Introduction
Angiogenesis, the creation of new blood vessels, is
an important effector of many physiological and pathological
processes (1). Angiogenesis occurs
to induce tumor growth and metastasis, and represents a key
hallmark of tumor development (2–5). Tumor
angiogenesis provides the nutrients and oxygen to maintain tumor
growth and invasion. Thus, inhibition of tumor angiogenesis may
decrease tumor cell growth and spread (6–8).
Angiogenesis is considered to be an effective therapeutic target;
hence, angiogenesis is a necessary area of biological research and
clinical oncology (4,9,10).
Tumor angiogenesis is an outcome of an imbalance between
pro-angiogenic factors, including the vascular endothelial growth
factor (VEGF) family, and anti-angiogenesis factors, such as
endostatin and other related factors (11–13).
VEGF effects the sprouting and endothelial cell proliferation, and
then VEGF can stimulate tumor angiogenesis (14). Many of anti-angiogenesis drugs serve
as inhibiting pro-angiogenic factors, such as the monoclonal
antibody bevacizumab binds to VEGF, or other small molecules that
inhibit the binding of VEGF (15,16).
Nevertheless, the mechanisms of tumor angiogenesis are not yet
entirely understood and specific, effective inhibitors of
angiogenesis are required for cancer therapy.
Hepatocellular carcinoma (HCC) includes >90% of
primary malignant liver cancers and is one of the most common
reasons for cancer-related mortality (17,18).
Because of the hepatitis B virus (HBV) and hepatitis C virus (HCV)
infection, the HCC incidence is increasing in Asia, especially in
China (19). HCC is regarded as
hypervascular, and tumor growth depends on angiogenesis (15,20).
Targeting angiogenesis by pharmacologic therapy has been used in
many other solid tumors. Therefore, the anti-angiogenic strategy
for HCC may increase the treatment outcomes for HCC patients.
C-X-C chemokine receptor type 7 (CXCR7), a new known
orphan receptor, has been shown to bind stromal cell-derived
factor-1 (SDF-1) (19,21). CXCR7 also has been demonstrated
important for primordial germ cell migration in zebra fish
(22). Recently, high level of
CXCR7 has been confirmed to be associated with aggressive tumors
(23). Moreover, overexpression of
CXCR7 was found to be connected to metastatic recurrence in
non-small cell lung cancer. Recently, overexpression of CXCR7 was
reported in tumor cell lines and tissues (24). However, the CXCR7 function in
angiogenesis of HCC is not yet clear.
We demonstrated that CXCR7 is highly expressed in
HCC cell lines. In addition, overexpression of CXCR7 promoted the
angiogenic capacity of HCC cells via ATK signaling pathway. This
study demonstrates that CXCR7 may induce angiogenesis in
vivo; therefore, CXCR7 may have potential therapeutic effects
in HCC.
Materials and methods
Cell lines
The HCC cell lines HCCLM3 (100% lung meta-static
potential), MHCC97-L (low meta static potential) and SMMC-7721
(without lung metastatic potential) were purchased from the Chinese
Academy of Science (Shanghai, China). The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) (both from Gibco, Carlsbad, CA, USA) and
100 U penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA) in a
5% CO2 humidified incubator at 37°C.
Real-time reverse transcription-PCR
analyses
The CXCR7 mRNA expression was calculated as follows.
Reactions were executed in 20 µl volumes, every sample
including 2 µl complementary DNA (cDNA) by using the primer
pairs: CXCR7 sense, 5′-GGGATGCAGCGGATAGTCAA-3′ and antisense,
5′-CGGTCGTTGTCCACATCCA-3′; Taqman probe,
5′-TCGGTCTCTCCCTGCCCGTCCT-3′. Real-time reverse transcription-PCR
used TaqMan PCR reagents and the ABI PRISM 7700 Sequence Detection
System (Applied Biosystems, Foster City, CA, USA) followed with an
initial denaturation step at 95°C for 10 min, then 28 cycles of
denaturation at 95°C for 60 sec, primer annealing at 58°C for 30
sec and primer extension at 72°C for 30 sec, with a final extension
step at 72°C for 5 min.
Western blotting
Western blotting was performed by using the Bio-Rad
Transfer Cell System (Bio-Rad, Mississauga, ON, Canada). Rabbit
anti-human CXCR7 antibody (1:200; R&D Systems, Inc.,
Minneapolis, MN, USA) followed by 1:3,000 horseradish
peroxidase-conjugated goat anti-rabbit IgG F(ab′)2 antibody
(Jackson ImmunoResearch, West Grove, PA, USA) was used.
Overexpression of CXCR7 in HCC cells
The HCC cell line HCCLM3 was transformed with human
full-length CXCR7 cDNA by using Lipofectamine 2000 (Invitrogen).
The HCC cell line HCCLM3 transformed with an empty plasmid was used
as a negative control. Stable cell lines expressing CXCR7 were
selected with G418 (K1, 250 µg/ml; TPC-1, 200 µg/ml;
and B-CPAP, 300 µg/ml). The CXCR7 expression was evaluated
by western blotting.
RNA interference (RNAi) in HCC cells
Downregulation of the expression of CXCR7 in HCCLM3
cells was performed using small interfering RNAs (siRNAs) as
follows: siCXCR7-286, 5′-CGCUCUCCUUCAUUUACAUdTdT-3′ (at position
286); negative control siRNA, 5′-UUCUCCGAACGUGUCACGUTT-3′;
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) positive control
siRNA, 5′-GUAUGACAACAGCCUCAAGTT-3′. siRNA transfection of HCCLM3
cells was performed using the protocol.
Human umbilical vein endothelial cell
(HUVEC) tube formation assay
The HUVEC tube formation assay was performed as
previously described. Firstly, 200 µl Matrigel were placed
into a 96-well plate for 30 min at 37°C. HUVECs (2×104)
in 200 µl conditioned medium (CM) from indicated HCC cells
were added to the well and incubated for 24 h at 37°C. Images were
attained by a bright-field microscope (×100), and formation of
capillary tubes was quantified by measuring their total number of
each image.
Chicken chorioallantoic membrane (CAM)
assay
The CAM assay was performed using 8-day-old chicken
embryo. About 1-cm diameter window was shaped in the shell of
chicken embryo. A diameter gelatin sponge with 100 µl CM
harvested from the indicated HCC cells was placed on the CAM. The
windows in the chicken embryo were closed by bandages. The chicken
embryos were incubated at 37°C for 48 h. Then the CAM was fixed
with stationary solution (1:1 v/v mixture of methanol and acetone)
for 15 min, the CAM was imaged by a digital camera. The number of
second- and third-order vessels in the test samples was compared to
control.
HUVEC Transwell migration assay
HUVECs (1×104) were cultured on the top
of polycarbonate Transwell filters (pore size, 8.0 µm;
Corning, Inc., Corning, NY, USA) in CM containing 5% FBS. The lower
chamber was filled with 500 µl of media containing SDF-1α
(100 ng/ml) and 15% FBS. The cells were incubated at 37°C for 8 h,
and the cells that migrated to the lower membrane surface were
fixed in 4% paraformaldehyde, stained using hematoxylin for 15 min,
and the number of cells in 10 randomly selected ×200 fields of view
per filter was counted and expressed relative to that of cells
treated with CM from vector control cells.
Matrigel plug assay in mice
C57BL/6 mice were injected 50 µl of
reconstituted CM with 50 U/ml heparin and SDF-1α (100 ng/ml) added
to 0.6 ml Matrigel. Matrigel polymerizes to a solid gel at body
temperature, and then the gel become vascularized after 10 days.
The gel was removed, photographed, stained, and also diluted in
water to measure the hemoglobin content by Drabkin's reagent kit
(Sigma).
Enzyme-linked immunosorbent assay
(ELISA)
The tumor necrosis factor (TNF)-α, interleukin
(IL)-6 and IL-8 ELISA assay were performed by the commercial kits.
All samples were added to the 96-well in triplicate, incubated at
36°C for 90 min, washed, incubated with a specific anti-antibody
(Cell Signaling Technology, Inc.) at 36°C for 1 h, washed,
incubated with secondary antibody at 36°C for 1 h, and then
substrate was added, incubated for 1 h and the absorbance values
were read at OD450 by an ELISA plate reader.
Statistical analysis
All experimental data are presented as the mean ± SD
of three independent biological replicates. Statistical analyses
were performed using SPSS 13.0 (IBM Corp., Armonk, NY, USA).
Analysis of variance (ANOVA) was used to evaluate the significance
of the differences between two groups. P≤0.05 was considered
statistically significant.
Results
CXCR7 is upregulated in HCC cell
lines
Western blotting and qRT-PCR assays demonstrated
CXCR7 protein and mRNA expression in HCCLM3 cells. The qRT-PCR
showed that CXCR7 mRNA level was obviously increased in HCCLM3
compared to the SMMC-7721 (Fig.
1A). Consistent with the mRNA results, the protein level also
was significantly upregulated in HCCLM3 compared to the SMMC-7721
(Fig. 1B). The data implied that
CXCR7 is overexpressed in HCC cells.
Overexpression of CXCR7 promotes the
angiogenic capacity in HCC cells
Firstly, overexpression of CXCR7 in the HCCLM3 was
confirmed by western blotting (Fig.
2A). The effect of CXCR7 inducing angiogenesis in the HCCLM3
was investigated by tube formation assay. CM from CXCR7
overexpression in HCCLM3 notably induced the tube formation
compared to the control (Fig. 2B).
In addition, CXCR7 overexpressing in HCCLM3 significantly induced
the HUVEC migration (Fig. 2C).
Moreover, CM from CXCR7 overexpression of HCCLM3 cells increased
the second- and third-order vessel number in the CAM (Fig. 2D). The results together indicated,
that CXCR7 induced the angiogenesis capacity of HCCLM3 cells in
vitro.
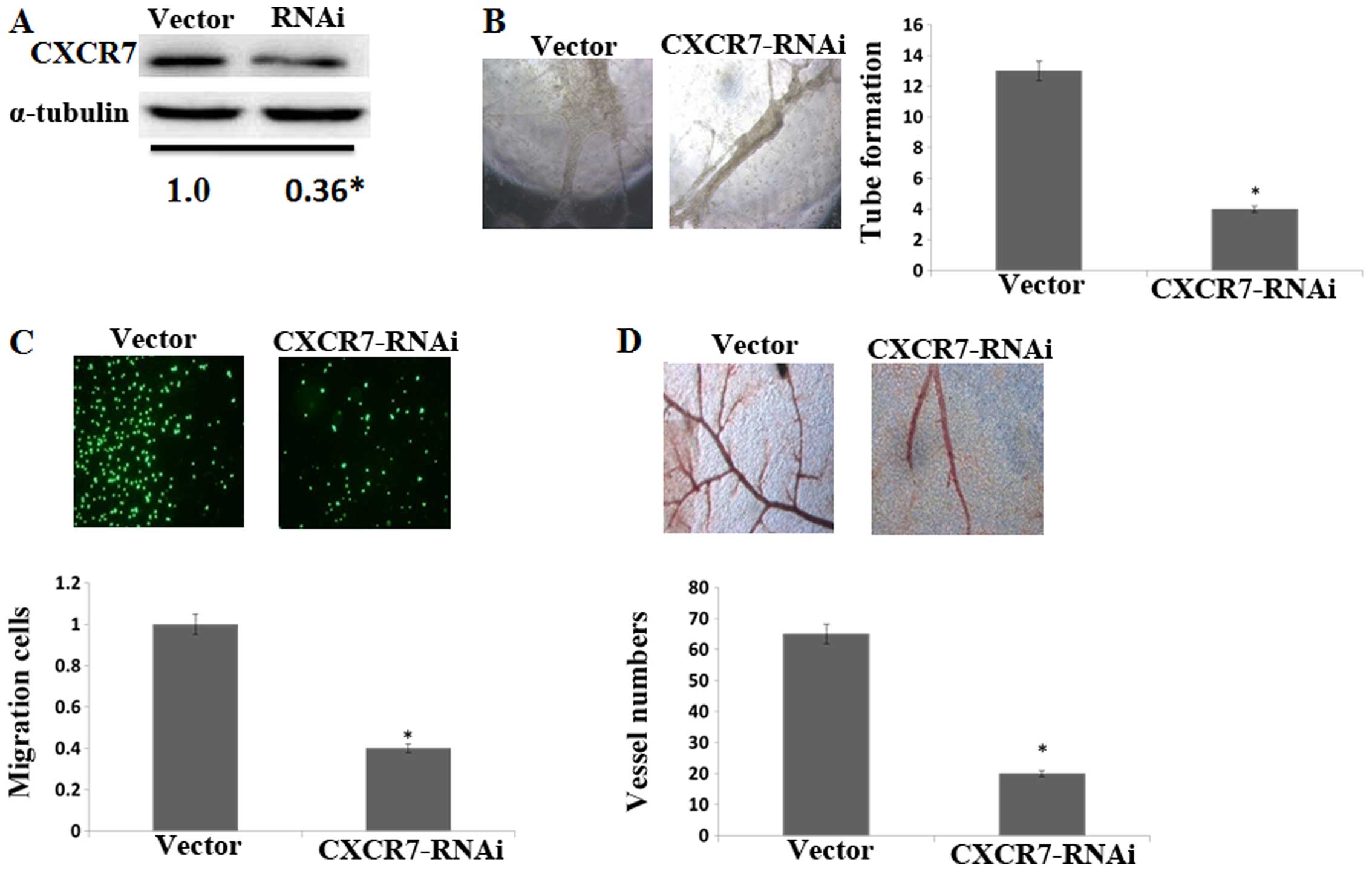
Silencing CXCR7 reduces the angiogenic
capacity in HCC cells
To better confirm the effect of CXCR7 on
angiogenesis in the HCC progression, CXCR7 was knocked down in the
HCCLM3 cells and was confirmed by western blotting (Fig. 3A). Compared to the control, CM from
CXCR7 knockdown cells decreased HUVEC tube formation (Fig. 3B) and migration (Fig. 3C). CM from CXCR7 knockdown cells
also decreased the second- and third-order vessel number in the CAM
assay (Fig. 3D). These data
illustrated that CXCR7 is involved in angiogenesis of HCCLM3
cells.
Overexpression of CXCR7 induces the
angiogenic capacity in Matrigel plug assay
Vascularization was calculated by Matrigel plug
assay (Fig. 4A). The C57BL/6 mice
were injected with Matrigel containing aliquots of control,
overexpressing or silenced CXCR7 in HCCLM3, plus heparin (50 U/ml)
and SDF-1α (100 ng/ml). As shown in Fig. 4B and C, the relative hemoglobin
content and vessel number showed that angiogenesis was inhibited by
CM containing CXCR7-silenced HCCLM3, whereas it was partially
promoted by CM containing overexpressing CXCR7.
CXCR7 promotes the angiogenic capacity of
HCC cells via activating the AKT signaling pathway
The CXCR7 affected the angiogenic capacity of HCCLM3
via activating AKT signaling. We used the AKT signaling inhibitor
LY294002. The stimulatory effects of CM derived from CXCR7
overexpressing HCCLM3 on HUVEC tube formation (Fig. 5A), migration (Fig. 5B) and the second- and third-order
vessel number in the CAM assay (Fig.
5C) was significantly reduced by using LY294002. Taken all
together, these results suggested that CXCR7 enhances the
angiogenic capacity of HCCLM3 cells via activation of the AKT
signaling pathway. The expression of AKT target protein, including
TNF-α, IL-6 and IL-8, was induced in CXCR7 overexpressing HCCLM3
and reduced in CXCR7 knockdown HCCLM3 (Fig. 5D). Moreover, western blotting
demonstrated that overexpression of CXCR7 upregulated the
phosphorylated AKT expression but did not significantly impact the
total AKT protein expression (Fig.
6). These data implied that the AKT pathway may trigger
angiogenesis of CXCR7 in HCCLM3 cells.
 | Figure 5Overexpression of CXCR7 enhances the
angiogenic capacity of HCC cells via activating the AKT pathway.
CXCR7-overexpressing HCC cells were inhibited with LY294002, which
uses as a specific AKT inhibitor. (A) The images and quantification
of tube formation by HUVECs on CM from the indicated cells. (B) The
migration images and quantification of HUVECs after incubation in
CM. (C) The images and quantification of blood vessels in the CAM
assay when stimulated by CM. (D) ELISA of TNF-α, IL-6 and IL-8
protein expression in the indicated cells *P<0.05.
CXCR7, C-X-C chemokine receptor type 7; HCC, hepatocellular
carcinoma; HUVECs, human umbilical vein endothelial cells; CM,
conditioned medium; CAM, chicken chorioallantoic membrane; ELISA,
enzyme-linked immunosorbent assay; TNF, tumor necrosis factor; IL,
interleukin. |
Discussion
There is abundant evidence to show that CXCR7
overexpression is involved in HCC progress, including breast, lung,
prostate and pancreatic cancers (23,25).
It has been demonstrated that knockdown of CXCR7 expression
significantly reduces SMMC-7721 cell invasion, adhesion and
angiogenesis. Moreover, decreased CXCR7 expression inhibited tumor
growth in a mouse model of HCC. The highly specific CXCR7
antagonist CCX771 also was used. In the past report, CXCL12 induced
Huh7, SNU449 and SNU475 cell migration, and the migration was
blocked by CCX771/anti-CXCR7. Similarly, the CCX771 significantly
inhibited glioma cell proliferation and invasion (26). The study provides new insights into
the significance of CXCR7 in invasion and angiogenesis of tumors
(27,28). Although the study shows the
importance of CXCR7 in HCC invasion, angiogenesis and tumor growth,
the role of CXCR7 effect the HUVEC function in tumor
microenvironment is not fully established. In the present study,
CXCR7 was confirmed to be involved in metastatic HCC. Both the past
studies and our data showed overexpression of CXCR7 in a highly
metastatic HCC. Also, increased expression of CXCR7 in HCCLM3
induced the angiogenic capacity in vitro. Furthermore,
silence of CXCR7 decreased the angiogenic capacity of HUVECs. The
data showed that CXCR7 may induce angiogenesis via activating the
AKT signaling pathway. Collectively the findings suggested the
potential effect of CXCR7 in angiogenic capacity of HCC cells.
The overexpression of CXCR7 suggested several
potential unknown functions. Although substantial research has
suggested that CXCR7 may serve as an oncogene in many tumor types,
the molecular mechanism of CXCR7 has not been accurately
demonstrated. In our study, we found that the overexpression of
CXCR7 induced the tube formation and migration of HUVECs and
significantly induced the second- and third-order vessel number of
CAM. The data showed the potential effect of CXCR7 angiogenic
activities in HCC cells. Furthermore, overexpression of CXCR7
notably enhanced the AKT pathway activity, suggesting that
phosphorylated AKT plays a key role in the CXCR7-induced
angiogenesis of HCC progression. AKT signaling pathway is known to
regulate inflammatory responses, and other physiological and
pathological functions including cancer progression. The findings
suggested that CXCR7 induces HCC angiogenesis via activating the
AKT signaling pathway.
Additionally, overexpression of CXCR7 induced TNF-α,
IL-6, and IL-8 expression. TNF-α promoted angiogenesis and
regulated blood vessel remodeling in vivo; IL-6 and IL-8
also promoted VEGF expression and tumor angiogenesis. Increasing
and compelling epidemiological evidence has been suggested that the
inflammatory microenvironment plays a key role in tumor cell
proliferation, angiogenesis and metastasis (29–31).
TNF-α is known as a key regulator of inflammation-related cancer,
including HCC (32). IL-6 is
necessary for HCC progression in animal models, with
hepatic-associated macro-phages representing a major paracrine IL-6
expression during HCC development and autocrine IL-6 inducing
notably HCC initiation (33–35).
IL-8 induced HCC grade, metastasis and recurrence (36,37).
It would be worth exploring the TNF-α, IL-6, and IL-8 functions in
HCC angiogenesis. The TNF-α, IL-6, and IL-8 expression in HCC cells
remained to be clarified and should be investigated further.
In conclusion, our study indicated that CXCR7 is
upregulated and induces angiogenic capacity in HCC by activation of
the AKT pathway. These data may give new insight into the
angiogenesis mechanism in HCC; and CXCR7 may be a new therapeutic
target for HCC.
Acknowledgments
This study was supported by grants (grant nos.
81400961 and 81301157) of the National Natural Science Foundation
of China (NSFC).
References
|
1
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29(Suppl 16): 15–18. 2002.
View Article : Google Scholar
|
|
3
|
Varinska L, Gal P, Mojzisova G, Mirossay L
and Mojzis J: Soy and breast cancer: Focus on angiogenesis. Int J
Mol Sci. 16:11728–11749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herbst RS and Fidler IJ: Angiogenesis and
lung cancer: Potential for therapy. Clin Cancer Res. 6:4604–4606.
2000.
|
|
5
|
Onishi M, Ichikawa T, Kurozumi K and Date
I: Angiogenesis and invasion in glioma. Brain Tumor Pathol.
28:13–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pollitt MJ, Hanby AM, Horgan K, Murphy CE,
Jones PF and Speirs V: Angiogenesis in breast cancer: How should we
measure this? (Review). Oncol Rep. 13:931–936. 2005.PubMed/NCBI
|
|
7
|
Herbst RS, Onn A and Sandler A:
Angiogenesis and lung cancer: Prognostic and therapeutic
implications. J Clin Oncol. 23:3243–3256. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sato Y: Molecular diagnosis of tumor
angiogenesis and anti-angiogenic cancer therapy. Int J Clin Oncol.
8:200–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Hinsbergh VW, Collen A and Koolwijk P:
Angiogenesis and anti-angiogenesis: Perspectives for the treatment
of solid tumors. Ann Oncol. 10(Suppl 4): 60–63. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duarte IG, Bufkin BL, Pennington MF, Gal
AA, Cohen C, Kosinski AS, Mansour KA and Miller JI: Angiogenesis as
a predictor of survival after surgical resection for stage I
non-small-cell lung cancer. J Thorac Cardiovasc Surg. 115:652–658;
discussion 658–659. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Folkman J: Endogenous inhibitors of
angiogenesis. Harvey Lect. 92:65–82. 1997.
|
|
12
|
Gyenge M, Amagase K, Kunimi S, Matsuoka R
and Takeuchi K: Roles of pro-angiogenic and anti-angiogenic factors
as well as matrix metalloproteinases in healing of NSAID-induced
small intestinal ulcers in rats. Life Sci. 93:441–447. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marjon PL, Bobrovnikova-Marjon EV and
Abcouwer SF: Expression of the pro-angiogenic factors vascular
endothelial growth factor and interleukin-8/CXCL8 by human breast
carcinomas is responsive to nutrient deprivation and endoplasmic
reticulum stress. Mol Cancer. 3:42004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hellberg C, Ostman A and Heldin CH: PDGF
and vessel maturation. Recent Results Cancer Res. 180:103–114.
2010. View Article : Google Scholar
|
|
15
|
Alfaro C, Suarez N, Gonzalez A, Solano S,
Erro L, Dubrot J, Palazon A, Hervas-Stubbs S, Gurpide A,
Lopez-Picazo JM, et al: Influence of bevacizumab, sunitinib and
sorafenib as single agents or in combination on the inhibitory
effects of VEGF on human dendritic cell differentiation from
monocytes. Br J Cancer. 100:1111–1119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thompson Coon J, Hoyle M, Green C, Liu Z,
Welch K, Moxham T and Stein K: Bevacizumab, sorafenib tosylate,
sunitinib and temsirolimus for renal cell carcinoma: A systematic
review and economic evaluation. Health Technol Assess. 14:1–184.
iii–iv. 2010. View
Article : Google Scholar
|
|
17
|
Willatt JM, Francis IR, Novelli PM,
Vellody R, Pandya A and Krishnamurthy VN: Interventional therapies
for hepatocellular carcinoma. Cancer Imaging. 12:79–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu AX, Duda DG, Sahani DV and Jain RK:
HCC and angiogenesis: Possible targets and future directions. Nat
Rev Clin Oncol. 8:292–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burns JM, Summers BC, Wang Y, Melikian A,
Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ,
et al: A novel chemokine receptor for SDF-1 and I-TAC involved in
cell survival, cell adhesion, and tumor development. J Exp Med.
203:2201–2213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Finn RS and Zhu AX: Targeting angiogenesis
in hepatocellular carcinoma: Focus on VEGF and bevacizumab. Expert
Rev Anticancer Ther. 9:503–509. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Balabanian K, Lagane B, Infantino S, Chow
KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M and
Bachelerie F: The chemokine SDF-1/CXCL12 binds to and signals
through the orphan receptor RDC1 in T lymphocytes. J Biol Chem.
280:35760–35766. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boldajipour B, Mahabaleshwar H, Kardash E,
Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q and Raz E:
Control of chemokine-guided cell migration by ligand sequestration.
Cell. 132:463–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Shiozawa Y, Wang J, Wang Y, Jung
Y, Pienta KJ, Mehra R, Loberg R and Taichman RS: The role of
CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate
cancer. J Biol Chem. 283:4283–4294. 2008. View Article : Google Scholar
|
|
24
|
Zheng K, Li HY, Su XL, Wang XY, Tian T, Li
F and Ren GS: Chemokine receptor CXCR7 regulates the invasion,
angiogenesis and tumor growth of human hepatocellular carcinoma
cells. J Exp Clin Cancer Res. 29:312010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iwakiri S, Mino N, Takahashi T, Sonobe M,
Nagai S, Okubo K, Wada H, Date H and Miyahara R: Higher expression
of chemokine receptor CXCR7 is linked to early and metastatic
recurrence in pathological stage I nonsmall cell lung cancer.
Cancer. 115:2580–2593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Y, Carson-Walter E and Walter KA:
Targeting chemokine receptor CXCR7 inhibits glioma cell
proliferation and mobility. Anticancer Res. 35:53–64.
2015.PubMed/NCBI
|
|
27
|
Lin L, Han MM, Wang F, Xu LL, Yu HX and
Yang PY: CXCR7 stimulates MAPK signaling to regulate hepatocellular
carcinoma progression. Cell Death Dis. 5:e14882014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xue TC, Jia QA, Bu Y, Chen RX, Cui JF,
Tang ZY and Ye SL: CXCR7 correlates with the differentiation of
hepatocellular carcinoma and suppresses HNF4α expression through
the ERK pathway. Oncol Rep. 32:2387–2396. 2014.PubMed/NCBI
|
|
29
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Castello G, Scala S, Palmieri G, Curley SA
and Izzo F: HCV-related hepatocellular carcinoma: From chronic
inflammation to cancer. Clin Immunol. 134:237–250. 2010. View Article : Google Scholar
|
|
31
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feldmann M: Development of anti-TNF
therapy for rheumatoid arthritis. Nat Rev Immunol. 2:364–371. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maeda S, Hikiba Y, Sakamoto K, Nakagawa H,
Hirata Y, Hayakawa Y, Yanai A, Ogura K, Karin M and Omata M: Ikappa
B kinasebeta/nuclear factor-kappaB activation controls the
development of liver metastasis by way of interleukin-6 expression.
Hepatology. 50:1851–1860. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Naugler WE, Sakurai T, Kim S, Maeda S, Kim
K, Elsharkawy AM and Karin M: Gender disparity in liver cancer due
to sex differences in MyD88-dependent IL-6 production. Science.
317:121–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park EJ, Lee JH, Yu GY, He G, Ali SR,
Holzer RG, Osterreicher CH, Takahashi H and Karin M: Dietary and
genetic obesity promote liver inflammation and tumorigenesis by
enhancing IL-6 and TNF expression. Cell. 140:197–208. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ren Y, Poon RT, Tsui HT, Chen WH, Li Z,
Lau C, Yu WC and Fan ST: Interleukin-8 serum levels in patients
with hepatocellular carcinoma: Correlations with
clinicopathological features and prognosis. Clin Cancer Res.
9:5996–6001. 2003.PubMed/NCBI
|
|
37
|
Welling TH, Fu S, Wan S, Zou W and Marrero
JA: Elevated serum IL-8 is associated with the presence of
hepatocellular carcinoma and independently predicts survival.
Cancer Invest. 30:689–697. 2012. View Article : Google Scholar : PubMed/NCBI
|