Introduction
The notion that aging and the quality of longevity
of living organisms including humans may be improved can be found
in century-old historical records (1). The topic is of interest to social and
public health experts as well as basic and clinical scientists. On
the one hand, life expectancy of humans has clearly benefitted from
modern day medical advances that have eradicated several diseases
that at one time plagued humankind (2,3); on
the other hand, it is known that two thirds of people die daily
from age-related causes pointing to aging as the single most
significant risk factor for many human diseases (4).
Do interventions and dietary modalities exist that
can delay the onset of aging, or counteract the deleterious effects
of environmental insults impinging on the integrity of our genome,
widely considered as a major risk factor for disease-associated
aging? It is our hypothesis that disease-associated, subclinical
aging (in relative terms) is a multistage biological process whose
duration and manifestation can be dynamically regulated by
environmental and dietary, as well as genetic factors. As a
corollary, therefore, age-related diseases can be managed in humans
using agents that attenuate cellular responses to external agents
capable of damaging the integrity of the DNA in the genome.
Accumulation of DNA damage is regarded as a cause
for aging, tumorigenesis and other inheritable diseases (5). Exposure of cells to ultraviolet (UV)
radiation results in the generation of DNA damage and lesions,
which, if left unrepaired, can directly or indirectly lead to
dysfunctional cellular events and possibly disease-associated
aging. Multiple changes occur to counteract UV-induced DNA damage,
including the upregulation and activation of transcription factor
p53. p53 is known to play an essential role in controlling various
downstream target genes, frequently as different sets by a
stimuli-specific [ionizing radiation, UV or reactive oxygen species
(ROS)] mechanism (6,7). Thus, UV-induced p53 mediates cell
cycle arrest and DNA repair and changes the expression of ataxia
telangiectasia mutated (ATM) protein kinase and γH2AX (H2AX
phosphorylated on Ser139) which then can be used as indicators to
monitor the ongoing DNA damage induced externally by exposure to UV
or by endogenously generated reactive oxygen species (ROS)
(8–11).
In this study, we used human A549 cells to test and
validate the ability of metformin and resveratrol, alone and in
combination, to confer protection against exposure to UVC, known to
contribute to aging by damaging genomic DNA. Metformin, with
demonstrated efficacy to restore insulin sensitivity in type II
diabetes (12,13), was selected for its activity in
managing age-related diseases including cardiovascular disorders
and cancer (14–19) by targeting the AMP-dependent protein
kinase (AMPK) (20,21), and extension of lifespan (22). The choice of resveratrol
(trans-3,42′,5-trihydroxystilbene), found abundantly in
grapes (23,24), was based on its plethora of
biological activities (14–19), and documented antioxidant,
anti-inflammatory (25–28) and anti-diabetic activities (29), as well as its ability to modulate
and activate SIRT1, a key protein for the aging process (30–33),
and prolongation of life span in mammals and other species
(33–35). Results of our studies support the
effectiveness of metformin, alone or combined with resveratrol, in
reducing the risk of aging by conferring protection against
UV-induced DNA damage.
Materials and methods
Reagents
Fetal calf serum, Eagle's minimum essential medium,
penicillin and streptomycin were purchased from Cellgro, Inc.
(Herndon, VA, USA). Metformin (1,1-dimeth-ylbiguanide chloride) and
resveratrol were obtained from Calbiochem (La Jolla, CA, USA) and
LKT Laboratories (St. Paul, MN, USA), respectively. All other
chemicals and solvents used were of analytical grade. Primary
antibodies: p53, cyclin B1, cyclin E, cdk1, cdk2, Rb, p53R2,
cdc25C, actin and secondary antibodies were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Other antibodies
for the present study were obtained from the following sources:
serine-139-phosphorylated histone H2AX (Upstate Biotechnology,
Inc., Lake Placid, NY, USA); p21 (Cell Signaling Technology, Inc.,
Beverly, MA, USA); plk1 (Invitrogen Corp., Carlsbad, CA, USA), and
p-chk2 (Cell Signaling Technology, Inc.). All other chemicals and
solvents used were of analytical grade.
Cell culture
The lung carcinoma cell line A549 was purchased from
the American Type Culture Collection (ATCC; Rockville, MD, USA).
Cells were maintained in Eagle's minimum essential medium
supplemented with 2 mM glutamine and Earle's BSS adjusted to
contain 1.5 g/l sodium bicarbonate, 0.1 mM non-essential amino
acids and 1 mM sodium pyruvate and supplemented with 0.01 mg/ml
bovine insulin and 10% fetal bovine serum. Cells were seeded at a
density of 5×104 cells/ml and passaged by washing the
monolayers with phosphate-buffered saline (PBS) followed by a brief
incubation with 0.25% trypsin/EDTA.
Preparation of chemicals and
treatment
Metformin and resveratrol were dissolved in dimethyl
sulfoxide (DMSO) and stored at −80°C as 500 and 50 mM stock,
respectively. Treatments included: 0, 2.5 or 25 µM of
resveratrol or 5 mM metformin alone or in combination (5 mM
metformin + 2.5 µM resveratrol or 5 mM metformin + 25
µM resveratrol). For UV irradiation experiments, the cells
were first primed with metformin or resveratrol for 48 h and washed
with PBS to remove the chemicals. The primed cells were exposed to
20 J/m2 UVC for 10 sec, after which the UVC-exposed
cells were maintained in culture for 4 h, and harvested for further
analysis.
Preparation of cell extracts and western
blot analysis
To determine the level of protein expression of
various genes examined in the present study, control and treated
cells were harvested and lysed in ice-cold RIPA buffer [50 mM Tris,
pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% deoxycholate,
0.1% SDS, 1 mM dithiothreitol and 10 µl/ml protease
inhibitor cocktail from Sigma Chemicals (St. Louis, MO, USA)]. The
protein concentration of the cell lysates was determined using the
Coomassie protein assay kit (Pierce, Rockford, IL, USA) with BSA as
the standard. The proteins in cell lysates were separated by 10%
SDS-PAGE and transferred to a nitrocellulose membrane as previously
described (36). The blots were
incubated overnight with various primary antibodies, followed by
incubation for 1 h with secondary antibodies. The blots were
detected with an ECL detection system (LumiGLO Peroxidase
Chemiluminescent Substrate kit; KPL Biotechnology, Inc.,
Gaithersburg, MD, USA), quantified by densitometry and normalized
against actin as the loading control as previously described
(37).
Cell cycle analysis
Cell cycle phase distribution was assayed by flow
cytometry as previously described (38–40);
the histograms obtained were quantified for the percentage of cells
in the respective phases (G1, S and G2/M) of
the cell cycle.
Results
Effects of resveratrol and metformin on
DNA damage response under normal and UVC-induced conditions
DNA damage is an important factor contributing to
carcinogenesis and the aging process. Resveratrol (18,19,23,41,42)
and metformin (14,43) have each been reported to have
beneficial effects against cancer cells, e.g., by suppressing
proliferation and induction of apoptosis (44–47),
and aging, e.g., prolonging life span in model systems (11,33,48–51).
However, the effects of these two chemicals alone or in combination
on p53 expression in the context of UV-induced DNA damage have not
been investigated. Accordingly we monitored changes in the level of
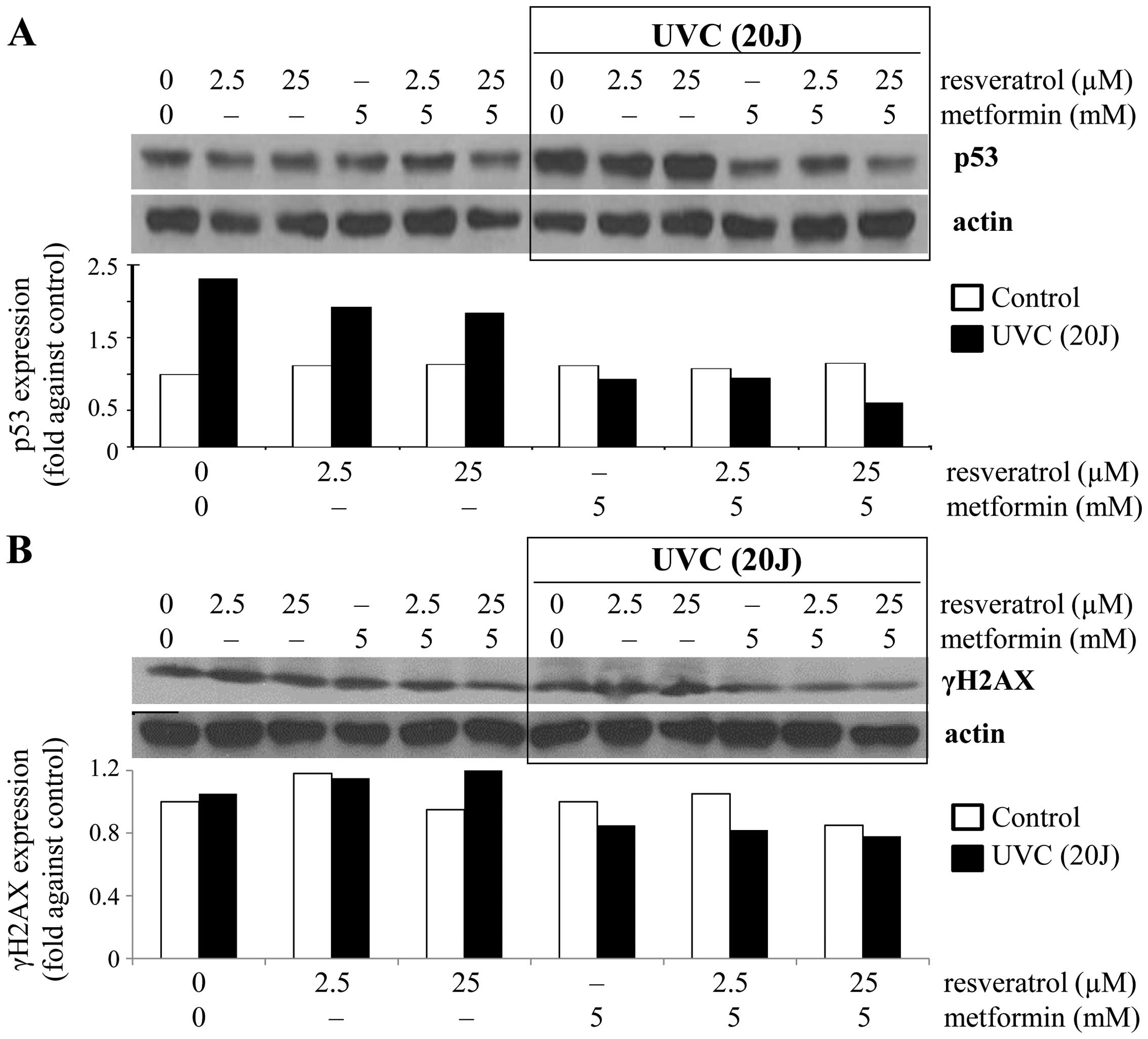
total p53. A pronounced increase (~2.3-fold) in p53 expression was
observed in the UVC-induced control cells compared to the
non-exposed control cells (Fig.
1A), suggesting that exposure to UVC resulted in the induction
of total p53. Correspondingly, no significant change in p53
expression was observed in control cells treated with either
resveratrol (2.5 and 25 µM) or metformin alone (5 mM) or in
combination (2.5 or 25 µM resveratrol combined with 5 mM
metformin) (Fig. 1A). In contrast,
under the UVC exposed condition, treatments resulted in a decrease
in p53 expression of 17–21% by resveratrol, ~60% by metformin and
59–74% by the combined treatment (Fig.
1A). These results are consistent with the interpretation that
metformin alone or in combination with resveratrol can prevent
UVC-induced p53 activation. Next, we tested whether resveratrol and
metformin may induce DNA damage by affecting the integrity of
genomic DNA by analyzing changes in the DNA damage marker γH2AX. In
non-stressed cells, the combination of 5 mM metformin and 25
µM resveratrol resulted in ~15% decrease in γH2AX expression
(Fig. 1B). Under the UVC-induced
condition, a slight increase in γH2AX expression was observed in
cells treated with 2.5 or 25 µM resveratrol (Fig. 1B). Surprisingly, metformin alone or
in combination with resveratrol inhibited UVC-induced γH2AX
expression (Fig. 1B). Thus, data on
prevention of DNA damage by metformin and/or resveratrol resulting
from the exposure to UVC as assayed by γH2AX expression generally
agreed with measurements of p53 changes.
Changes in cell cycle phase transition
and expression of specific signaling proteins impinging on cell
cycle control by resveratrol and metformin under UVC-induced
conditions
Alteration in p53 expression could induce an arrest
in cell cycle progression. Since minimum effects on p53 resulted
from treatment by resveratrol or metformin under non-UVC-induced
conditions, we next focused only on cells exposed to UVC. We first
determined the effects of resveratrol and metformin on cell cycle
progression by flow cytometry. Metformin alone and in combination
with a low dose of resveratrol caused a significant decrease in the
S phase cell population (13.7% in control vs. 8.8 and 7.3% in cells
treated with 5 mM metformin alone and combined with 2.5 µM
resveratrol, respectively). This decrease was accompanied by a
concomitant accumulation in the G1 phase cell population
(59.1% in control vs. 69.2 and 70.9% in 5 mM metformin without and
with addition of 2.5 µM resveratrol) (Table I). To gain additional information on
the underlying causes for the observed cell cycle phase transition
change, we measured levels of cell cycle regulatory proteins cyclin
E/cdk2 specifically required for G1 and S phase
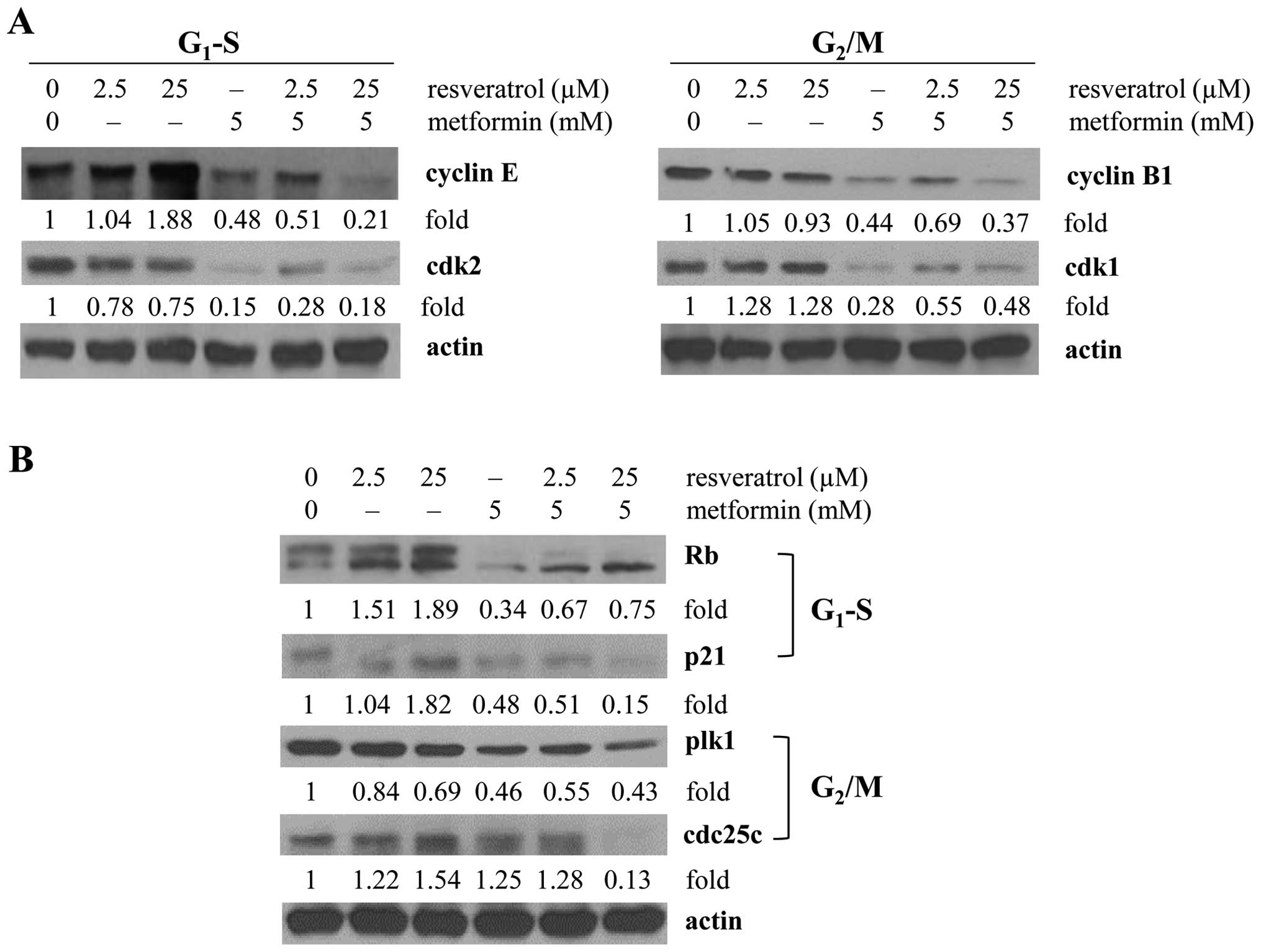
transition by western blot analysis. Results in Fig. 2A showed that metformin alone or in
combination with resveratrol resulted in 52–79 and 72–85%
suppression of cyclin E and cdk2, respectively. Since resveratrol
alone did not significantly change cell cycle phase distribution
under the conditions of exposure to UVC, the observed increase in
cyclin E expression along with decreasing cdk2 expression following
treatment by resveratrol may reflect may reflect a compensatory
regulatory adjustment by cyclin E/cdk2 (Table I). As expected, however, a more
pronounced decrease in the expression of cyclin E as well as cdk2
was detected in the cells treated with 5 mM metformin alone or with
the addition of 25 µM resveratrol (Fig. 2A). Since metformin-treated cells
also showed alterations in G2/M progression, we assayed
the changes in cyclin B/cdk1 expression. Following metformin
treatment, downregulation of cyclin B1 (~56%) and cdk1 (~72%) was
observed, but no further reduction on cdk1 expression occurred in
cells treated with metformin combined with 2.5 or 25 µM
resveratrol (Fig. 2A).
 | Table IEffect of resveratrol or metformin on
cell cycle distribution. |
Table I
Effect of resveratrol or metformin on
cell cycle distribution.
| Treatment | G1 | UV (20
J/m2) S |
G2/M |
|---|
| Control | 59.1 | 13.73 | 22.60 |
| Resveratrol (2.5
µM) | 60.90 | 14.00 | 21.01 |
| Resveratrol (25
µM) | 53.90 | 13.14 | 21.13 |
| Metformin (5
mM) | 69.24 | 8.82 | 13.68 |
| Resveratrol (2.5
µM) + metformin (5 mM) | 70.87 | 7.26 | 14.81 |
| Resveratrol (25
µM) + metformin (5 mM) | 65.48 | 11.57 | 15.54 |
Cyclin E/cdk2 also plays a pivotal role
in controlling Rb and entry into the S phase
We also examined whether control of cyclin E/cdk2 by
metformin may result in a change in Rb. We found that under the
same treatment conditions a ~66% suppression of Rb was observed
that could contribute to the partial G1 and S arrest
elicited by metformin (Fig. 2B).
Additionally, we also investigated the effects of metformin and
resveratrol on the p53-p21 axis of the G1 and S checkpoint control
in response to the UVC stimuli. Resveratrol (25µM) increased
p21 expression (1.8-fold); whereas metformin alone or in
combination with resveratrol resulted in >50% downregulation of
p21 (metformin alone or with 2.5 µM resveratrol) and ~85%
decrease of p21 (metformin with 25 µM resveratrol) (Fig. 2B). Activation of cyclin B1 and the
cyclin B1/cdk1 complex is tightly controlled by phosphorylation and
de-phosphorylation via plk1 and cdc25c, respectively (52,53).
Therefore, cyclin B/cdk1-mediated G2/M progression by
metformin and resveratrol was further analyzed by the changes in
plk1/cdc25c. A more pronounced decrease in the expression of
plk1/cdc25c was detected in the cells treated with 5 mM metformin
when combined with 25 µM resveratrol (Fig. 2B); in agreement with the cyclin
B1/cdk1 changes we observed (Fig.
2A).
Control of DNA damage checkpoint and
repair by resveratrol and metformin under UVC-induced
conditions
DNA repair plays an important role in DNA damage
responses during anti-carcinogenesis and anti-aging. Two tumor
suppressor proteins, checkpoint kinase 2 (Chk2) and p53R2,
associated with DNA damage checkpoint and DNA repair were further
analyzed in response to UVC. In cells treated with resveratrol,
p-chk2 and p53R2 were upregulated (Fig.
3), suggesting the activation of DNA repair by resveratrol
under UVC treatment as a cellular protective mechanism from DNA
damage. Metformin alone or in combination with resveratrol also
resulted in the upregulation of p53R2 (Fig. 3) while downregulation of p-chk2 was
found in cells treated with metformin alone or combined with
resveratrol (Fig. 3). These results
suggest that in cells exposed to UVC, metformin alone or with
addition of resveratrol likely induces DNA repair via the
upregulation of p53R2 without concomitantly invoking the activation
of chk2.
Discussion
In previous studies, we examined multiple
gero-preventive agents by focusing on their activity in controlling
the mTOR/S6 signaling pathway (54). Both metformin and resveratrol were
found to reduce constitutive DNA damage as indicated by the
inhibition of the phosphorylation of H2AX (γH2AX) and ribosomal S6
protein expression (54). These
results suggest that metformin and resveratrol, previously regarded
as prime candidates for treating and preventing type II diabetes
(12,13) and coronary heart disease (25–28),
may offer the potential to be repositioned as candidate anti-aging
drugs via the modulation of intrinsic aging factors. Indeed, the
interest in resveratrol and metformin as gero-active chemicals may
have started much earlier stemming from efforts to identify and
develop caloric restriction mimetics (CRMs) based on the
longstanding observation of McCay in the 1930s that a reduction in
caloric intake retarded aging and extended median and maximal life
span (55,56). Metformin, a biguanide currently
considered the primary stay of management for diabetes, acts as a
CRM in whole organisms by a multitude of mechanisms including at
the metabolic level, the facilitation of fatty acid oxidation and
glucose uptake in peripheral tissues as well as the suppression of
hepatic gluconeogenesis. Molecularly, metformin not only serves as
a sensor/modulator of cellular energy status, but also as an
activator and inhibitor of AMPK and mTOR, respectively, in line
with the vital role it plays in growth control. In tumorigenic
settings, ample evidence has been obtained that exposure to
metformin shows efficacy in patients diagnosed with malignant
diseases including pancreatic (57)
and breast cancer (58), and
colorectal polyps (59). Since
cancer is a disease associated with aging, it is not totally
surprising that metformin also harnesses the capacity to improve
life expectancy as befitting of a gero-protective agent. The same
considerations may apply to resveratrol (14–19);
its efficacy has been verified in all three stages of
carcinogenesis (initiation, promotion and progression) in UVB and
chemically induced skin tumor growth in mice (23,42)
and in numerous animal models of human types of cancers (18,19).
To date, the roles of resveratrol and metformin in preventing
environmentally induced external damage that contribute to aging
remain largely unknown.
In the present study, we focused on the roles
resveratrol and metformin play in modulating the cellular and
molecular changes in cancer cells elicited in response to UV
challenge using as a model UVC-stressed and unstressed A549 cells.
Specifically, DNA damage responses by resveratrol and metformin
were assessed using changes in the level of expression of p53 and
γH2AX. Our results showed that metformin at 5 mM significantly
prevented the UVC-induced upregulation of p53; relatively, much
less inhibition on UVC-induced p53 expression was observed in cells
treated with resveratrol (Fig. 1A).
In addition, inhibition of UVC-induced γH2AX expression was only
observed in metformin and not resveratrol treatment conditions
(Fig. 1B). These findings suggest
that metformin has better preventive potential against UVC-induced
DNA damage compared to resveratrol.
Flow cytometric analysis showed differential effects
on cell cycle control by metformin and resveratrol in UVC-exposed
cells (Table I). Metformin resulted
in G1 arrest and prevention of S and G2/M
entry accompanied by the inhibition in cell cycle-associated
regulatory proteins, vis-à-vis, cyclin E/cdk2, Rb, p21 cyclin
B1/cdk1 and plk1 (Fig. 2A and B).
As well, metformin downregulated p-chk2, known to be involved in a
p53-dependent cell cycle checkpoint for DNA damage (Fig. 3). In contrast, no significant change
in cell cycle transition occurred in UVC-induced cells following
resveratrol treatment (Table I),
and only moderate changes to the above mentioned G1 and
S and G2/M cell cycle regulatory proteins as well as
p-chk2 were observed (Figs. 2A and
B, and 3). It is also notable
that the metformin induced cell cycle arrest at the G1
and S checkpoint under UVC conditions was not mediated via the
p53-p21 axis, but did show a correlation with the reduction in
cyclin E/cdk2 and Rb (Fig. 2).
Since metformin-mediated cell cycle control is decoupled from
p53/p21, we also tested control of p53-mediated DNA repair by the
changes in p53R2, a recently discovered DNA repair regulatory
protein (60–62). Upregulation of p53R2 expression by
metformin after UVC exposure was also observed (Fig. 3). The effects of metformin on
UVC-induced cells may therefore be summarized as to include: i) the
prevention of UVC-induced DNA damage as supported by downregulation
of p-chk2, p53 and γH2AX (Figs. 1
and 3); ii) induction of cell cycle
arrest (Table I) decoupled from
p53/p21; and iii) fortification of DNA repair through
p53-independent control of p53R2 (Fig.
3).
Compared to metformin, resveratrol as a single agent
is marginally effective in UVC-exposed cells, suggesting that it
operates by a different mechanism. This possibility is supported by
our results showing that, as related to the prevention of DNA
damage in UVC-exposed A549 cells, synergism occurs between these
two agents since cells are more susceptible to the co-treatment
regimen than to each individual agent. This conclusion is made
evident by the following results: i) suppression of DNA damage
based on the downregulation of γH2AX/p53/p-chk2 (Figs. 1 and 3); ii) inhibition of cell cycle
progression via modulation of cyclin E/cdk2, Rb, p21 cyclin B1/cdk1
and plk1/cdc25c (Fig. 2A and B);
and iii) enhancement of DNA repair indicated by the upregulation of
p53R2 (Fig. 3).
In conclusion, our results revealed the mechanistic
aspects that underlie or contribute to the beneficial effects of
metformin and resveratrol, two readily available and widely used
agents, regarding their potential as single or combined candidates
for conferring protection against UV-induced DNA damage and hence
reducing the risk of aging.
Acknowledgments
The present study was supported in part by the
Intramural Sponsored Research Program of New York Medical College
to T.C.H., and by the Seed funding grant program jointly sponsored
by New York Medical College and the Touro College and University
System to J.M.W.
References
|
1
|
Bromley DB: The idea of ageing: An
historical and psychological analysis. Compr Gerontol C. 2:30–41.
1988.PubMed/NCBI
|
|
2
|
Callaway E: Race to stamp out animal
plague begins. Nature. 520:139–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clifford CB and Watson J: Old enemies,
still with us after all these years. ILAR J. 49:291–302. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dillin A, Gottschling DE and Nyström T:
The good and the bad of being connected: The integrons of aging.
Curr Opin Cell Biol. 26:107–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marrot L and Meunier JR: Skin DNA
photodamage and its biological consequences. J Am Acad Dermatol.
58(Suppl 2): S139–S148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cawley S, Bekiranov S, Ng HH, Kapranov P,
Sekinger EA, Kampa D, Piccolboni A, Sementchenko V, Cheng J,
Williams AJ, et al: Unbiased mapping of transcription factor
binding sites along human chromosomes 21 and 22 points to
widespread regulation of noncoding RNAs. Cell. 116:499–509. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei CL, Wu Q, Vega VB, Chiu KP, Ng P,
Zhang T, Shahab A, Yong HC, Fu Y, Weng Z, et al: A global map of
p53 transcription-factor binding sites in the human genome. Cell.
124:207–219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka T, Halicka HD, Huang X, Traganos F
and Darzynkiewicz Z: Constitutive histone H2AX phosphorylation and
ATM activation, the reporters of DNA damage by endogenous oxidants.
Cell Cycle. 5:1940–1945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao H, Tanaka T, Halicka HD, Traganos F,
Zarebski M, Dobrucki J and Darzynkiewicz Z: Cytometric assessment
of DNA damage by exogenous and endogenous oxidants reports
aging-related processes. Cytometry A. 71:905–914. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Halicka HD, Zhao H, Li J, Traganos F,
Zhang S, Lee M and Darzynkiewicz Z: Genome protective effect of
metformin as revealed by reduced level of constitutive DNA damage
signaling. Aging. 3:1028–1038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Darzynkiewicz Z, Zhao H, Halicka HD, Li J,
Lee YS, Hsieh TC and Wu JM: In search of antiaging modalities:
Evaluation of mTOR- and ROS/DNA damage-signaling by cytometry.
Cytometry A. 85:386–399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bennett WL, Maruthur NM, Singh S, Segal
JB, Wilson LM, Chatterjee R, Marinopoulos SS, Puhan MA, Ranasinghe
P, Block L, et al: Comparative effectiveness and safety of
medications for type 2 diabetes: An update including new drugs and
2-drug combinations. Ann Intern Med. 154:602–613. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inzucchi SE, Bergenstal RM, Buse JB,
Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R and
Matthews DR; American Diabetes Association (ADA); European
Association for the Study of Diabetes (EASD): Management of
hyperglycemia in type 2 diabetes: A patient-centered approach:
Position statement of the American Diabetes Association (ADA) and
the European Association for the Study of Diabetes (EASD). Diabetes
Care. 35:1364–1379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zakikhani M, Dowling R, Fantus IG,
Sonenberg N and Pollak M: Metformin is an AMP kinase-dependent
growth inhibitor for breast cancer cells. Cancer Res.
66:10269–10273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kasznicki J, Sliwinska A and Drzewoski J:
Metformin in cancer prevention and therapy. Ann Transl Med.
2:572014.PubMed/NCBI
|
|
16
|
Chandel N: Four key questions about
metformin and cancer. BMC Biol. 12:852014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Azvolinsky A: Repurposing to fight cancer:
The metformin-prostate cancer connection. J Natl Cancer Inst.
106:dju0302014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Athar M, Back JH, Tang X, Kim KH,
Kopelovich L, Bickers DR and Kim AL: Resveratrol: A review of
preclinical studies for human cancer prevention. Toxicol Appl
Pharmacol. 224:274–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: The in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buzzai M, Jones RG, Amaravadi RK, Lum JJ,
DeBerardinis RJ, Zhao F, Viollet B and Thompson CB: Systemic
treatment with the antidiabetic drug metformin selectively impairs
p53-deficient tumor cell growth. Cancer Res. 67:6745–6752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Algire C, Amrein L, Bazile M, David S,
Zakikhani M and Pollak M: Diet and tumor LKB1 expression interact
to determine sensitivity to anti-neoplastic effects of metformin in
vivo. Oncogene. 30:1174–1182. 2011. View Article : Google Scholar
|
|
22
|
Ulgherait M, Rana A, Rera M, Graniel J and
Walker DW: AMPK modulates tissue and organismal aging in a
non-cell-autonomous manner. Cell Reports. 8:1767–1780. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soleas GJ, Diamandis EP and Goldberg DM:
Wine as a biological fluid: History, production, and role in
disease prevention. J Clin Lab Anal. 11:287–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goldberg DM: More on antioxidant activity
of resveratrol in red wine. Clin Chem. 42:113–114. 1996.PubMed/NCBI
|
|
26
|
Wu JM, Lu X, Guo J and Hsieh TC: Vascular
effects of resveratrol. Phytochemicals Mechanisms of Action.
Bidlack WR, Davies AJ, Lewis DS and Randolph RK: CRC Press. Meskin,
MS; pp. 145–161. 2004
|
|
27
|
Wu JM and Hsieh TC: Resveratrol: A
cardioprotective substance. Ann NY Acad Sci. 1215:16–21. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu JM, Hsieh TC and Wang Z:
Cardioprotection by resveratrol: A review of effects/targets in
cultured cells and animal tissues. Am J Cardiovasc Dis. 1:38–47.
2011.
|
|
29
|
Wang L, Waltenberger B, Pferschy-Wenzig
EM, Blunder M, Liu X, Malainer C, Blazevic T, Schwaiger S,
Rollinger JM, Heiss EH, et al: Natural product agonists of
peroxisome proliferator-activated receptor gamma (PPARγ): A review.
Biochem Pharmacol. 92:73–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anisimov VN, Egormin PA, Bershtein LM,
Zabezhinskii MA, Piskunova TS, Popovich IG and Semenchenko AV:
Metformin decelerates aging and development of mammary tumors in
HER-2/neu transgenic mice. Bull Exp Biol Med. 139:721–723. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anisimov VN, Berstein LM, Egormin PA,
Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV,
Kovalenko IG, Poroshina TE, et al: Metformin slows down aging and
extends life span of female SHR mice. Cell Cycle. 7:2769–2773.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Blagosklonny MV: An anti-aging drug today:
From senescence-promoting genes to anti-aging pill. Drug Discov
Today. 12:218–224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Blagosklonny MV: Validation of anti-aging
drugs by treating age-related diseases. Aging. 1:281–288. 2009.
View Article : Google Scholar
|
|
34
|
Howitz KT, Bitterman KJ, Cohen HY, Lamming
DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL,
et al: Small molecule activators of sirtuins extend Saccharomyces
cerevisiae lifespan. Nature. 425:191–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bauer JH, Goupil S, Garber GB and Helfand
SL: An accelerated assay for the identification of
lifespan-extending interventions in Drosophila melanogaster. Proc
Natl Acad Sci USA. 101:12980–12985. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hsieh TC, Yang CJ, Lin CY, Lee YS and Wu
JM: Control of stability of cyclin D1 by quinone reductase 2 in
CWR22Rv1 prostate cancer cells. Carcinogenesis. 33:670–677. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hsieh TC, Wu P, Park S and Wu JM:
Induction of cell cycle changes and modulation of
apoptogenic/anti-apoptotic and extracellular signaling regulatory
protein expression by water extracts of I'm-Yunity (PSP). BMC
Complement Altern Med. 6:302006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hsieh TC, Kunicki J, Darzynkiewicz Z and
Wu JM: Effects of extracts of Coriolus versicolor (I'm-Yunity) on
cell-cycle progression and expression of interleukins-1 beta,-6,
and -8 in promyelocytic HL-60 leukemic cells and mitogenically
stimulated and nonstimulated human lymphocytes. J Altern Complement
Med. 8:591–602. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
DiPietrantonio AM, Hsieh TC, Olson SC and
Wu JM: Regulation of G1/S transition and induction of apoptosis in
HL-60 leukemia cells by fenretinide (4HPR). Int J Cancer. 78:53–61.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Darzynkiewicz Z, Bedner E and Smolewski P:
Flow cytometry in analysis of cell cycle and apoptosis. Semin
Hematol. 38:179–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hsieh TC and Wu JM: Differential effects
on growth, cell cycle arrest, and induction of apoptosis by
resveratrol in human prostate cancer cell lines. Exp Cell Res.
249:109–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aziz MH, Reagan-Shaw S, Wu J, Longley BJ
and Ahmad N: Chemoprevention of skin cancer by grape constituent
resveratrol: Relevance to human disease? FASEB J. 19:1193–1195.
2005.PubMed/NCBI
|
|
43
|
Gwinn DM, Shackelford DB, Egan DF,
Mihaylova MM, Mery A, Vasquez DS, Turk BE and Shaw RJ: AMPK
phosphorylation of raptor mediates a metabolic checkpoint. Mol
Cell. 30:214–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hadad SM, Hardie DG, Appleyard V and
Thompson AM: Effects of metformin on breast cancer cell
proliferation, the AMPK pathway and the cell cycle. Clin Transl
Oncol. 16:746–752. 2014. View Article : Google Scholar
|
|
45
|
Silvestri A, Palumbo F, Rasi I, Posca D,
Pavlidou T, Paoluzi S, Castagnoli L and Cesareni G: Metformin
induces apoptosis and downregulates pyruvate kinase M2 in breast
cancer cells only when grown in nutrient-poor conditions. PLoS One.
10:e01362502015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang X, Li X and Ren J: From French
Paradox to cancer treatment: Anti-cancer activities and mechanisms
of resveratrol. Anticancer Agents Med Chem. 14:806–825. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Coperchini F, Leporati P, Rotondi M and
Chiovato L: Expanding the therapeutic spectrum of metformin: From
diabetes to cancer. J Endocrinol Invest. 38:1047–1055. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang T, Wang L, Zhu M, Zhang L and Yan L:
Properties and molecular mechanisms of resveratrol: A review.
Pharmazie. 70:501–506. 2015.PubMed/NCBI
|
|
49
|
Pryor R and Cabreiro F: Repurposing
metformin: An old drug with new tricks in its binding pockets.
Biochem J. 471:307–322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Miles JM, Rule AD and Borlaug BA: Use of
metformin in diseases of aging. Curr Diab Rep. 14:4902014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Burkewitz K, Zhang Y and Mair WB: AMPK at
the nexus of energetics and aging. Cell Metab. 20:10–25. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gheghiani L and Gavet O: Spatiotemporal
investigation of phosphorylation events during cell cycle
progression. Methods Mol Biol. 1342:157–171. 2016. View Article : Google Scholar
|
|
53
|
Peter M, Le Peuch C, Labbé JC, Meyer AN,
Donoghue DJ and Dorée M: Initial activation of cyclin-B1-cdc2
kinase requires phosphorylation of cyclin B1. EMBO Rep. 3:551–556.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Halicka HD, Zhao H, Li J, Lee YS, Hsieh
TC, Wu JM and Darzynkiewicz Z: Potential anti-aging agents suppress
the level of constitutive mTOR- and DNA damage-signaling. Aging.
4:952–965. 2012. View Article : Google Scholar
|
|
55
|
McCay CM: Is longevity compatible with
optimum growth? Science. 77:410–411. 1933. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Park HW: Longevity, aging, and caloric
restriction: Clive Maine McCay and the construction of a
multidisciplinary research program. Hist Stud Nat Sci. 40:79–124.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sadeghi N, Abbruzzese JL, Yeung SC, Hassan
M and Li D: Metformin use is associated with better survival of
diabetic patients with pancreatic cancer. Clin Cancer Res.
18:2905–2912. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jiralerspong S, Palla SL, Giordano SH,
Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L, Hung MC, Hortobagyi
GN and Gonzalez-Angulo AM: Metformin and pathologic complete
responses to neoadjuvant chemotherapy in diabetic patients with
breast cancer. J Clin Oncol. 27:3297–3302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hosono K, Endo H, Takahashi H, Sugiyama M,
Sakai E, Uchiyama T, Suzuki K, Iida H, Sakamoto Y, Yoneda K, et al:
Metformin suppresses colorectal aberrant crypt foci in a short-term
clinical trial. Cancer Prev Res. 3:1077–1083. 2010. View Article : Google Scholar
|
|
60
|
Tanaka H, Arakawa H, Yamaguchi T,
Shiraishi K, Fukuda S, Matsui K, Takei Y and Nakamura Y: A
ribonucleotide reductase gene involved in a p53-dependent
cell-cycle checkpoint for DNA damage. Nature. 404:42–49. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shao J, Zhou B, Zhu L, Qiu W, Yuan YC, Xi
B and Yen Y: In vitro characterization of enzymatic properties and
inhibition of the p53R2 subunit of human ribonucleotide reductase.
Cancer Res. 64:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yen Y, Chu B, Yen C, Shih J and Zhou B:
Enzymatic property analysis of p53R2 subunit of human
ribonucleotide reductase. Adv Enzyme Regul. 46:235–247. 2006.
View Article : Google Scholar : PubMed/NCBI
|