycling parameters of 94°C for 25 sec, 55°C for 1 min and
72°C for 1 min (Stratagene, La Jolla, CA, USA) with SYBR-Green.
Western blotting
Sorted MSCs from MMTV-Wnt1 tumors were lysed in 1X
Laemmli buffer. Fifty micrograms of total cellular proteins was
separated by SDS-PAGE. Proteins were electroblotted to PVDF
membranes (Roche Applied Sciences, Indianapolis, IN, USA). Blots
were incubated with blocking solution followed by anti-histone
H3K27me3 and anti-β-actin antibodies for 16 h at 4°C. After washing
in Tris-buffered saline containing 0.1% Tween-20, blots were
incubated for 30 min at room temperature with an anti-IgG secondary
antibody conjugated to horseradish peroxidase. Bands were
visualized by the enhanced chemiluminescence method and quantitated
by densitometry. Data were analyzed by t-test.
Histopathology and
immunohistochemistry
Formalin-fixed tumor tissue was dehydrated in
ethanol, cleared in xylene and embedded in paraffin. Sections were
deparaffinized and stained with hematoxylin and eosin. For
immunohistochemical studies, the sections were rehydrated in PBS
(pH 7.4) and blocked with 10% normal serum. For
immunohistochemistry studies, the sections were incubated with PCNA
primary antibody overnight at room temperature. Following washing
in PBS, the sections were incubated with biotinylated secondary
antibody and streptavidin-conjugated horseradish peroxidase.
Antigen-antibody complexes were detected by incubation with
peroxide substrate solution containing aminoethylcarbazole
chromogen followed by hematoxylin counterstaining. The percentage
of PCNA cells in 10 random high power fields was determined by
counting. Data were analyzed by t-test.
Cell death analysis
Tumor tissue sections were incubated with terminal
deoxynucleotidyl transferase and dUTP-fluorescein for 1 h at 37°C
according to the manufacturer’s recommendations (Roche Applied
Sciences). After washing, apoptotic cells were visualized by
fluorescence microscopy following coverslipping with anti-fade
mounting medium containing DAPI. The percentage of fluorescent
cells in 10 random high power fields was determined by counting.
Data were analyzed by t-test.
Results
To determine the role of EZH2 in mammary cancer stem
cell function, we transduced MSCs from MMTV-Wnt1 mammary tumors
with EZH2, JMJD3 or control lentiviral shRNAs. JMJD3 is a histone
demethylase which counteracts EZH2 activity by removing methyl
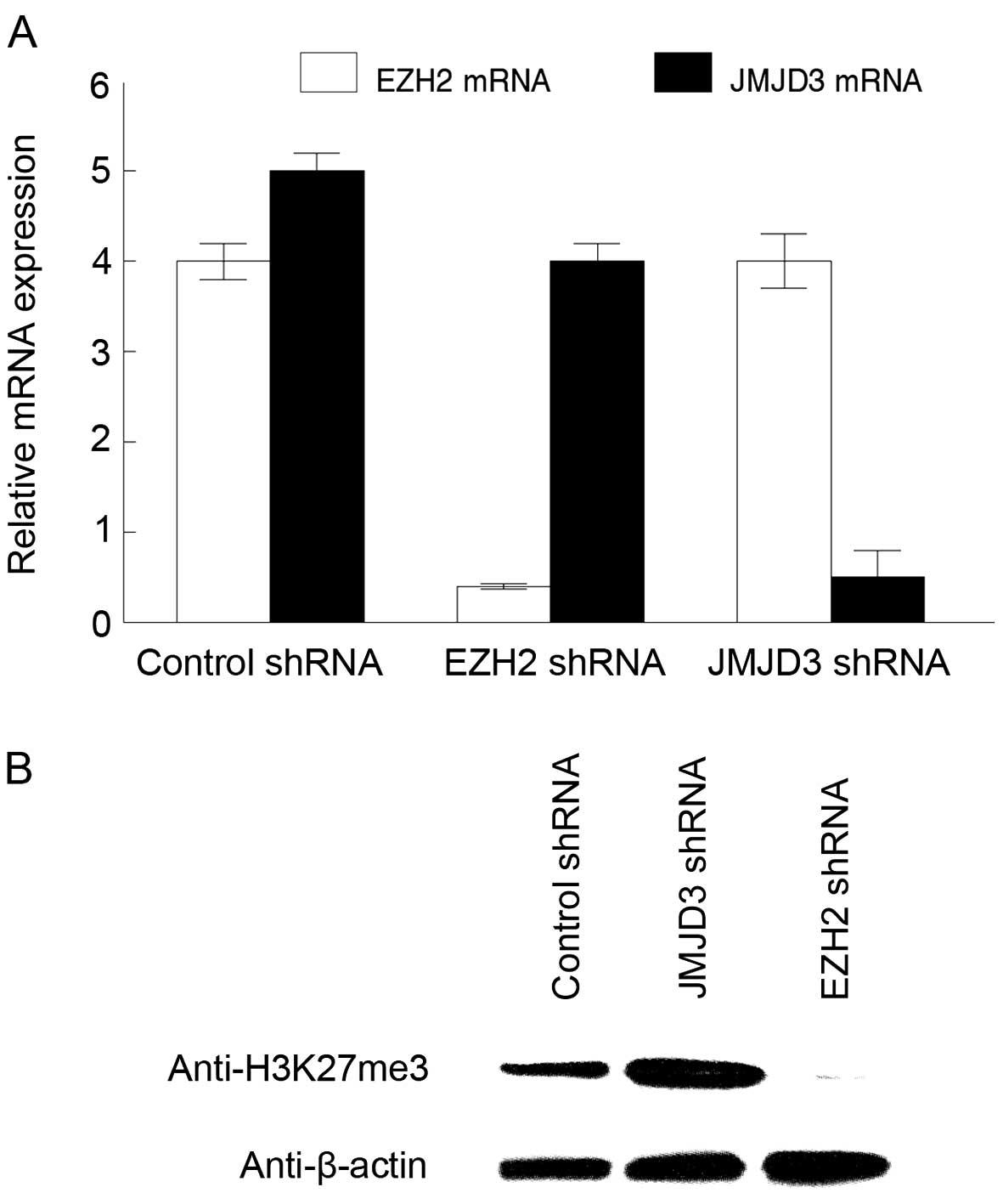
groups from lysine 27 of histone H3. EZH2 and JMJD3 shRNAs reduced
expression of these mRNAs in the tumorigenic MSCs by 80% compared
to the control shRNAs (Fig. 1A). We
examined trimethylation of lysine 27 in histone H3 in the
transduced tumorigenic MSCs by western blotting. JMJD3 inhibition
produced a 3-fold upregulation of H3K27me3 levels in the
tumorigenic MSCs, while EZH2 shRNAs resulted in >95% reduction
of H3K27me3 (Fig. 1B). We concluded
that EZH2 and JMJD3 regulate histone H3K27me3 levels in tumorigenic
MSCs.
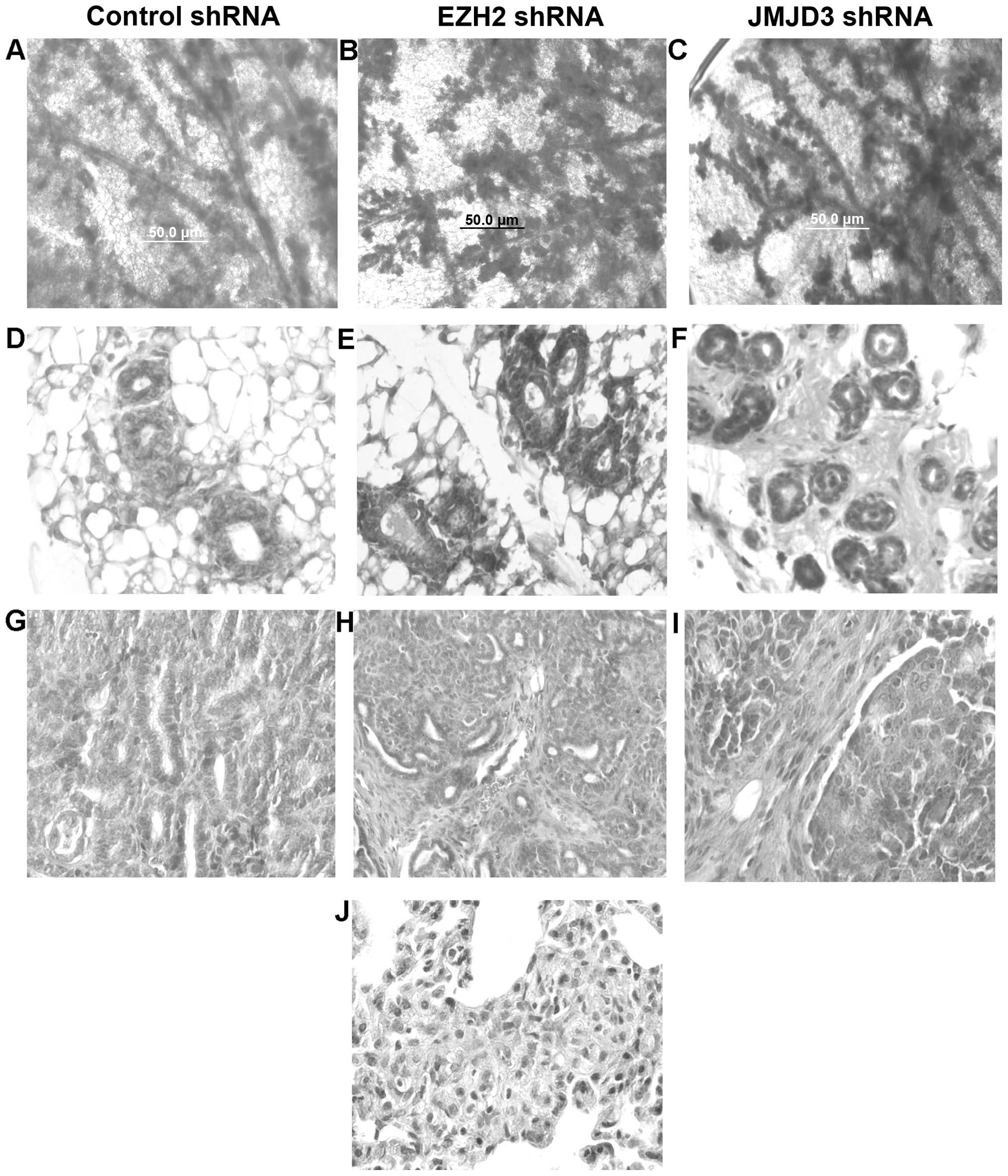
We transplanted tumorigenic MSCs transduced with
control, EZH2 or JMJD3 shRNAs to the cleared fat pads of
immunodeficient mice. We examined mammary gland reconstitution by
these MSCs using whole mount staining. As shown in Fig. 2A–C, transplanted mammary glands
exhibited hyperplastic branching characteristic of the MMTV-Wnt1
mice in all three transduced groups. There were no statistically
significant differences in the number of branches or terminal end
buds between the three groups. We also examined these reconstituted
mammary glands by histopathologic sectioning, and hematoxylin and
eosin (H&E) staining. Reconstituted mammary glands exhibited
ductal and basal cell hyperplasia characteristic of MMTV-Wnt1 mice
(Fig. 2D–F). Some glands exhibited
early signs of stromal cell hyperplasia and fibrosis. However, we
did not detect significant differences in the histopathology of
reconstituted mammary glands transduced with the control, EZH2 nor
JMJD3 shRNAs. Mammary glands transplanted with the tumorigenic MSCs
transduced with the control, EZH2 or JMJD3 shRNAs developed mammary
tumors. We did not detect significant differences in tumor latency
or growth rate between the three groups. Tumors from each group
were classified as poorly differentiated adenocarcinoma exhibiting
ductal and stromal hyperplasia (Fig.
2G–I). Metastatic tumors were not detected in cancers arising
from the MSCs transduced with the control or EZH2 shRNAs. However,
lung metastasis was detected in all immunodeficient mice bearing
tumors derived from the MSCs transduced with the JMJD3 shRNAs. Lung
metastases were classified as poorly differentiated adenocarcinoma
(Fig. 2J). We concluded that
increased EZH2 activity promotes lung metastasis in MMTV-Wnt1
mammary cancer.
To determine whether EZH2 activity alters
tumorigenic MSC and LP cell fractions in MMTV-Wnt1 mammary tumors,
we sorted these populations by fluorescence-activated cell sorting
(FACS) from cancers derived from the MSCs transduced with the
control, EZH2 or JMJD3 shRNAs. As shown in Fig. 3A, the MSC fraction in the mammary
tumors transduced with the EZH2 shRNAs was reduced by 2.5-fold
(P<0.01). Similarly the tumorigenic LP fraction in the tumors
transduced with the EZH2 shRNAs was reduced by 2-fold (P<0.03).
However, the ER+ luminal cell fraction increased from 42
to 61% (P<0.05). We also sorted the tumorigenic MSC and LP cell
fractions from tumors derived from the MSCs transduced with JMJD3
shRNAs. The MSC fraction in mammary tumors transduced with JMJD3
shRNAs increased from 25 to 56% (P<0.002), and the LP cell
fraction also was elevated by 2-fold (P<0.04). However the
ER+ luminal cell fraction was reduced from 42 to 28%
(P<0.02). We concluded that EZH2 activity promotes tumorigenic
MSC and LP cell expansion in mammary tumors yet represses
ER+ cellular differentiation.
To determine the mechanism of increased MSC and LP
cell fractions in these tumors, we examined proliferation and
programmed cell death in cancers from each group. The
PCNA+ cell fraction was reduced in the mammary tumors
derived from the MSCs transduced with EZH2 shRNAs compared to the
control transplants (20 vs. 41%; P<0.01; Fig. 3B, C and E). The PCNA+
cell fraction was significantly increased in the mammary tumors
derived from the MSCs transduced with the JMJD3 shRNAs compared to
the control transplants (69 vs. 41%; P<0.003; Fig. 3B, D and E). There were no
significant differences in the TUNEL+ cell fractions in
the mammary tumors derived from the MSCs transduced with the EZH2
or JMJD3 shRNAs compared to the control transplants (Fig. 3E). We concluded that EZH2 promotes
tumor cell proliferation in our model of basal mammary cancer.
Our results demonstrated that EZH2 promoted MSC and
LP expansion and inhibited ER+ cellular differentiation
(Fig. 3). To determine whether
ER+ cells inhibited MSC expansion as a possible
mechanism for these observations, we transplanted tumor-derived
ER+ or ER− luminal cells with the MSCs into
the cleared mammary fat pads of immunodeficient mice. Tumors
resulting from these transplants were sorted by FACS to determine
the MSC and LP cell fractions. As shown in Fig. 4, the mammary tumors derived from
transplants of the MSCs and ER+ cells exhibited
significantly reduced MSC fractions compared to those derived from
the MSCs transplanted with the ER− cells (13 vs. 24%;
P<0.002). Similarly, mammary tumors derived from transplants of
the MSCs and ER+ cells exhibited significantly reduced
LP fractions compared to those derived from the MSCs transplanted
with the ER− cells (0.4 vs. 2%; P<0.04). We conclude
that ER+ cells inhibit MSC and LP expansion in a model
of basal mammary cancer.
Discussion
EZH2 is associated with younger age at breast cancer
diagnosis (29), increased tumor
size, high histopathologic grade, negative hormone receptor status,
epidermal growth factor receptor and Her2 overexpression, p53
mutations, lymphatic invasion, poor survival and metastasis
(30–32). Our results demonstrated that EZH2
activity was associated with lung metastasis in a model of basal
breast cancer. Future studies will determine which of these genetic
changes are the direct result of EZH2-mediated regulation of target
genes.
Our results demonstrated that EZH2 results in
expansion of tumorigenic MSCs and LP cells. Previous studies
demonstrated that expansion of breast tumor-initiating cells
resulted from Raf1 or Notch gene expression in cancers
overexpressing EZH2 (33,34). The present study was the first to
determine that EZH2 also induced expansion of the tumorigenic LP
population in basal mammary tumors. Our results also demonstrated
that EZH2 suppresses ER+ cellular differentiation.
Previous retrospective studies using human breast cancer tissue
demonstrated that patients with high tumoral EZH2 expression had
the least clinical benefit from anti-estrogen therapy (35). We also demonstrated that EZH2
activity resulted in increased cell proliferation which was
observed in human breast cancers (36). A previous in vitro study
demonstrated that EZH2 inhibition resulted in decreased
proliferation of human breast cancer cell lines (37). The present study demonstrated a
novel mechanism by which tumor-derived ER+ cells repress
tumorigenic MSC and LP expansion.
EZH2 overexpression was shown to induce ductal
hyper-plasia in the mouse mammary gland (24). MMTV-Wnt1 mammary glands exhibit
ductal hyperplasia (13), and we
did not detect changes in glandular hyperplasia resulting from
transplantation of the Wnt1 tumor-derived MSCs transduced with the
EZH2 or JMJD3 shRNAs. Similarly, mammary tumors derived from these
transplants did not exhibit signifi-cant differences in
histopathologic appearance. These results are likely due to
Wnt1-induced hyperplasia which overrides the effects of EZH2.
In summary, EZH2 promotes tumorigenic MSC and LP
cell expansion and metastasis while suppressing ER+
cellular differentiation. Future studies will determine how EZH2
induces mammary tumor cell proliferation, expansion of the
tumorigenic LP cell population, and suppresses ER+
cellular differentiation.
Acknowledgments
This study was supported by the Department of
Defense Breast Cancer Research Program award W81XWH-10-1-0081.
References
|
1
|
American Cancer Society: Cancer Facts and
Figures 2013. American Cancer Society, Inc.; Atlanta: 2013
|
|
2
|
Mariotto AB, Yabroff KR, Shao Y, Feuer EJ
and Brown ML: Projections of the cost of cancer care in the United
States: 2010–2020. J Natl Cancer Inst. 103:117–128. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crowe DL, Parsa B and Sinha UK:
Relationships between stem cells and cancer stem cells. Histol
Histopathol. 19:505–509. 2004.PubMed/NCBI
|
|
4
|
Stingl J, Raouf A, Emerman JT and Eaves
CJ: Epithelial progenitors in the normal human mammary gland. J
Mammary Gland Biol Neoplasia. 10:49–59. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Visvader JE: Keeping abreast of the
mammary epithelial hierarchy and breast tumorigenesis. Genes Dev.
23:2563–2577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Visvader JE and Lindeman GJ: Mammary stem
cells and mammopoiesis. Cancer Res. 66:9798–9801. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Keymeulen A, Rocha AS, Ousset M, Beck
B, Bouvencourt G, Rock J, Sharma N, Dekoninck S and Blanpain C:
Distinct stem cells contribute to mammary gland development and
maintenance. Nature. 479:189–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shehata M, Teschendorff A, Sharp G, Novcic
N, Russell IA, Avril S, Prater M, Eirew P, Caldas C and Watson CJ:
Phenotypic and functional characterisation of the luminal cell
hierarchy of the mammary gland. Breast Cancer Res. 14:R1342012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shackleton M, Vaillant F, Simpson KJ,
Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ and
Visvader JE: Generation of a functional mammary gland from a single
stem cell. Nature. 439:84–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stingl J, Eirew P, Ricketson I, Shackleton
M, Vaillant F, Choi D, Li HI and Eaves CJ: Purification and unique
properties of mammary epithelial stem cells. Nature. 439:993–997.
2006.PubMed/NCBI
|
|
12
|
Li Y, Welm B, Podsypanina K, Huang S,
Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, et al:
Evidence that transgenes encoding components of the Wnt signaling
pathway preferentially induce mammary cancers from progenitor
cells. Proc Natl Acad Sci USA. 100:15853–15858. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu BY, McDermott SP, Khwaja SS and
Alexander CM: The transforming activity of Wnt effectors correlates
with their ability to induce the accumulation of mammary progenitor
cells. Proc Natl Acad Sci USA. 101:4158–4163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Charafe-Jauffret E, Ginestier C, Iovino F,
Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F,
Dutcher J, et al: Breast cancer cell lines contain functional
cancer stem cells with metastatic capacity and a distinct molecular
signature. Cancer Res. 69:1302–1313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han JS and Crowe DL: Tumor initiating
cancer stem cells from human breast cancer cell lines. Int J Oncol.
34:1449–1453. 2009.PubMed/NCBI
|
|
18
|
Liu H, Patel MR, Prescher JA, Patsialou A,
Qian D, Lin J, Wen S, Chang YF, Bachmann MH, Shimono Y, et al:
Cancer stem cells from human breast tumors are involved in
spontaneous metastases in orthotopic mouse models. Proc Natl Acad
Sci USA. 107:18115–18120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Woodward WA, Chen MS, Behbod F, Alfaro MP,
Buchholz TA and Rosen JM: WNT/beta-catenin mediates radiation
resistance of mouse mammary progenitor cells. Proc Natl Acad Sci
USA. 104:618–623. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pece S, Tosoni D, Confalonieri S, Mazzarol
G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG and Di Fiore
PP: Biological and molecular heterogeneity of breast cancers
correlates with their cancer stem cell content. Cell. 140:62–73.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vaillant F, Asselin-Labat ML, Shackleton
M, Forrest NC, Lindeman GJ and Visvader JE: The mammary progenitor
marker CD61/beta3 integrin identifies cancer stem cells in mouse
models of mammary tumorigenesis. Cancer Res. 68:7711–7717. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lim E, Vaillant F, Wu D, Forrest NC, Pal
B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, et al:
Aberrant luminal progenitors as the candidate target population for
basal tumor development in BRCA1 mutation carriers. Nat Med.
15:907–913. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bhan A, Hussain I, Ansari KI, Bobzean SA,
Perrotti LI and Mandal SS: Histone methyltransferase EZH2 is
transcriptionally induced by estradiol as well as estrogenic
endocrine disruptors bisphenol-A and diethylstilbestrol. J Mol
Biol. 426:3426–3441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Gonzalez ME, Toy K, Filzen T,
Merajver SD and Kleer CG: Targeted overexpression of EZH2 in the
mammary gland disrupts ductal morphogenesis and causes epithelial
hyperplasia. Am J Pathol. 175:1246–1254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding L, Erdmann C, Chinnaiyan AM, Merajver
SD and Kleer CG: Identification of EZH2 as a molecular marker for a
precancerous state in morphologically normal breast tissues. Cancer
Res. 66:4095–4099. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding L and Kleer CG: Enhancer of Zeste 2
as a marker of preneoplastic progression in the breast. Cancer Res.
66:9352–9355. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kleer CG, Cao Q, Varambally S, Shen R, Ota
I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, et al: EZH2
is a marker of aggressive breast cancer and promotes neoplastic
transformation of breast epithelial cells. Proc Natl Acad Sci USA.
100:11606–11611. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raaphorst FM, Meijer CJ, Fieret E,
Blokzijl T, Mommers E, Buerger H, Packeisen J, Sewalt RA, Otte AP
and van Diest PJ: Poorly differentiated breast carcinoma is
associated with increased expression of the human polycomb group
EZH2 gene. Neoplasia. 5:481–488. 2003. View Article : Google Scholar
|
|
29
|
Kunju LP, Cookingham C, Toy KA, Chen W,
Sabel MS and Kleer CG: EZH2 and ALDH-1 mark breast epithelium at
risk for breast cancer development. Mod Pathol. 24:786–793. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alford SH, Toy K, Merajver SD and Kleer
CG: Increased risk for distant metastasis in patients with familial
early-stage breast cancer and high EZH2 expression. Breast Cancer
Res Treat. 132:429–437. 2012. View Article : Google Scholar :
|
|
31
|
Hussein YR, Sood AK, Bandyopadhyay S,
Albashiti B, Semaan A, Nahleh Z, Roh J, Han HD, Lopez-Berestein G
and Ali-Fehmi R: Clinical and biological relevance of enhancer of
zeste homolog 2 in triple-negative breast cancer. Hum Pathol.
43:1638–1644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong M, Fan XJ, Chen ZH, Wang TT, Li X,
Chen J, Lin Q, Wen JY, Ma XK, Wei L, et al: Aberrant expression of
enhancer of zeste homologue 2, correlated with HIF-1α, refines
relapse risk and predicts poor outcome for breast cancer. Oncol
Rep. 32:1101–1107. 2014.PubMed/NCBI
|
|
33
|
Chang CJ, Yang JY, Xia W, Chen CT, Xie X,
Chao CH, Woodward WA, Hsu JM, Hortobagyi GN and Hung MC: EZH2
promotes expansion of breast tumor initiating cells through
activation of RAF1-β-catenin signaling. Cancer Cell. 19:86–100.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gonzalez ME, Moore HM, Li X, Toy KA, Huang
W, Sabel MS, Kidwell KM and Kleer CG: EZH2 expands breast stem
cells through activation of NOTCH1 signaling. Proc Natl Acad Sci
USA. 111:3098–3103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reijm EA, Jansen MP, Ruigrok-Ritstier K,
van Staveren IL, Look MP, van Gelder ME, Sieuwerts AM, Sleijfer S,
Foekens JA and Berns EM: Decreased expression of EZH2 is associated
with upregulation of ER and favorable outcome to tamoxifen in
advanced breast cancer. Breast Cancer Res Treat. 125:387–394. 2011.
View Article : Google Scholar
|
|
36
|
Collett K, Eide GE, Arnes J, Stefansson
IM, Eide J, Braaten A, Aas T, Otte AP and Akslen LA: Expression of
enhancer of zeste homologue 2 is significantly associated with
increased tumor cell proliferation and is a marker of aggressive
breast cancer. Clin Cancer Res. 12:1168–1174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gonzalez ME, Li X, Toy K, DuPrie M,
Ventura AC, Banerjee M, Ljungman M, Merajver SD and Kleer CG:
Downregulation of EZH2 decreases growth of estrogen
receptor-negative invasive breast carcinoma and requires BRCA1.
Oncogene. 28:843–853. 2009. View Article : Google Scholar :
|