Introduction
Prostate cancer (PCa), one of the most prevalent
malignancies in male patients worldwide, causes considerable
morbidity and mortality, and a great deal of attention is currently
given to its tumorigenesis. Androgen signaling based on the
androgen receptor is an essential oncogenic pathway for PCa
progression (1) and deprivation of
androgen is used widely as the basic therapeutic strategy for
patients with androgen-dependent PCa (2). Nevertheless, most patients have a
recurrence with a more aggressive form, known as
castration-resistant prostate cancer (CRPC) (3), with 80% of CRPC patients experiencing
the presence of bony metastases. Due to its frequent metastasis and
significant heterogeneity, CRPC is difficult to cure, and it has
become a vexing problem for the oncologists and clinicians
(4). Therefore, it is of vital
importance to identify appropriate agents to kill selectively or
sensitize prostate cancer.
Tetrandrine (TET) is a bisbenzylisoquinoline
alkaloid with the molecular structural formula
C38H42N2O6 and a
molecular weight of 622.74988 g/mol. Tetrandrine isolated from the
Chinese herbal medicine Stephaaniae has been applied to a very
broad spectrum of pharmacological events (5), such as antihypertension,
antiarrhythmia, and antirheumatism. In recent decades, tetrandrine
has been used as an antifibrotic agent in the treatment of
silicosis (6), and accumulating
evidence suggests that tetrandrine exerts strong anticancer effects
on diverse cancers in vitro, including colon (7,8),
hepatoma (9), bladder (10), and lung cancer (11). The beneficial impact of tetrandrine
on tumor cell multidrug resistance (12), radiosensitization (13), and angiogenesis (14) has also attracted a great deal of
attention. Also, tetrandrine has been shown to modulate multiple
cellular signaling events, including the Wnt/β signaling pathway
(15), mitogen-activated protein
kinase activation (16), and NF-κB
signaling pathway.
In our previous study, we found that tetrandrine
exhibited anticancer effects against PCa in vitro by
suppressing cell proliferation, inducing apoptosis and inhibiting
cell migration and invasion. Despite its potential as an anticancer
constituent, the underlying mechanism of tetrandrine on PCa
metastasis has not yet been elucidated. Therefore, we investigated
the possible mechanism of tetrandrine on the inhibitory effect of
metastasis in PCa DU145 and PC-3 cells.
Materials and methods
Cell culture
Human PCa cell lines DU145 and PC-3 were obtained
from the American Type Culture Collection (Manassas, VA, USA). The
cells were cultured in Dulbecco's modified Eagle's medium/1640
supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY,
USA) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA,
USA), in a humidified atmosphere with 5% CO2 at
37°C.
Reagents
Tetrandrine
(C38H42N2O6) and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Tetrandrine was diluted with 0.1 mol/l HCl at a concentration of 25
mg/ml−1, then added to the cell culture supernatant in
appropriate proportions. Antibodies against Akt, phospho-Akt, the
mammalian target of rapamycin (mTOR), matrix metalloproteinase-9
(MMP-9) and peroxidase-conjugated secondary antibodies were
obtained from Cell Signaling Technology, Inc. (Beverly, MA, USA).
LY294002 (Akt inhibitor) and rapamycin (mTOR inhibitor) were
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
The enhanced chemiluminescence (ECL) detection system was obtained
from Amersham Life Science, Inc. (Arlington Heights, IL, USA).
MTT assay
A modified MTT assay was used to assess cell
proliferation viability. Briefly, DU145 and PC-3 cells were seeded
in 96-well plates (8×103 cells/well, 90% density) and
incubated in the presence or absence of tetrandrine for various
periods of time. Then, 0.5 mg/ml MTT dye solution was added to each
well and incubated at 37°C for 4 h. After incubation, the culture
medium was discarded and the cells were lysed with dimethyl
sulfoxide to dissolve the formazan crystals. Absorbance at a
wavelength of 490 nm was detected using a 96-well microplate reader
(Bio-Rad, Hercules, CA, USA). The experiments were performed in
triplicate.
Transwell migration assay
Transwell migration assays were performed using PCa
DU145 and PC3 cells after treatment with tetrandrine. Cells (DU145:
6×104 or PC-3: 5×104 in 200 μl medium
of serum starvation, respectively) were seeded into the top
chamber, and 800 μl of medium supplemented with 10% fetal
calf serum were added to the lower chamber. After incubation at
37°C for various times, cells adhering to the top chambers were
removed with a cotton swab. The migratory cells on the lower
surface of the membrane were fixed with 4% paraformaldehyde and
stained with 0.1% crystal violet (Beyotime, Shanghai, China). Cells
that migrated to the lower surface were counted in five randomly
chosen visual fields under a microscope at ×100 magnification. The
data were obtained from three independent experiments.
Matrigel invasion assay
The Transwell chambers (polycarbonic membrane,
6.5-mm diameter, 8 μm pore size) were coated with 50
μl Matrigel (Matrigel:serum-free medium 1:5). After
incubation at 37°C for five hours, the cells (DU145:
10×104 or PC-3: 12×104 in 200 μl
medium of serum starvation, respectively) were treated according to
the protocol procedure, which was similar to the Transwell
migration assay.
Western blotting
The PCa cells were harvested 24 h after the
tetrandrine treatment, and the total cell lysates were denatured
with lysis buffer [10 mmol/l Tris-HCl (pH 7.4), 150 mmol/l NaCl,
0.1% sodium dodecyl sulfate (SDS), 1 mmol/l−1
ethylenediaminetetraacetic acid, 1 mmol/l ethylene glycol
tetraacetic acid, 0.3 mmol/l phenylmethylsulfonyl fluoride, 0.2
mmol/l sodium orthovanadate, 1% NP-40, 10 mg/ml leupeptin, and 10
mg/ml aprotinin]. Then clarified protein lysates (~30–60 μg
whole-cell lysates, mitochondrial and cytosolic fractions) were
resolved electrophoretically on denaturing SDS-polyacrylamide gel
(10%) and transferred to nitrocellulose membranes. Immunoblotting
was performed with the primary antibody, anti-MMP-9, as well as
anti-mTOR, and p-Akt overnight at 4°C. The membranes were washed
and incubated with peroxidase-conjugated secondary antibody at room
temperature (25°C). Ultimately, the proteins of interest were
visualized with ECL Substrate and exposed to X-ray film.
Gelatin zymography
Gelatin zymography was performed following the
standardized protocol (17).
Briefly, 5×105 cells were treated with tetrandrine for
24 h, after which the supernatants were collected to load onto 10%
polyacrylamide gels and co-polymerized with 0.1% gelatin (Sigma).
After undergoing electrophoresis, the gels were washed twice for 40
min in 2.5% Triton X-100 and 50 mmol/l Tris-HCl. Then, the gels
were incubated for the next 24 h at 37°C in buffer containing 50
mmol/l Tris-HCl, 5 mmol/l CaCl2 and 0.02%
NaN3, followed by staining with Coomassie brilliant
G-250 and destaining with 20% methanol and 10% acetic acid.
Finally, the gels were visualized with a molecular imager (ChemiDoc
XRS+, Bio-Rad).
Statistical analysis
SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) was
used for statistical analyses. Statistical differences between the
vehicle and drug treatment groups were compared by one-way analysis
of variance, and Dunnett's t-test was used for multiple
comparisons. Student's t-test (two-sided) was used for comparisons
involving only two groups. A value of P<0.05 was considered
statistically significant.
Results
Effects of tetrandrine on cell migration
and invasion in prostate cancer DU145 and PC-3 cells
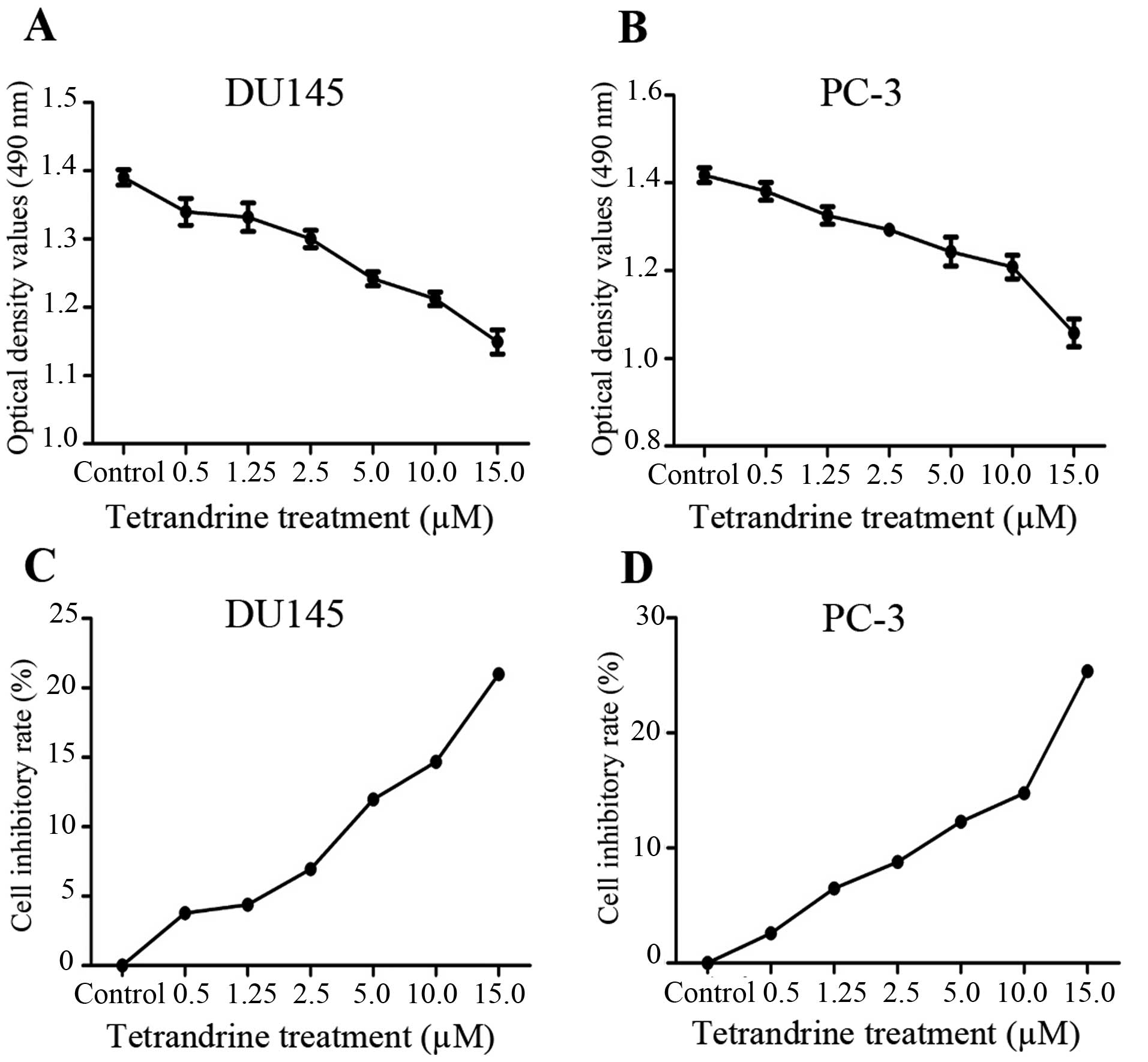
The MTT assays showed that cell proliferation was
significantly inhibited by tetrandrine at a concentration of ≥2.5
μM in a cell density >90% (Fig. 1). In view of these results,
tetrandrine at 2.5 μM (a <10% inhibitory rate) was chosen
as the representative dose in the subsequent in vitro
studies, to exclude the suppressing interference from PCa
proliferation by tetrandrine.
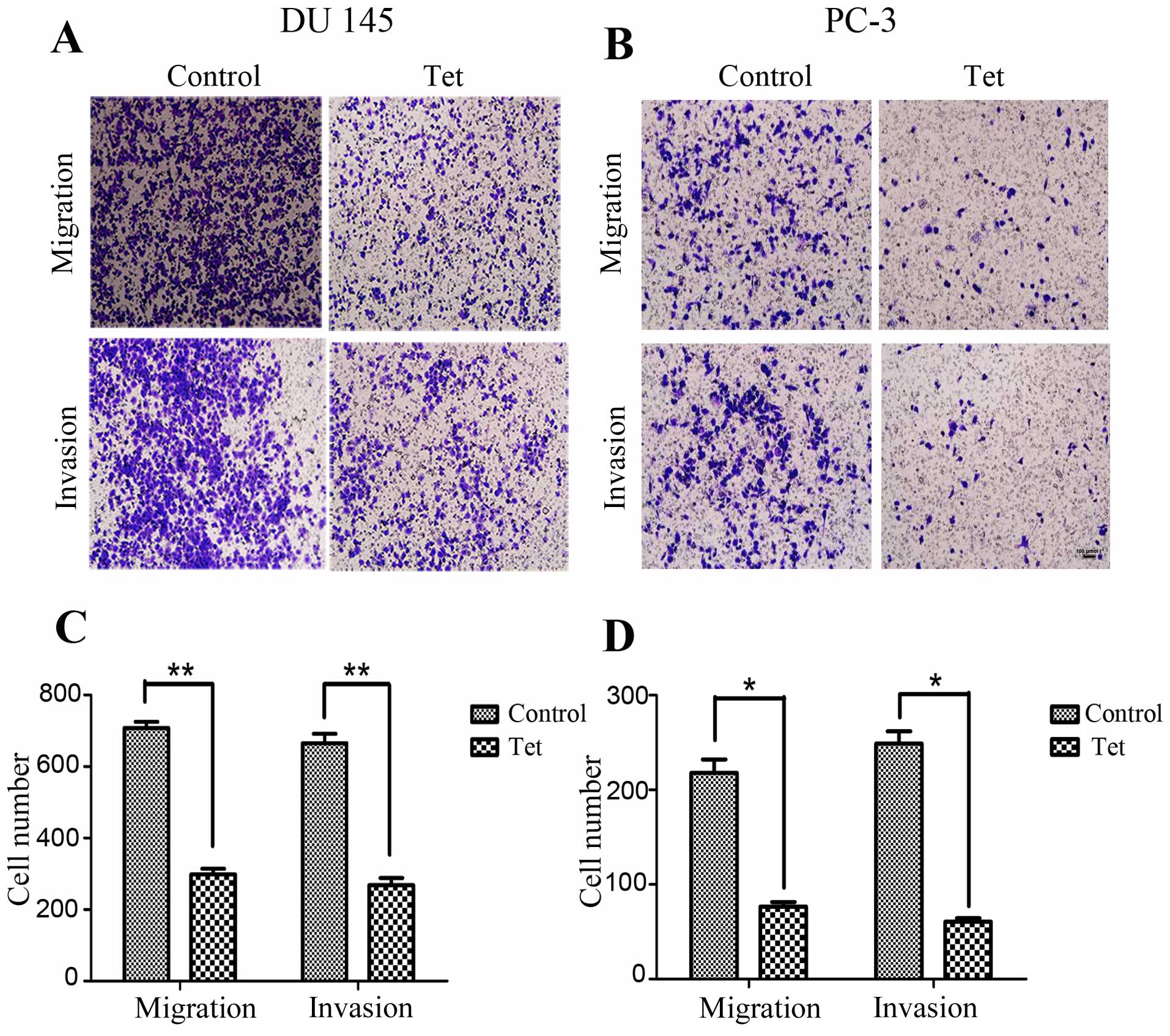
To determine whether tetrandrine regulates cellular
metastatic processes, we detected the effects of tetrandrine on PCa
cell migration. Using a Transwell migration assay, we found that
tetrandrine functioning in the DU145 cell line decreased cell
migration approximately 2.5-fold after 24 h (Fig. 2A and C). Similarly, treatment of the
PC-3 cells with tetrandrine resulted in a greater than 2.8-fold
decrease in migration compared to the control group (Fig. 2B and D).
Next, using a Matrigel invasion assay, we explored
whether tetrandrine could affect the invasiveness of PCa cells.
Serum-starved cells were added to the upper chambers of the
Transwell and the total number of cells invading through the
Matrigel barrier in response to a chemoattractant (serum) at
various times were counted. Tetrandrine significantly decreased the
number of invading cells by >2.4-fold and 4-fold in the DU145
and PC-3 cells, respectively (Fig.
2).
These results suggest that tetrandrine might play a
vital role in inhibiting the migration and invasion potential of
human PCa cells, as indicated by the observation in the Transwell
migration and Matrigel invasion assays.
Identification of the signaling pathway
regulated by tetrandrine in prostate cancer
To define the underlying mechanism of
tetrandrine-mediated inhibition of metastatic processes in PCa, we
investigated the expression of several key factors in cancer cell
migration and invasion, including E-cadherin, N-cadherin, vimentin,
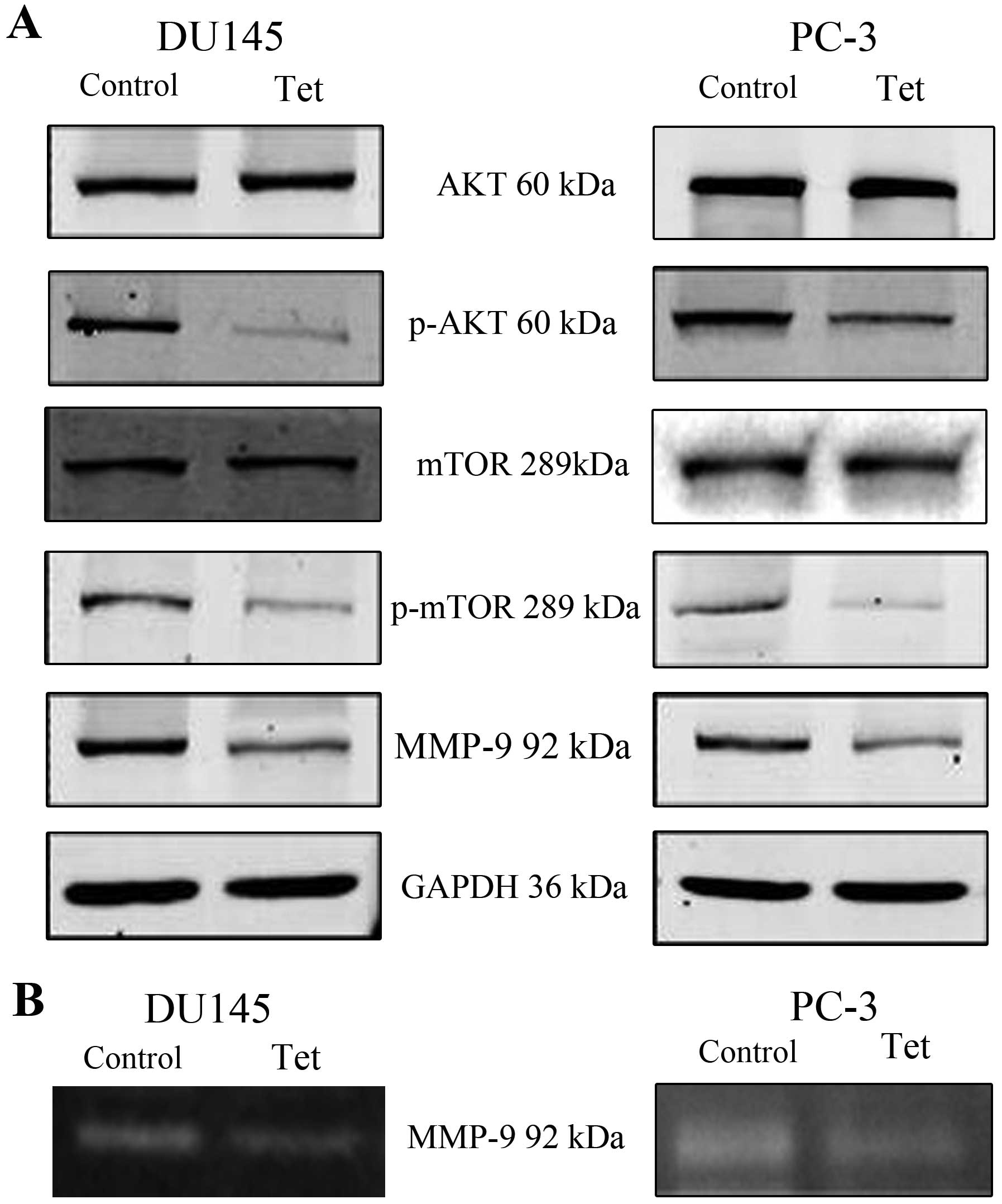
and MMP-9. To determine the impact of tetrandrine on the production
of proteinases by DU145 and PC-3, protein lysates were collected
and subjected to SDS-polyacrylamide gel electrophoresis. As shown
in Fig. 3, the presence of
proteinases led to a clear band at 92 kDa in the nitrocellulose
membranes, which was assigned to MMP-9, and tetrandrine reduced
MMP-9 protein levels (Fig. 3A) and
decreased MMP-9 activity (Fig. 3B),
which corresponded to the inhibition of invasiveness in the DU145
and PC-3 cells. However, the protein levels of E-cadherin,
N-cadherin and vimentin in the tetrandrine treatment remained the
same (data not shown).
To detect the upstream of MMP-9, we found that the
pretreatment with tetrandrine resulted in a marked decrease in the
protein levels of phosphorylated Akt (p-Akt) and phosphorylated
mTOR (p-mTOR) in both the DU145 and PC-3 cells (Fig. 3). To gain further insights into the
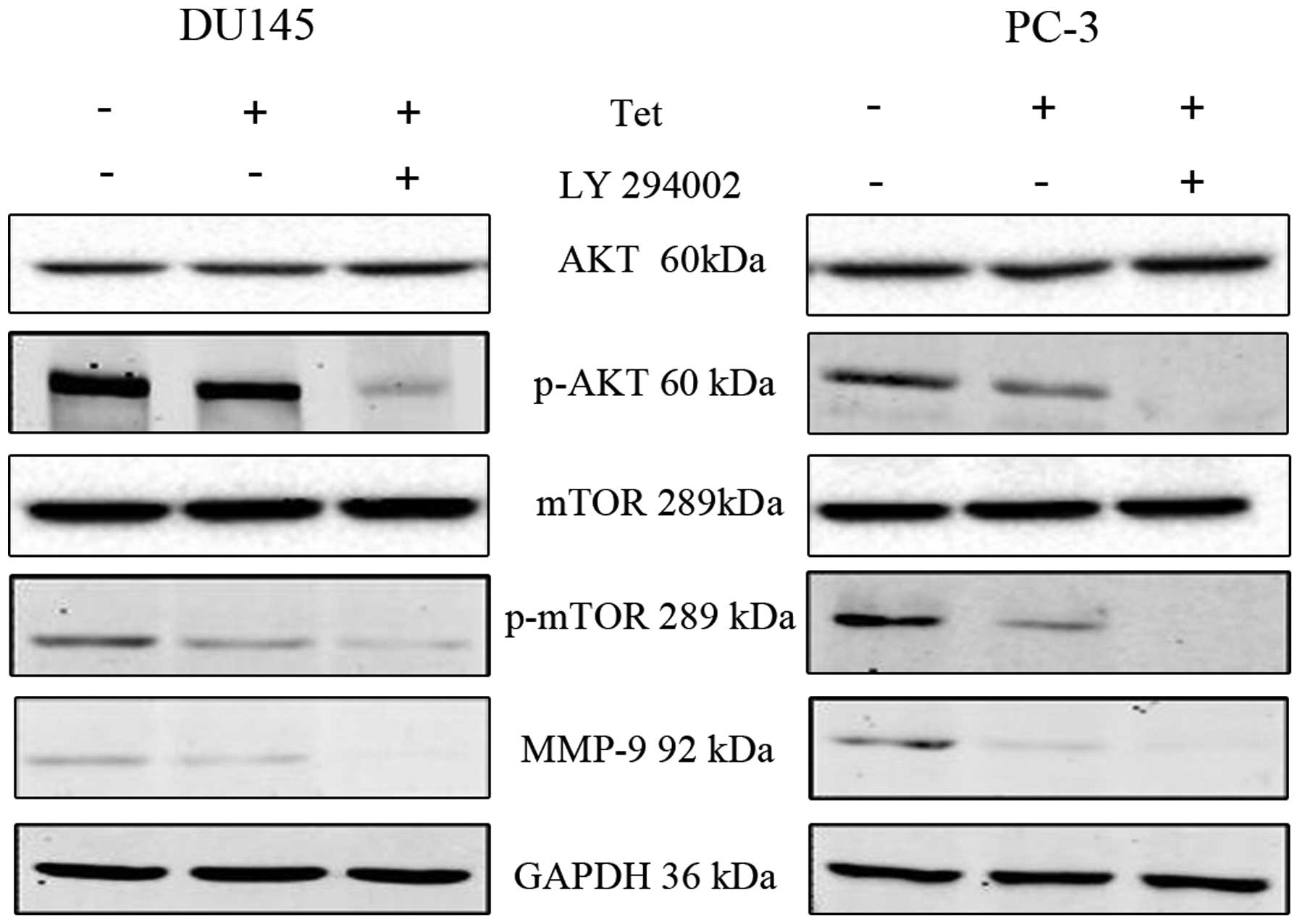
molecular mechanisms of tetrandrine in the PCa metastasis process,
LY294002 (Akt inhibitor) or rapamycin (mTOR inhibitor) was selected
to combine with tetrandrine for functional and mechanism research.
The data showed that pretreatment with LY294002 decreased the
tetrandrine-inhibited migration and invasion in the DU145 and PC-3
cells (Fig. 4), and reduced the
p-mTOR and MMP-9 protein levels (Fig.
5) much more significantly compared with the tetrandrine alone
group. This result suggests that Akt protein was passably related
to the antimetastasis effect of tetrandrine.
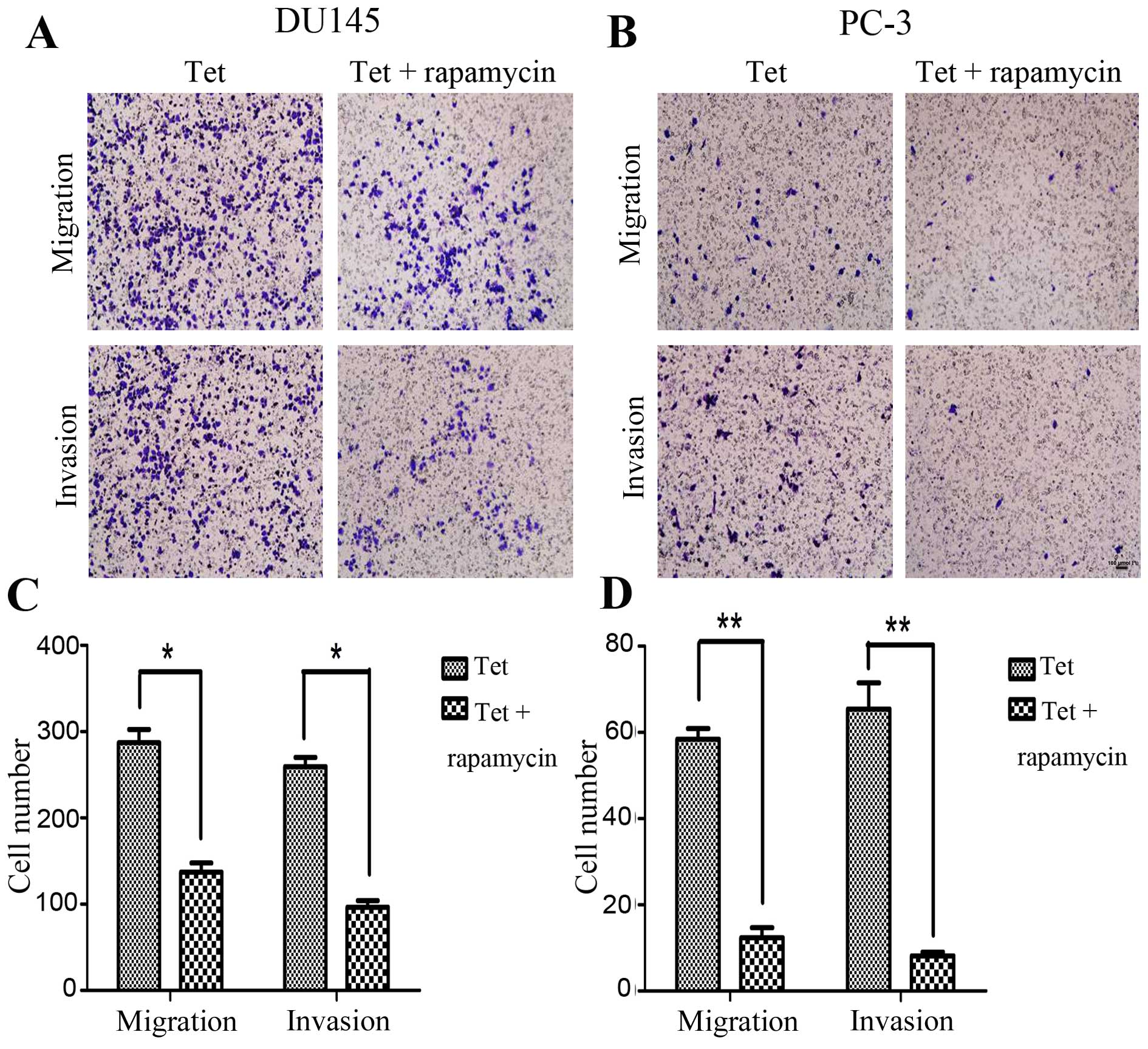
Given that Akt protein might contribute to the
anticancer activity of tetrandrine, the downstream of Akt was
evaluated to define further the molecular basis of
tetrandrine-inhibited metastasis. Similar results were observed in
a combination of tetrandrine and rapamycin. The combined effect of
the two agents was found to suppress prostate cancer cell migration
and invasion (Fig. 6), and diminish
of MMP-9 protein levels more effectively (Fig. 7); there was no significant change in
NF-κB protein level (data not shown).
Taken together, our findings show that the
Akt/mTOR/MMP-9 signaling pathway was partially associated with
tetrandrine-inhibited metastasis in CRPC cells, suggesting that
tetrandrine might be a potential promising agents in the
suppression of metastatic phenotypes in PCa treatment.
Discussion
In recent decades, the incidence of PCa has
increased sharply in Asian countries (18). Until now, the majority of clinical
trials have achieved limited benefits in the treatment of advanced
PCa, mainly because of the high incidence of invasion and
metastasis. Metastasis, a symbol of malignancy, is the migration of
cancer cells from the original tumor site to distant organs through
the bloodstream or lymph system. Metastasis development is a
multi-step process that is implicated in such activities as local
invasion, transfer, extravasation, and tumor deposit (19). Shedding light on the mechanisms that
facilitate tumor cell migration and invasion is of major concern in
cancer research, as approximately 90% of deaths from solid tumors
arise from metastasis. The median survival rate for patients with
metastatic PCa is far less than five years while the opposite is
true for men with localized disease (19). Based on these factors, it is known
that the tendency to invade and transfer is one of the obstacles
facing PCa treatments. Hence, there is an urgent need to develop
new agents for PCa therapy.
Tetrandrine, a traditional Chinese medicine, has
been shown to exhibit a wide range of uses. Accumulating evidence
indicates that tetrandrine exerts antitumor effects against various
cancer cells in vitro by inducing cell cycle arrest and
inhibiting angiogenesis. In our previous study, we found that the
induction of PCa cell apoptosis by tetrandrine might be mediated
partially by the activation of caspase cascade. Nevertheless, it is
conceivable that the anticancer activity of tetrandrine might be
involved with many other signaling pathways. It has been reported
that tetrandrine induces apoptosis in hepatocellular carcinoma
cells by generating reactive oxygen species, followed by the
repression of Akt activity (20).
Tetrandrine also exhibits anti-proliferation effects by targeting
β-catenin activity to a certain extent. In addition, it has been
reported that tetrandrine inhibited in vivo tumor metastasis
in a mouse model of stage IV breast cancer. The inhibitory effect
of tetrandrine on breast cancer metastasis might be mediated partly
by regulating endothelial cell-specific molecule-1 (ESM-1),
integrin β5 protein and intercellular cell adhesion molecule-1
(ICAM-1) levels (21).
Metastasis in patients with PCa is one of the main
lethal factors blocking direct treatment. In our studies, the
impact of tetrandrine resulted in decreased cell migration and
invasion in the metastatic CRPC cell lines DU145 and PC-3.
Identifying the molecular mechanisms of tetrandrine on the
metastatic process underlying PCa is crucial in order to clarify
our understanding of tumorigenesis and metastasis. As such, we have
detected the expression of several key factors in the cell
metastatic process and found that tetrandrine might decrease MMP-9
protein levels and activity in two independent CRPC cell lines;
however, we found no significant changes in MMP-2 protein levels.
These findings indicate that MMP-9 deregulation could be a vital
event in PCa progression and are consistent with our data that
tetrandrine expression resulted in decreased MMP-9 levels and a
decline in metastatic traits.
In addition to speculating that MMP-9 might be
associated with anti-metastasis activity displayed by tetrandrine,
we also measured the Akt signaling pathway, which is upstream of
MMP-9. The results showed that p-Akt and p-mTOR protein levels
decreased significantly with tetrandrine treatment. Next, to
determine whether the ability of tetrandrine to inhibit PCa
metastasis was mediated through a decrease in p-Akt and p-mTOR
kinase activity, we treated DU145 and PC-3 cells with tetrandrine
in combination with LY294002 and rapamycin, respectively, and then
documented cell migration and invasion using Transwell assay and
western blotting. Our data in the present study demonstrated that
the inhibition of metastasis in DU145 and PC3 cells by tetrandrine
was markedly enhanced in combination with LY294002 or rapamycin
pre-treatment.
Furthermore, tetrandrine and LY294002
synergistically decreased p-mTOR and MMP-9 protein levels, and
rapamycin worked in the same manner. However, whether the decrease
in p-mTOR protein level is directly related to downregulation of
MMP-9 protein needs to be investigated further. These data suggest
that, at least partially, the Akt/mTOR/MMP-9 signaling pathway is
involved in the tetrandrine-mediated metastatic inhibition of PCa.
However, several limitations exist in our study. On the one hand,
the lack of animal models in our studies is a short coming, and
further considerations should be taken about tetrandrine's function
on animal models in our future study. On the other hand, we only
observe the anti-metastatic effect of tetrandrine on prostate
cancer cells by negatively regulating Akt/mTOR/MMP-9 signaling
pathway. Whether alternative signaling pathways participated in
this effect of tetrandrine has not yet been elucidated. Hence, it
is of necessity for us to resolve these questions in further
research.
While our studies have shown that tetrandrine is
valid as a single agent for tumor therapy, it might be rational to
speculate that it will be used widely in combination with other
agents in the clinical setting. Our findings demonstrated that
tetrandrine decreased not only p-Akt and p-mTOR activity, but also
showed good synergy with LY294002 or rapamycin in the inhibition of
PCa cells. Thus, our studies suggest that tetrandrine might be a
stronger chemotherapeutic agent when combined with LY294002 or
rapamycin.
To the best of our knowledge, our studies provide
the first evidence that tetrandrine inhibits PCa cells metastasis
by repressing Akt/mTOR and inactivating MMP-9 and that Akt/mTOR
appear to be the upstream regulators of MMP-9 inactivation. In
conclusion, it is suggested that tetrandrine suppresses metastasis
by negatively regulating the Akt/mTOR/MMP-9 signaling pathway, and
that it could serve as a potential inhibitor of tumor metastasis in
future therapeutic interventions in PCa patients.
Acknowledgments
This study was partially supported by the Science
and Technology Research Projects of Shaanxi
(2014K-11-03-01-03).
References
|
1
|
Goto Y, Kojima S, Nishikawa R, Enokida H,
Chiyomaru T, Kinoshita T, Nakagawa M, Naya Y, Ichikawa T and Seki
N: The microRNA-23b/27b/24-1 cluster is a disease progression
marker and tumor suppressor in prostate cancer. Oncotarget.
5:7748–7759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chaudhary P and Vishwanatha JK: c-Jun
NH2-terminal kinase-induced proteasomal degradation of c-FLIPL/S
and Bcl2 sensitize prostate cancer cells to Fas- and
mitochondria-mediated apoptosis by tetrandrine. Biochem Pharmacol.
91:457–473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lamont KR and Tindall DJ: Minireview:
Alternative activation pathways for the androgen receptor in
prostate cancer. Mol Endocrinol. 25:897–907. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shamash J, Dancey G, Barlow C, Wilson P,
Ansell W and Oliver RT: Chlorambucil and lomustine (CL56) in
absolute hormone refractory prostate cancer: Re-induction of
endocrine sensitivity an unexpected finding. Br J Cancer. 92:36–40.
2005. View Article : Google Scholar
|
|
5
|
Chen YJ: Potential role of tetrandrine in
cancer therapy. Acta Pharmacol Sin. 23:1102–1106. 2002.PubMed/NCBI
|
|
6
|
Pang L and Hoult JR: Cytotoxicity to
macrophages of tetrandrine, an antisilicosis alkaloid, accompanied
by an overproduction of prostaglandins. Biochem Pharmacol.
53:773–782. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu JM, Chen Y, Chen JC, Lin TY and Tseng
SH: Tetrandrine induces apoptosis and growth suppression of colon
cancer cells in mice. Cancer Lett. 287:187–195. 2010. View Article : Google Scholar
|
|
8
|
Wu K, Zhou M, Wu QX, Yuan SX, Wang DX, Jin
JL, Huang J, Yang JQ, Sun WJ, Wan LH, et al: The role of IGFBP-5 in
mediating the anti-proliferation effect of tetrandrine in human
colon cancer cells. Int J Oncol. 46:1205–1213. 2015.
|
|
9
|
Ng LT, Chiang LC, Lin YT and Lin CC:
Antiproliferative and apoptotic effects of tetrandrine on different
human hepatoma cell lines. Am J Chin Med. 34:125–135. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Su B, Liu R, Wu D and He D:
Tetrandrine induces apoptosis and triggers caspase cascade in human
bladder cancer cells. J Surg Res. 166:e45–e51. 2011. View Article : Google Scholar
|
|
11
|
Lee JH, Kang GH, Kim KC, Kim KM, Park DI,
Choi BT, Kang HS, Lee YT and Choi YH: Tetrandrine-induced cell
cycle arrest and apoptosis in A549 human lung carcinoma cells. Int
J Oncol. 21:1239–1244. 2002.PubMed/NCBI
|
|
12
|
Sun YF and Wink M: Tetrandrine and
fangchinoline, bisbenzylisoquinoline alkaloids from Stephania
tetrandra can reverse multidrug resistance by inhibiting
P-glycoprotein activity in multidrug resistant human cancer cells.
Phytomedicine. 21:1110–1119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang TH, Wan JY, Gong X, Li HZ and Cheng
Y: Tetrandrine enhances cytotoxicity of cisplatin in human
drug-resistant esophageal squamous carcinoma cells by inhibition of
multidrug resistance-associated protein 1. Oncol Rep. 28:1681–1686.
2012.PubMed/NCBI
|
|
14
|
Xiao W, Jiang Y, Men Q, Yuan L, Huang Z,
Liu T, Li W and Liu X: Tetrandrine induces G1/S cell cycle arrest
through the ROS/Akt pathway in EOMA cells and inhibits angiogenesis
in vivo. Int J Oncol. 46:360–368. 2015.
|
|
15
|
He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim
SH, Huang E, Gao Y, Yang K, Wagner ER, et al: Tetrandrine inhibits
Wnt/β-catenin signaling and suppresses tumor growth of human
colorectal cancer. Mol Pharmacol. 79:211–219. 2011. View Article : Google Scholar :
|
|
16
|
Qin R, Shen H, Cao Y, Fang Y, Li H, Chen Q
and Xu W: Tetrandrine induces mitochondria-mediated apoptosis in
human gastric cancer BGC-823 cells. PLoS One. 8:e764862013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Toth M, Sohail A and Fridman R: Assessment
of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol
Biol. 878:121–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sim HG and Cheng CW: Changing demography
of prostate cancer in Asia. Eur J Cancer. 41:834–845. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu C, Gong K, Mao X and Li W: Tetrandrine
induces apoptosis by activating reactive oxygen species and
repressing Akt activity in human hepatocellular carcinoma. Int J
Cancer. 129:1519–1531. 2011. View Article : Google Scholar
|
|
21
|
Gao JL, Ji X, He TC, Zhang Q, He K, Zhao
Y, Chen SH and Lv GY: Tetrandrine suppresses cancer angiogenesis
and metastasis in 4T1 tumor bearing mice. Evid Based Complement
Alternat Med. 2013:2650612013. View Article : Google Scholar : PubMed/NCBI
|