Introduction
Colorectal cancer (CRC) has one of the greatest
incidences and mortality rates of any type of cancer in Europe and
the United States. In China, the mortality of CRC ranks fifth in
malignant tumors (1), but a rapid
rise has been observed in recent years (2). Nearly 70% of CRC patients survive no
more than 5 years after diagnosis. Surgical excision combined with
chemotherapy is the preferred treatment for early-stage CRC, and
thus can lead to good prognosis and higher overall survival rates.
However, in advanced cases of CRC with multi-organ metastasis, only
one-tenth of CRC patients survive to the 5-year mark (3). Fluorouracil and capecitabine are
widely used chemotherapeutic drugs for CRC (4). However, their use is usually limited
due to the drug resistance of cancer. Although targeted agents
anti-VEGF and anti-EGFR have been introduced for treatment of CRC,
the overall death rate in advanced CRC remains high since EGFR
therapeutic antibodies are only effective in ~25% of advanced CRC
patients (5). EGFR-targeted therapy
fails mainly due to mutations in KRAS, NRAS and BRAF. Furthermore,
amplification of ERBB2 and MET caused primary resistance (5,6). Cells
that acquired resistance to cetuximab remained sensitive to the
combination of anti-EGFR and anti-MEK (6). Thus, early identification of molecular
heterogeneity is crucial for the treatment of metastatic CRC.
ANP32A, known as PP32, I-1PP2A, Lanp and
PHAPI, is an acidic phosphate protein consisting of a leucine-rich
repeat (LRR) amino terminal, and stretched acid-rich amino acids at
the carboxy terminus (7), expressed
in normal tissues as well as in pancreatic, breast and prostate
cancer and other malignant tumors (8–10).
ANP32A plays an important role in cell proliferation, apoptosis,
transcriptional regulation, signal transduction and other processes
(11). Most previous studies had
shown that ANP32A is a tumor suppressor (9,12–14),
while recent studies found that ANP32A is highly expressed in
hepatocellular carcinoma (15), and
oral squamous cell carcinoma (OSCC) (16). Velmurugan et al reported that
ANP32A is a potential prognostic factor and closely related to the
survival rate of OSCC patients (16). In CRC, however, the roles and
mechanisms of ANP32A are still not clear.
Acidic leucine-rich nuclear phosphoprotein-32A
(ANP32A) has been identified as an inhibitor of protein phosphatase
2A (PP2A) (17), which is a
serine/threonine protein phosphatase expressed in most mammalian
tissues. PP2A has been shown to be a critical cellular regulator of
cell metabolism, transcription, translation, RNA splicing, DNA
replication, cell cycle progression, transformation and apoptosis
(18). Studies have shown that PP2A
activation induces apoptosis via the p38 MAPK-mediated pathway
(18–22). The p38 MAP kinase is thought to be a
promising cancer drug target, but its real therapeutic effect is
unsatisfactory in CRC treatment (23). Zhang et al reported opposite
responses in two subgroups of CRC cells to p38 inhibitors, which
can explain why p38 inhibitors showed no positive effects on CRC in
clinical settings (23). These
findings are consistent with those reporting that p38 signaling has
a dual function in colorectal tumorigenesis (24), and that the role and function of p38
in tumorigenesis may be affected by other unpredictable factors
(25) including PP2A (23). Akt signaling has also been shown to
be upregulated to promote colon cell growth required for the
activity of PP2A (26). Numerous
studies have revealed the relationship between ANP32A and PP2A, but
there are few studies that have focused on the correlation between
p38, Akt and ANP32A, which possesses the opposite effect of
PP2A.
In the present study, we collected clinical
specimens, analyzed the expression of ANP32A in CRC patients and
its relationship with the differentiation of CRC. Meanwhile, due to
the important role of p38 and Akt in CRCs, the relationship between
ANP32A and these two proteins was investigated. The clarification
of these results may be helpful in the treatment of CRC.
Material and methods
Clinical specimens
A total of 68 cases of CRC patients and matched
tumor adjacent tissues were obtained from the Affiliated Hospital
of Guilin Medical College to evaluate the relationship between the
expression levels of ANP32A and the clinical/pathological factors.
Tumor tissues were classified according to cancer
tumor-node-metastasis (TNM) classification system issued by the
Union for International Cancer Control. In order to avoid any
factors that may affect the results of the experiment, all the
selected specimens were examined without any treatment. All samples
were studied with the consent of the patient and approval of the
Guilin Medical College Ethics Committee.
CRC cell line
The CRC cell lines LoVo and SW480 were obtained from
Xiangya Medical College of Central South University and stored in
our laboratory. Cells were cultured in Dulbeccos modified Eagles
medium (DMEM) (HyClone, Shanghai, China) containing 10% fetal
bovine serum (FBS) (Gemini Biological Products, Calabasas, CA, USA)
and 100 U/ml penicillin and streptomycin (Solarbio, Beijing, China)
at 37°C in humidified atmosphere with 5% CO2.
Immunohistochemistry (IHC)
analysis
Tissue sections were fixed by formalin and embedded
by paraffin in this experiment. The cancer tissues were cut into
4-µm sections, and then hematoxylin/eosin staining was performed to
confirm the presence of the original cancer as shown by its
morphology. Tissue sections were dewaxed using xylene, and then
tissue blocks were dehydrated in an ascending series of ethanol
solutions. A 0.3% solution of hydrogen peroxide in methanol was
added for 10 min to eliminate the effects of endogenous peroxidase
and incubated with 10 mmol/l citrate buffer (pH 6.0). The tissues
were blocked at 37°C with 5% goat serum (Solarbio). The sections
were then incubated with ANP32A rabbit polyclonal antibody (Abcam,
Cambridge, UK) in room temperature for 1 h. The secondary antibody
used was a horseradish peroxidase-conjugated antibody (MXB, Fuzhou,
China). Each tumor was scored based on the ratio of the intensity
of the nucleus and cytoplasm: negative staining and weak positive
staining, ±; moderate staining, 1+; and strong staining, 2+.
Staining intensity was confirmed by two pathologists. For the
Allred scoring system, cut-off scores ≤4 and >4 were defined as
low and high expression levels, respectively.
Cell transfection
Double-stranded small interfering RNA (siRNA)
oligonucleotides were obtained from Bioligo, (Shanghai, China). The
siRNA sequence targeting ANP32A (si-ANP32A) was
5-CAAUCGCAAACUUACCAAAGT-3; a fluorescently-labeled non-specific
pri-miR sequence was used as a negative control (sequence targeting
was 5-UUCUCCGAA CGUGUCACGUTT-3).
Total RNA isolation and quantitative
real-time PCR
Total RNA was isolated from SW480 cells transfected
with si-ANP32A and CRC tissue using TRIzol reagent (Tiangen Biotech
Co., Ltd., Beijing, China). The isolated RNA was used as a template
for the reverse transcription reaction. The primers were designed
according to the corresponding cDNA sequences in GenBank. These
primers listed in Table I were
designed to detect ANP32A, AKT, p53 and β-actin (positive
control).
 | Table I.Sequences of primers used in
quantitative real-time PCR. |
Table I.
Sequences of primers used in
quantitative real-time PCR.
| RNA species |
| Primer pairs |
|---|
| ANP32A | Sense |
5′-CACCTCAATCGCAAACTTACCA-3′ |
|
| Antisense |
5′-AACACATTTTCTCGGTAGTCGTT-3′ |
| β-actin | Sense |
5′-AAAGACCTGTACGCCAACAC-3′ |
|
| Antisense |
5′-GTCATACTCCTGCTTGCTGAT-3′ |
| Akt | Sense |
5′-AGAACCTCATRCTGGACAA-3′ |
|
| Antisense |
5′-CTCATGGTCCTGGTTGTAGA-3′ |
| p53 | Sense |
5′-TCAACAAGATGTTTTGCCAACTG-3′ |
|
| Antisense |
5-ATGTGCTGTGACTGCTTGTAGATG-3 |
Western blotting
Total protein was extracted from SW480 and LoVo
cells, and CRC tissues. The primary antibodies of ANP32A (Abcam),
p-Akt, p38, and p-p38 (Wanleibio, Shenyang, China) were used to
detect the protein expression. β-actin (ZSGB-BIO, Beijing, China)
was used as a loading control. Proteins of different molecular
weights were isolated using 12% SDS-PAGE. A nitrocellulose membrane
was then used to transfer the target protein. The membrane was
incubated in 5% skim milk overnight at 4°C, and then treated with
horseradish peroxidase-labeled anti-mouse or anti-rabbit secondary
antibodies for 1 h at 37°C and detected with an enhanced
chemiluminescence (ECL) reagent (Bio-Rad, Hercules, CA, USA).
MTT analysis
SW480 cells were divided into a si-ANP32A, negative
and blank control group. Each group had quadruplicate wells for
treatment, respectively. Cells at 2×104 cells/ml were
seeded into 96-well plates at a final volume of 100 µl and
incubated at 37°C and 5% CO2 in saturated humidity.
Cells were incubated with 20 µl of MTT for 4 h at 37°C and 5%
CO2 in saturated humidity and treated with 150 µl of
dimethyl sulfoxide (DMSO) after removal of the supernatant, and
then shaken for 10 min on a plate shaker. Dissolved the optical
density (OD) value of each well was measured at 490 nm wavelength
by TECAN M200 (Tecan Group Ltd., Männedorf, Switzerland). All
experiments were performed at least three times. Growth inhibition
rate = (control group OD value - OD value of experimental
group)/control group OD values × 100%.
Statistical analysis
All data in the present study were evaluated using
SPSS version 18.0 software (SPSS, Inc., Chicago, IL, USA). The
statistical significance of the data was evaluated using the
Student's t-test. All data are expressed as mean ± SD. The
relationship between the expression of ANP32A and the
clinicopathological parameters of CRC was assessed by the
χ2 test. For all tests, the level of significance was
set at P<0.05.
Results
ANP32A expression in tumor and normal
tissues of CRC patients
The clinical data of 68 patients with CRC are
summarized in Table II. According
to the histological grade of CRC, 23 patients well-differentiated
tumors, whereas the remaining 45 patients had moderately or poorly
differentiated tumors. In order to investigate the expression of
ANP32A in CRC, we performed IHC. As shown in Fig. 1, positive IHC staining was mainly
localized in the cytoplasm and nucleus of CRC cells, and ANP32A was
highly expressed in poorly differentiated CRC. Total protein was
extracted from CRC and matched tumor adjacent tissues, then
subjected to western blot analysis was used to detect ANP32A
expression. The results revealed that the expression of ANP32A was
higher in CRC, which was consistent with the aforementioned IHC
data in which a high level of ANP32A expression was detected. In
addition, high ANP32A protein expression was found to have a
statistically significant relationship to tumor differentiation
(P=0.021) (Table III).
 | Table II.Demographics and characteristics
among CRC patients. |
Table II.
Demographics and characteristics
among CRC patients.
| Factors | No. | % |
|---|
| Sex |
|
Female | 43 | 63.24 |
|
Male | 25 | 36.76 |
| Age, years |
|
≤49 | 12 | 17.65 |
|
50–59 | 22 | 32.35 |
|
60–69 | 23 | 33.82 |
|
≥70 | 11 | 16.18 |
| T (tumor size) |
|
TIS | 3 | 4.41 |
| I | 9 | 13.24 |
| II | 19 | 27.94 |
|
III | 36 | 52.94 |
| IV | 1 | 1.47 |
| N (lymph node) |
| N0 | 48 | 70.59 |
| N1 | 14 | 20.59 |
| N2 | 6 | 8.82 |
| M (metastasis) |
| M0 | 62 | 91.18 |
| M1 | 6 | 8.82 |
| AJCC cancer
stage |
| 0 | 3 | 4.41 |
| I | 25 | 36.76 |
| II |
|
IIA | 18 | 26.47 |
| III |
|
IIIA | 1 | 1.47 |
|
IIIB | 12 | 17.65 |
|
IIIC | 3 | 4.41 |
| IV |
|
IVA | 4 | 5.88 |
|
IVB | 2 | 2.94 |
| Histological
grade |
|
Well | 23 | 33.82 |
|
Moderate | 42 | 61.76 |
|
Poor | 3 | 4.41 |
 | Table III.Correlation of ANP32A expression with
clinical-pathological characteristics using the Allred scoring
system among CRC patients. |
Table III.
Correlation of ANP32A expression with
clinical-pathological characteristics using the Allred scoring
system among CRC patients.
|
| Allred |
|
|
|---|
|
|
|
|
|
|---|
| Factors | Low no. (n=37) | High no.
(n=31) | χ2 | P-value |
|---|
| Histological
grade |
|
| 5.328 | 0.021 |
| Well | 17 | 6 |
|
|
| Moderate/poor | 20 | 25 |
|
|
Cell proliferation is detected by MTT
assay
In order to investigate the effect of ANP32A on cell
proliferation, the cell viability was assessed in the present study
via silencing of ANP32A in SW480 cells. The MTT assay revealed that
the growth rate of SW480 cells transfected with siRNA targeting
ANP32A was lower than that of the negative or blank control groups
(P<0.05). Τhe results revealed that the growth inhibition rates
of the siRNA treatment group were 8.4, 15.6 and 14.1% at 24, 48 and
72 h, respectively (Fig. 2B). There
was no significant difference between the negative or blank control
groups. Collectively, these results indicated that knockdown of
ANP32A can prevent proliferation of SW480 cells effectively.
Silencing of ANP32A affects the
expression of genes associated with cell proliferation in SW480
cells
The expression of ANP32A was detected by western
blotting. Higher levels of p-Akt expression were observed in CRC
tissues than in normal tumor-adjacent tissues. Unlike the
expression patterns of p-Akt, p38 and p-p38 were overexpressed in
normal tumor-adjacent tissues (Fig.
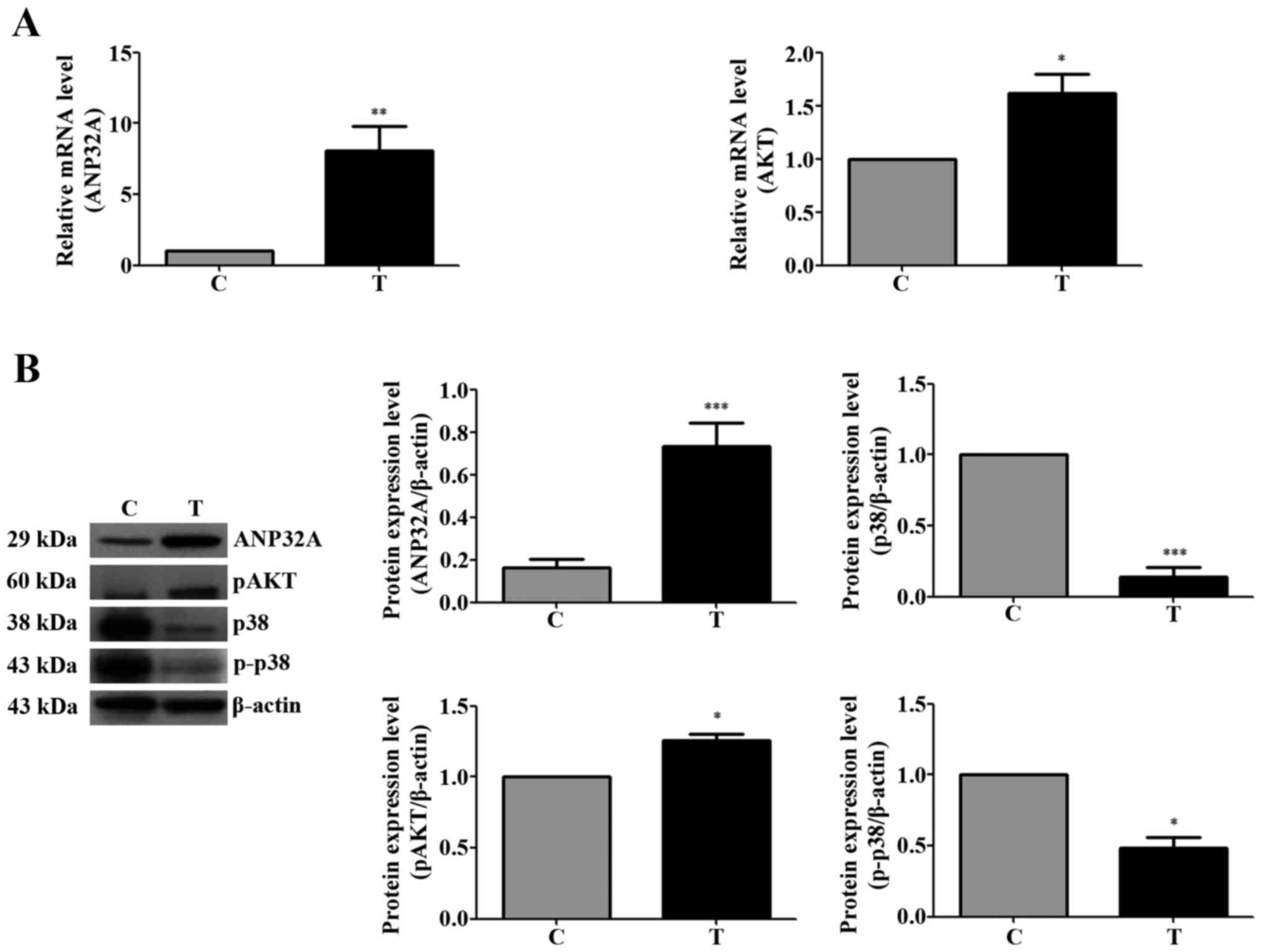
3). As shown in Fig. 3A, the
mRNA expression of total Akt was higher in the CRC tissues, as was
the expression of ANP32A in the CRC tissues. The protein expression
levels of ANP32A and p-Akt were higher in the CRC tissues and the
level of phosphorylated p-38 was lower (Fig. 3B).
To elucidate the details underlying the molecular
mechanism of ANP32A in CRC, SW480 cells with higher ANP32A
expression were selected for the current investigation of the
effects of ANP32A knockdown (Fig.
4A). For this reason, pre-treatment with siRNA targeting ANP32A
for 48 h, and then quantitative real-time PCR experiments were
performed to determine whether knockdown of ANP32A subsequently
regulates Akt transcription. The data revealed that Akt mRNA
expression was decreased by the inhibition of the expression of
ANP32A, whereas the levels of p53 were increased (Fig. 4B). When normalizing to β-actin,
ANP32A mRNA expression in the CRC samples was nearly 8.09-fold
higher than that in the matched non-cancerous tissues, and the data
were consistent with the previous western blotting test in which
high expression of ANP32A was detected (Fig. 4B). The results indicated that
knockdown of ANP32A resulted in the decrease of p-Akt expression,
but the increase of the expression of p-p38 (Fig. 4C). All of these data strongly
demonstrated that silencing of ANP32A inhibited the Akt activation
and increased the expression of p-p38 in SW480 cells. These results
demonstrated that the expression of ANP32A is associated with the
level of Akt and that it may promote the proliferation of CRC
cells.
Discussion
Colorectal cancer (CRC) is a high-incidence and
high-mortality form of cancer (27). The findings of numerous different
studies have shown that several factors induce the formation of
CRC, included genetic and epigenetic alterations (28,29).
Some of these gene alterations can be applied as biomarkers for
cancer diagnosis and provide strategies for cancer therapy
(30). The human ANP32A gene is
expressed differently in different tumor tissues. Low expression
has been reported in prostate (31), pancreatic (9,32) and
breast cancer (33,34), and B cell lymphoma (13). Early studies have shown that ANP32A
is expressed as a modulator of cancer cell apoptosis in
vitro and in vivo (35,36),
including human non-small cell lung cancer (NSCLC) (37). In pancreatic cancer, Brody et
al demonstrated that considerable expression of ANP32A was
found in well-differentiated tumors, however decreased or absent
expression of ANP32A was found to be related to poor
differentiation (38). Notably,
Velmurugan et al found ANP32A levels to be higher in tumor
tissues and that high levels of ANP32A expression were associated
with poor tumor differentiation in OSCC (16). Shi et al found that ANP32A
was overexpressed in CRC using laser capture microdissection (LCM)
and two-dimensional difference gel electrophoresis (2D-DIGE)
(39), but no further information
is available concerning ANP32A expression with tumor
differentiation or its significance to the development of CRC. In
the present study, the expression of ANP32A was evaluated in CRC
and adjacent tumor-free tissue samples. IHC, western blotting, and
real-time PCR analysis demonstrated that CRC specimens exhibited
significantly higher ANP32A protein and mRNA levels than normal
tissues nearby. There is a statistically positive correlation
between the ANP32A protein expression levels and the degrees of
tumor differentiation in cancer tissues. Overexpression of ANP32A
has been shown to suppress tumor cell growth and induce apoptosis
in breast and non-small-cell lung cancer (33,37).
It has been reported that artificially introducing ANP32A
expression into pancreatic cell lines in which it has been absent
can increase the rate of G1 arrest relative to control cells
(38). The present study
demonstrated that ANP32A knockdown by RNA interference suppresses
the proliferation of CRC cells. These results revealed that ANP32A
promotes tumor development in CRC cells by accelerating cell
proliferation and that overexpression of ANP32A has an important
role in tumorigenesis and progression. These results were
inconsistent with results associated with other tumors. The results
indicated that the expression of ANP32A varies in different types
of cancer, and may have the opposite effects in different tumor
tissues.
To identify the possible mechanisms by which ANP32A
enhances the survival of colon cancer cells, the effects of ANP32A
on molecules involved in the proliferation of CRC were
investigated. Numerous studies have shown that p38 has a promotive
effect in CRC, but newer studies have shown that p38 MAPK can act
as a tumor suppressor in CRC (40),
and that it constitutes a potential molecular target in the
inhibition of colorectal carcinogenesis (41,42). A
previous study revealed that the activation of p38 can lead to an
increase in apoptosis in the LoVo CRC cell line (43). The p38 inhibitor SB203580 can
reverse the effect of apoptosis upon curcumol treatment in LoVo
cells (43). PP2A, an inhibitor of
ANP32A, is a negative regulator of p38 activation (22,44–46).
It can be easily hypothesized that ANP32A may have a positive
effect in p38 regulation. Habrukowich et al revealed that
knockdown of ANP32A expression induced p38 phosphorylation in human
umbilical vein endothelial cells (HUVEC) (47). To confirm the activity of p38 in
colon cancer tissues, the expression of phosphorylated p38 was
assessed in CRC specimens and ANP32A silenced SW480 cells. Protein
analysis data revealed the p38 phosphorylation levels to be lower
in CRC than in normal cells, and that silencing of ANP32A
upregulated p38 activation. Akt, according to previous studies,
plays a key role in protein synthesis, cell metabolism and
survival, and it is a key regulator of proliferation and metastasis
in CRC cells (48–50). Phosphorylated Akt (p-Akt) can be
used as a tissue biomarker to identify patients with favorable
prognosis and to identify suitable therapeutic targets (41,51).
It has been demonstrated that in addition to activation of p38, the
activity of PP2A was also significantly increased by triptolide and
hydroxycamptothecin, and the Akt survival pathway was also
inhibited in A549 cells (46). Van
Kanegan et al found that PP2A inhibition potentiates Akt
phosphorylation in PC12 cells (52). To further confirm the effect of
ANP32A in CRC and determine the correlation between ANP32A and
p-Akt, function analysis of ANP32A was performed in SW480 cells.
These protein and mRNA results revealed that, as the expression of
ANP32A increased in colorectal tissues, phosphorylated Akt was
upregulated. The results also revealed there to be less expression
of Akt in the ANP32A-silenced cells than in other cells at both the
mRNA and protein levels.
In summary, ANP32A was overexpressed in CRC
patients. Knockdown of ANP32A was found to inhibit CRC SW480 cell
growth and the net effect of ANP32A silencing was
hyperphosphorylation of p38 and dephosphorylation of Akt. These
results demonstrated that ANP32A may be a suitable molecule for the
development of CRC, and the mechanism by which ANP32A promotes
colon cancer growth may involve p38 inactivation and Akt
activation. Further studies are required to improve our
understanding of the specific effect of ANP32A in cancer. The
involvement of other factors and signaling pathways in ANP32A
interaction remains to be explored.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 31460229), the
Natural Science Fund of Guangxi Province (grant no.
2015GXNSFAA139313), the Scientific Research and Technology
Development Projects of Guilin (grant no. 20140105-8), and the
Small Talent Highland Fund in Guangxi (grant nos. 1415 and 201707).
The authors would like to thank Professor Junfei Jin for his
valuable suggestions and editorial contributions.
References
|
1
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang H, Chen Y and Wu G: SDHB deficiency
promotes TGFβ-mediated invasion and metastasis of colorectal cancer
through transcriptional repression complex SNAIL1-SMAD3/4. Transl
Oncol. 9:512–520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jin XQ, Liu P and Zhang QP: [Genetic
susceptibility in children with incomplete Kawasaki disease.
Zhongguo Dang Dai Er Ke Za Zhi. 17:663–667. 2015.PubMed/NCBI
|
|
4
|
Segal NH and Saltz LB: Evolving treatment
of advanced colon cancer. Annu Rev Med. 60:207–219. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Misale S, Di Nicolantonio F,
Sartore-Bianchi A, Siena S and Bardelli A: Resistance to anti-EGFR
therapy in colorectal cancer: From heterogeneity to convergent
evolution. Cancer Discov. 4:1269–1280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Misale S, Yaeger R, Hobor S, Scala E,
Janakiraman M, Liska D, Valtorta E, Schiavo R, Buscarino M,
Siravegna G, et al: Emergence of KRAS mutations and acquired
resistance to anti-EGFR therapy in colorectal cancer. Nature.
486:532–536. 2012.PubMed/NCBI
|
|
7
|
Opal P, Garcia JJ, McCall AE, Xu B, Weeber
EJ, Sweatt JD, Orr HT and Zoghbi HY: Generation and
characterization of LANP/pp32 null mice. Mol Cell Biol.
24:3140–3149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adegbola O and Pasternack GR:
Phosphorylated retinoblastoma protein complexes with pp32 and
inhibits pp32-mediated apoptosis. J Biol Chem. 280:15497–15502.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Williams TK, Costantino CL, Bildzukewicz
NA, Richards NG, Rittenhouse DW, Einstein L, Cozzitorto JA, Keen
JC, Dasgupta A, Gorospe M, et al: pp32 (ANP32A) expression inhibits
pancreatic cancer cell growth and induces gemcitabine resistance by
disrupting HuR binding to mRNAs. PLoS One. 5:e154552010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brody JR, Kadkol SS, Hauer MC, Rajaii F,
Lee J and Pasternack GR: pp32 reduction induces differentiation of
TSU-Pr1 cells. Am J Pathol. 164:273–283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Wang Y, Lu Q, Liu X, Wang F, Ma X,
Cui C, Shi C, Li J and Zhang D: The expression and distributions of
ANP32A in the developing brain. Biomed Res Int.
2015:2073472015.PubMed/NCBI
|
|
12
|
Pan W, da Graca LS, Shao Y, Yin Q, Wu H
and Jiang X: PHAPI/pp32 suppresses tumorigenesis by stimulating
apoptosis. J Biol Chem. 284:6946–6954. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schramedei K, Mörbt N, Pfeifer G, Läuter
J, Rosolowski M, Tomm JM, von Bergen M, Horn F and Brocke-Heidrich
K: MicroRNA-21 targets tumor suppressor genes ANP32A and SMARCA4.
Oncogene. 30:2975–2985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mazroui R, Di Marco S, Clair E, von Roretz
C, Tenenbaum SA, Keene JD, Saleh M and Gallouzi IE:
Caspase-mediated cleavage of HuR in the cytoplasm contributes to
pp32/PHAP-I regulation of apoptosis. J Cell Biol. 180:113–127.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li C, Ruan HQ, Liu YS, Xu MJ, Dai J, Sheng
QH, Tan YX, Yao ZZ, Wang HY, Wu JR, et al: Quantitative proteomics
reveal up-regulated protein expression of the SET complex
associated with hepatocellular carcinoma. J Proteome Res.
11:871–885. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Velmurugan BK, Yeh K-T, Lee C-H, Lin SH,
Chin MC, Chiang SL, Wang ZH, Hua CH, Tsai MH, Chang JG, et al:
Acidic leucine-rich nuclear phosphoprotein-32A (ANP32A) association
with lymph node metastasis predicts poor survival in oral squamous
cell carcinoma patients. Oncotarget. 7:10879–10890. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li M, Makkinje A and Damuni Z: Molecular
identification of I1PP2A, a novel potent
heat-stable inhibitor protein of protein phosphatase 2A.
Biochemistry. 35:6998–7002. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schönthal AH: Role of serine/threonine
protein phosphatase 2A in cancer. Cancer Lett. 170:1–13. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grethe S and Pörn-Ares MI: p38 MAPK
regulates phosphorylation of Bad via PP2A-dependent suppression of
the MEK1/2-ERK1/2 survival pathway in TNF-alpha induced endothelial
apoptosis. Cell Signal. 18:531–540. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng G, Sun Y, Fu W, Guo Z and Xu L:
Microcystin-LR induces cytoskeleton system reorganization through
hyperphosphorylation of tau and HSP27 via PP2A inhibition and
subsequent activation of the p38 MAPK signaling pathway in
neuroendocrine (PC12) cells. Toxicology. 290:218–229. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garcia A, Cayla X, Guergnon J, Dessauge F,
Hospital V, Rebollo MP, Fleischer A and Rebollo A: Serine/threonine
protein phosphatases PP1 and PP2A are key players in apoptosis.
Biochimie. 85:721–726. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boudreau RT, Conrad DM and Hoskin DW:
Apoptosis induced by protein phosphatase 2A (PP2A) inhibition in T
leukemia cells is negatively regulated by PP2A-associated p38
mitogen-activated protein kinase. Cell Signal. 19:139–151. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shang C and Huang S: PP2A level in
colorectal cancer cells predicts the response of p38 targeted
therapy. EBioMedicine. 2:1848–1849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gupta J, del Barco Barrantes I, Igea A,
Sakellariou S, Pateras IS, Gorgoulis VG and Nebreda AR: Dual
function of p38α MAPK in colon cancer: Suppression of
colitis-associated tumor initiation but requirement for cancer cell
survival. Cancer Cell. 25:484–500. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumar A, Pandurangan AK, Lu F, Fyrst H,
Zhang M, Byun HS, Bittman R and Saba JD: Chemopreventive
sphingadienes downregulate Wnt signaling via a PP2A/Akt/GSK3β
pathway in colon cancer. Carcinogenesis. 33:1726–1735. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smith RA, Andrews K, Brooks D, DeSantis
CE, Fedewa SA, Lortet-Tieulent J, Manassaram-Baptiste D, Brawley OW
and Wender RC: Cancer screening in the United States, 2016: A
review of current American Cancer Society guidelines and current
issues in cancer screening. CA Cancer J Clin. 66:96–114. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okugawa Y, Grady WM and Goel A: Epigenetic
alterations in colorectal cancer: Emerging biomarkers.
Gastroenterology. 149:1204–1225.e12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bae JM, Kim JH and Kang GH: Epigenetic
alterations in colorectal cancer: The CpG island methylator
phenotype. Histol Histopathol. 28:585–595. 2013.PubMed/NCBI
|
|
30
|
Weng KP, Hsieh KS, Huang SH, Wu HW, Chien
JH, Lin CC, Tang CW, Ou SF, Huang SJ and Ger LP: Myeloperoxidase
genetic polymorphisms and susceptibility to Kawasaki disease in
Taiwanese children. J Microbiol Immunol Infect. 49:788–796. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bai J, Brody JR, Kadkol SS and Pasternack
GR: Tumor suppression and potentiation by manipulation of pp32
expression. Oncogene. 20:2153–2160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li W, Chen Z, Gong FR, Zong Y, Chen K, Li
DM, Yin H, Duan WM, Miao Y, Tao M, et al: Growth of the pancreatic
cancer cell line PANC-1 is inhibited by protein phosphatase 2A
inhibitors through overactivation of the c-Jun N-terminal kinase
pathway. Eur J Cancer. 47:2654–2664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schafer ZT, Parrish AB, Wright KM,
Margolis SS, Marks JR, Deshmukh M and Kornbluth S: Enhanced
sensitivity to cytochrome c-induced apoptosis mediated by
PHAPI in breast cancer cells. Cancer Res. 66:2210–2218. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kadkol SS, El Naga GA, Brody JR, Bai J,
Gusev Y, Dooley WC and Pasternack GR: Expression of pp32 gene
family members in breast cancer. Breast Cancer Res Treat. 68:65–73.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hill MM, Adrain C, Duriez PJ, Creagh EM
and Martin SJ: Analysis of the composition, assembly kinetics and
activity of native Apaf-1 apoptosomes. EMBO J. 23:2134–2145. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim HE, Jiang X, Du F and Wang X: PHAPI,
CAS, and Hsp70 promote apoptosome formation by preventing Apaf-1
aggregation and enhancing nucleotide exchange on Apaf-1. Mol Cell.
30:239–247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hoffarth S, Zitzer A, Wiewrodt R, Hähnel
PS, Beyer V, Kreft A, Biesterfeld S and Schuler M: pp32/PHAPI
determines the apoptosis response of non-small-cell lung cancer.
Cell Death Differ. 15:161–170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brody JR, Witkiewicz A, Williams TK,
Kadkol SS, Cozzitorto J, Durkan B, Pasternack GR and Yeo CJ:
Reduction of pp32 expression in poorly differentiated pancreatic
ductal adenocarcinomas and intraductal papillary mucinous neoplasms
with moderate dysplasia. Mod Pathol. 20:1238–1244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi H, Hood KA, Hayes MT and Stubbs RS:
Proteomic analysis of advanced colorectal cancer by laser capture
microdissection and two-dimensional difference gel electrophoresis.
J Proteomics. 75:339–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pekarčíková L, Knopfová L, Beneš P and
Šmarda J: c-Myb regulates NOX1/p38 to control survival of
colorectal carcinoma cells. Cell Signal. 28:924–936. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li H, Huang K, Gao L, Wang L, Niu Y, Liu
H, Wang Z, Wang L, Wang G and Wang J: TES inhibits colorectal
cancer progression through activation of p38. Oncotarget.
7:45819–45836. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fassetta M, D'Alessandro L, Coltella N, Di
Renzo MF and Rasola A: Hepatocyte growth factor installs a survival
platform for colorectal cancer cell invasive growth and overcomes
p38 MAPK-mediated apoptosis. Cell Signal. 18:1967–1976. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang J, Huang F, Bai Z, Chi B, Wu J and
Chen X: Curcumol inhibits growth and induces apoptosis of
colorectal cancer LoVo cell line via IGF-1R and p38 MAPK pathway.
Int J Mol Sci. 16:19851–19867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu LG, Packman LC, Weldon M, Hamlett J and
Rhodes JM: Protein phosphatase 2A, a negative regulator of the ERK
signaling pathway, is activated by tyrosine phosphorylation of
putative HLA class II-associated protein I (PHAPI)/pp32 in response
to the antiproliferative lectin, jacalin. J Biol Chem.
279:41377–41383. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang Z, Yang H, Tachado SD, Capó-Aponte
JE, Bildin VN, Koziel H and Reinach PS: Phosphatase-mediated
crosstalk control of ERK and p38 MAPK signaling in corneal
epithelial cells. Invest Ophthalmol Vis Sci. 47:5267–5275. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Meng G, Wang W, Chai K, Yang S, Li F and
Jiang K: Combination treatment with triptolide and
hydroxycamptothecin synergistically enhances apoptosis in A549 lung
adenocarcinoma cells through PP2A-regulated ERK, p38 MAPKs and Akt
signaling pathways. Int J Oncol. 46:1007–1017. 2015.PubMed/NCBI
|
|
47
|
Habrukowich C, Han DK, Le A, Rezaul K, Pan
W, Ghosh M, Li Z, Dodge-Kafka K, Jiang X, Bittman R, et al:
Sphingosine interaction with acidic leucine-rich nuclear
phosphoprotein-32A (ANP32A) regulates PP2A activity and
cyclooxygenase (COX)-2 expression in human endothelial cells. J
Biol Chem. 285:26825–26831. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Da Silva M, Jaggers GK, Verstraeten SV,
Erlejman AG, Fraga CG and Oteiza PI: Large procyanidins prevent
bile-acid-induced oxidant production and membrane-initiated ERK1/2,
p38, and Akt activation in Caco-2 cells. Free Radic Biol Med.
52:151–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Johnson SM, Gulhati P, Rampy BA, Han Y,
Rychahou PG, Doan HQ, Weiss HL and Evers BM: Novel expression
patterns of PI3K/Akt/mTOR signaling pathway components in
colorectal cancer. J Am Coll Surg. 210:767–776, 776–768. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen B, Zeng X, He Y, Wang X, Liang Z, Liu
J, Zhang P, Zhu H, Xu N and Liang S: STC2 promotes the
epithelial-mesenchymal transition of colorectal cancer cells
through AKT-ERK signaling pathways. Oncotarget. 7:71400–71416.
2016.PubMed/NCBI
|
|
51
|
Baba Y, Nosho K, Shima K, Hayashi M,
Meyerhardt JA, Chan AT, Giovannucci E, Fuchs CS and Ogino S:
Phosphorylated AKT expression is associated with PIK3CA
mutation, low stage, and favorable outcome in 717 colorectal
cancers. Cancer. 117:1399–1408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Van Kanegan MJ, Adams DG, Wadzinski BE and
Strack S: Distinct protein phosphatase 2A heterotrimers modulate
growth factor signaling to extracellular signal-regulated kinases
and Akt. J Biol Chem. 280:36029–36036. 2005. View Article : Google Scholar : PubMed/NCBI
|