Introduction
Lenvatinib is an oral, multitargeted tyrosine kinase
inhibitor (TKI) of vascular endothelial growth factor receptors 1–3
(VEGFR1-VEGFR3), fibroblast growth factor receptors 1–4
(FGFR1-FGFR4), PDGFRα, RET and v-kit Hardy-Zuckerman 4 feline
sarcoma viral oncogene homolog (KIT) signaling networks involved in
tumor angiogenesis (1).
In vitro studies have evaluated lenvatinib in
preclinical models. Lenvatinib decreased the auto-phosphorylation
of KIF5B-RET, CCDC6-RET and NcoA4-RET, inhibited the proliferation
of CCDC6-RET human thyroid and lung cancer cell lines and blocked
the tumorigenicity of RET gene fusion-transformed NIH3T3 cells
(2). Orally administered lenvatinib
exhibited antitumor activity in xenograft models of five
differentiated thyroid cancer (DTC) cell lines, five anaplastic
thyroid cancer (ATC) cell lines and one medullary thyroid cancer
cell line (3). Lenvatinib exhibited
antiangiogenic activity in five DTC and five ATC xenografts, while
its antiproliferative activity was shown in vitro only in
2/11 thyroid cancer cell lines (i.e. RO82-W-1 and TT cells).
Lenvatinib was also able to inhibit RET phosphorylation in TT cells
with the activating mutation C634W (3).
In vivo phase II (4,5) and
phase III (6) studies in patients
with aggressive DTC not responsive to radioiodine have demonstrated
that the administration of lenvatinib is associated with an
improvement in progression-free survival (PFS) compared with
placebo (median PFS 18.2 vs. 3.6 months). Following the results of
this phase III study, lenvatinib has been approved for the
treatment of patients with locally recurrent or metastatic,
progressive, radioactive iodine refractory DTC (7).
ATC is one of the most aggressive human types of
tumor. Lymph node or distant metastases are present in ~80% of
patients at diagnosis (8–10) and the median survival rate is 6
months (11,12). Multimodal treatment, including
debulking, hyperfractionated accelerated external beam radiotherapy
and chemotherapy (doxorubicin, paclitaxel, docetaxel and cisplatin)
is the most effective treatment strategy, and improves median
survival rate to ~10 months (13,14).
Several genetic alterations have been identified in
ATC molecular pathways, involving p53, BRAF, RAS, RET/PTC, VEGFR1,
VEGFR2, EGFR, PDGFRα, PDGFRβ, KIT, MET, PIK3Ca, PIK3Cb and PDK1,
that lead to tumor aggressiveness and progression (14,15).
New drugs targeting these molecular alterations have been recently
evaluated in ATC (14).
Recent anecdotal evidence and a phase II clinical
study have reported the antineoplastic activity of lenvatinib in
ATC (16–20). In the present study, we aimed to
evaluate the antineoplastic activity of lenvatinib in ATC
continuous cell lines and in primary ATC cell cultures both in
vitro and in vivo.
Materials and methods
Chemicals and supplements
Lenvatinib (1 and 100 nM; and 1, 10, 25 and 50 µM)
was evaluated in primary ATC cell cultures, in 8305C cells (DSMZ,
Braunschweig, Germany) and AF cells, and in AF cells in CD nu/nu
mice. Chemicals and supplements were obtained from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). RPMI-1640 medium was purchased
from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). PCR
reagents for quantitative PCR were purchased from Applied
Biosystems (Thermo Fisher Scientific, Inc.).
Thyroid tissues
Thyroid samples were surgically collected from 9 ATC
patients and from 5 healthy subjects undergoing parathyroidectomy.
The diagnosis was made on the basis of clinical and histological
criteria by a recognized laboratory (21–23).
By immunohistochemistry it was demonstrated that TSH receptor,
sodium/iodide symporter (NIS), thyroperoxidase (TPO) and
thyroglobulin (Tg) were not expressed in thyroid tissues.
DNA extraction and microdissection and detection of
BRAF mutation were conducted through PCR single strand
conformation polymorphism assays, using accepted protocols such as
direct DNA sequencing (21–23). All patients agreed to take part in
the study and provided written informed consent. The study was
authorized by the local Ethics Committee of the University of
Pisa.
Cell cultures
Human primary ATC cell cultures
ATC cell cultures were established as previously
described (21–23). Tumor samples were divided into
pieces of 1–3 mm with a lancet or clippers. The obtained fragments
were washed 3–5 times in M-199 media containing penicillin (500,000
U/l), streptomycin (500,000 U/l) and nystatin (1,000,000 U/l).
Then, neoplastic samples were suspended in Dulbecco's modified
Eagle's medium (DMEM) with penicillin/streptomycin (50 mg/l),
glutamine (1% w/v) and fetal calf serum (FCS) (20% v/v), at 37°C
and 5% CO2.
As primary cultures reached confluence, the cells
were separated with a trypsin solution, then moved into flasks for
the primary tissue cultures (Becton-Dickinson Labware, Bedford, MA,
USA). After reaching the third passage, the cells were coated with
methocel (24) for the evaluation
of colony-forming efficiency. Subsequently, the biggest colonies
were isolated and amplified in flasks for tissue cultures (21–23)
and the required tests were performed at the fourth passage.
The absence of expression of the TSH receptor
(25), Tg, NIS (26) and TPO (27) was investigated by
immunocytochemistry, as was the presence of cytokeratin (26), which exhibited a partial and focal
positivity. A pattern similar to that of the original neoplastic
tissue was reported by DNA fingerprinting (21–23).
Thyroid follicular cell (TFC)
culture
Primary TFC cultures were established as previously
described (28). The specimens were
minced and digested with collagenase (1 mg/ml; Roche Diagnostics,
Almere, The Netherlands) in RPMI-1640 (Whittaker Bioproducts, Inc.,
Walkersville, MD, USA) for 1 h at 37°C. Semi-digested follicles
were removed, sedimented for 2 min, washed and cultured in
RPMI-1640 with 10% fetal bovine serum (FBS; Seromed, Biochrom,
Germany), 2 mM glutamine and 50 mg/ml penicillin/streptomycin at
37°C and 5% CO2 in plastic 75-cm2 flasks
(Sarstedt, Verona, Italy).
AF cell line
Nine primary ATC cell cultures were established and
among them the AF cell line grew in nu/nu mice, after being
subcutaneously inoculated.
8305C cell line
As the control, 8305C cells, an undifferentiated
thyroid cancer continuous cell line (DSMZ), with a papillary
component, were seeded in RPMI-1640 with 15% FBS and 2 mM
L-glutamine.
Evaluation of cell viability and
proliferation
In order to investigate cell proliferation, we
conducted an MTT assay, using
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
(WST-1; Roche Diagnostics) (22,23,28).
The 8305C, AF, ATC and TFC cell lines were plated (35,000 cells/ml)
at 100 µl/well and treated for 24 h with lenvatinib at different
concentrations or the vehicle alone (four wells for each
concentration). The IC50 value was determined with
linear interpolation. Triplicate experiments were conducted for
each cell preparation (22,23,28).
The absorbance at 450 nm was estimated at 1 and 2 h from the
beginning of the tetrazolium reaction.
Furthermore, to assess the proliferation rate of the
TFC, ATC, AF and 8305C cell lines, cell number counting was also
performed (22,23,28).
Apoptosis: Hoechst uptake and Annexin
V binding assay
The 8305C, AF and ATC cells were plated in wells
(35,000 cells/ml, in 100 µl/well) and treated for 48 h with
lenvatinib in a humidified atmosphere (37°C, 5% CO2).
The cells were then dyed with Hoechst 33342 (28). Subsequently, the apoptosis index
(apoptotic cells/total cells × 100) was determined. The apoptosis
evaluation was conducted using the Annexin V binding method. The
cells were plated in the Lab-Tek II Chamber Slide system (Nalge
Nunc International, Penfield, NY, USA) and treated with lenvatinib
for 48 h. The apoptosis index was calculated as previously reported
(28).
Migration and invasion tests
Migration and invasion assays were performed using
Transwell permeable supports (Corning Life Sciences, Corning, NY,
USA) (29,30). Cell cultures were starved for 5 h in
serum-free medium at 37°C, 5% CO2, then collected with a
solution of PBS and 5 mM EDTA. Total cell number was calculated.
After centrifugation, the cells were seeded at a concentration of
0.5×105 cells/well in serum-free medium.
To produce a gradient, 10% v/v FCS (or serum-free
medium as negative control) was added to receiver wells with
increasing concentrations of lenvatinib and then, the medium was
removed from the lower compartments, and calcein AM (2 µg/ml;
Sigma-Aldrich) was added for 1 h. An ELISA reader, with filters set
to 485 nm for excitation and 520 nm for emission, was used to
assess the intracellular fluorescence.
For the migration assay, cells were incubated for 12
h and for the invasion assay, cells were incubated for 24 h. A
basement membrane extract (Trevigen, Gaithersburg, MD, USA) was
used overnight (37°C, 5% CO2) for invasion. To obtain
the number of migrated or invasive cells with respect to the
fluorescence values, a standard curve with various cell
concentrations was generated.
ELISA tests in ATC cells
Phospho-EGFR inhibition cell-based
assay
ATC cells were plated (5×104 cells/well)
in 1% FBS medium and treated for 72 h (after 24 h of incubation)
with lenvatinib at a concentration close to the experimental
IC50 of the cell proliferation test (25 µM for ATC), or
with a higherx (50 µM), or lower (1 µM) concentration or with
vehicle. Cell lysates were then harvested (31) and evaluated using PathScan
phospo-EGFR (Tyr1173) and total EGFR ELISA kits (Cell Signaling
Technology, Inc., Danvers, MA, USA). Optical density (OD) was
assessed at 450 nm.
ERK1/2 (pTpY185/187) and
Akt (pThr308) ELISA
ATC cells were plated (5×104 cells/well)
and treated with lenvatinib for 72 h (31). Then, cell lysates were evaluated for
human ERK1/2 and Akt phosphorylation using PhosphoDetect ERK1/2
(pThr185/pTyr187) and the PhosphoDetect Akt
(pThr308) ELISA kits (Calbiochem; EMD Millipore,
Billerica, MA, USA). To normalize the obtained data, total protein
ERK1/2 and Akt concentrations were determined with ERK1/2 and Akt
ELISA kits, respectively. OD was estimated at 450 nm.
Cyclin D1 protein expression is
quantified in lenvatinib-treated ATC cells
To evaluate the effect of lenvatinib on protein
cyclin D1 modulation, ATC cells were treated with lenvatinib for 72
h (at the previously indicated concentrations) or with vehicle
alone (31). The amount of cyclin
D1 was quantified in cell lysates, obtained using lysis buffer
(ice-cold 1X; 0.5 ml), with sonication on ice for 10 sec. After
microcentrifugation for 10 min at 4°C, supernatants were collected
and assessed using a human cyclin D1 ELISA kit (USCN Life Science
and Technology Co., Wuhan, China). OD was assessed at 450 nm and
the obtained data were reported as cyclin D1 ng/mg of total
protein.
In vivo studies
Animals and treatment
Six-week-old CD nu/nu male mice, provided by Envigo
(Milan, Italy), were housed in microisolator cages on vented racks
and manipulated using aseptic techniques. Housing and procedures
involving animals were conducted according to the protocol approved
by the Academic Organization Responsible for Animal Welfare
[Organismo Preposto per il Benessere Animale (OPBA)] at the
University of Pisa, according to the Italian law D.lgs. 26/2014,
and with the approval of the Italian ministry of Health
(authorization no. 613/2015-PR).
Each experiment employed the minimum number of mice
needed to obtain statistically meaningful results. On day 0,
4×106±5% viable AF cells/mouse were subcutaneously
inoculated. Animal weights were monitored and tumor volume
(mm3) was defined as: [(w1 × w1 ×
w2) × (π/6)], where w1 and w2 were
the smallest and the largest tumor diameter (mm), respectively.
Treatment (n=6 mice/group) was initiated 20 days after cell
inoculation, when the mean volume was ~100 mm3. All mice
were randomized shortly before the initiation of treatment. Control
mice received vehicle alone. Lenvatinib was administered at 25
mg/kg by gavage daily without interruption for 16 days. Mice were
sacrificed using an anesthetic overdose, after which tumors were
excised and measured.
Tumor tissue: Immunohistochemistry and
microvessel density determination
Neoplastic samples from the two treatment groups
were weighed, then fixed in formalin and subsequently embedded in
paraffin. Sections of 5-µm thickness were stained by hematoxylin
and eosin (H&E), as previously described (29).
VEGF expression was evaluated with an anti-VEGF
rabbit polyclonal antibody (cat. no. sc-152; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) at 1:50 dilution.
Expression was presented as a percentage of positive cells out of
at least 1,000 tumor cells. Microvascular count (MVC) was evaluated
using anti-FVIII polyclonal antibody (cat. no. 760-2642; Ventana
Medical Systems) as previously reported (29).
Statistical analysis
Data are presented as the mean (± SD) for normally
distributed variables, or as the median and interquartile range.
Experiments were conducted in triplicate from each subject and the
mean of the samples was reported for TFC and ATC cells. One-way
ANOVA, Mann-Whitney U or Kruskal-Wallis test were used to compare
mean group values for normally distributed variables. The
χ2 test was used to compare group proportions. Post hoc
comparisons on normally distributed variables were performed using
the Bonferroni-Dunn test. Analysis of apoptosis results was
performed using one-way ANOVA with the Newman-Keuls multiple
comparison test.
Results
In vitro studies in ATC cells
Evaluation of cell proliferation
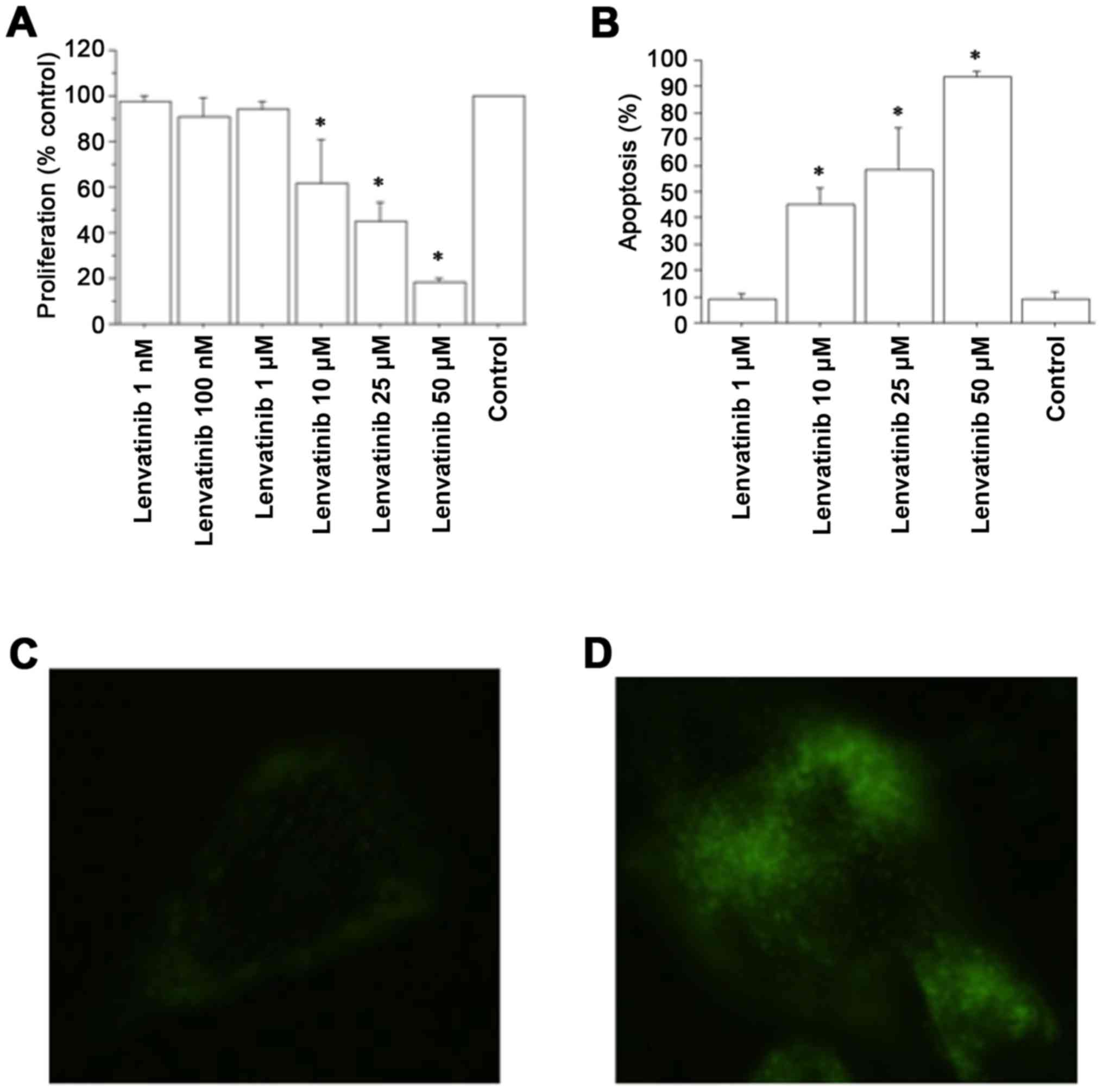
Data obtained from the WST-1 test in ATC cells
demonstrated a significant reduction in cell proliferation rate vs.
the control group with lenvatinib at 1 h from the beginning of the
tetrazolium reaction. Cell counting confirmed these results. After
1 h, the cell number was 11,850±620/100 µl/well in the ATC control
group; 11,613±680 (98%) with lenvatinib 1 nM; 11,496±890 (97%) with
lenvatinib 100 nM; 11,376±790 (96%) with lenvatinib 1 µM; 9,480±600
(80%) with lenvatinib 10 µM; 7,229±460 (61%) with lenvatinib 25 µM;
and 4,503±450 (38%) with lenvatinib 50 µM (P<0.01, ANOVA). The
WST-1 assay in ATC cells also demonstrated a significant reduction
in the proliferation rate vs. the control group with lenvatinib at
2 h from the beginning of the tetrazolium reaction (P<0.01, for
both, ANOVA) (Fig. 1A). Cell
counting confirmed these results; after 2 h, the cell number was
18,720±820/100 µl/well in the ATC control group; 18,532±780 (99%)
with lenvatinib 1 nM; 17,784±990 (95%) with lenvatinib 100 nM;
18,158±810 (97%) with lenvatinib 1 µM; 11,232±1,100 (60%) with
lenvatinib 10 µM; 8,425±960 (45%) with lenvatinib 25 µM; and
2,995±750 (16%) with lenvatinib 50 µM; (P<0.01, ANOVA). The
IC50 value for lenvatinib, obtained by linear
interpolation, was 19±2.5 µM.
Data obtained from the WST-1 assay in TFC cells
following lenvatinib treatment demonstrated a slight but
significant reduction in the proliferation rate vs. the control
group both at 1 h (P<0.01, ANOVA) with lenvatinib 10 µM (96% vs.
control), 25 µM (90% vs. control) and 50 µM (85% vs. control) and
at 2 h (P<0.01, ANOVA) with lenvatinib 10 µM (90% vs. control),
25 µM (85% vs. control) and 50 µM (81% vs. control). Cell counting
confirmed these results: after 1 h, the cell number was
10,150±620/100 µl/well in the TFC control; 9,642±1,100 (95%) with
lenvatinib 10 µM; 9,238±960 (91%) with lenvatinib 25 µM; and
8,625±950 (85%) with lenvatinib 50 µM; (P<0.01, ANOVA); after 2
h, the cell number was 17,500±820/100 µl/well; 15,925±1,120 (91%)
with lenvatinib 10 µM; 14,874±1,060 (85%) with lenvatinib 25 µM;
and 14,350±980 (82%) with lenvatinib 50 µM (P<0.01, ANOVA).
Proliferation and BRAF
The V600EBRAF mutation was
detected in three ATC samples. RET/PTC1 and RET/PTC3,
N-RAS or H-RAS mutations evaluated by quantitative PCR
were not detected in primary ATC cell cultures. Proliferation was
inhibited in a similar manner in ATC from tumors in the
presence/absence of V600EBRAF mutation (data not
shown).
Apoptosis evaluation
Lenvatinib dose-dependently increased apoptotic ATC
cells (P<0.001, ANOVA; Fig. 1B).
The Annexin V assay corroborated these results (Fig. 1C and D).
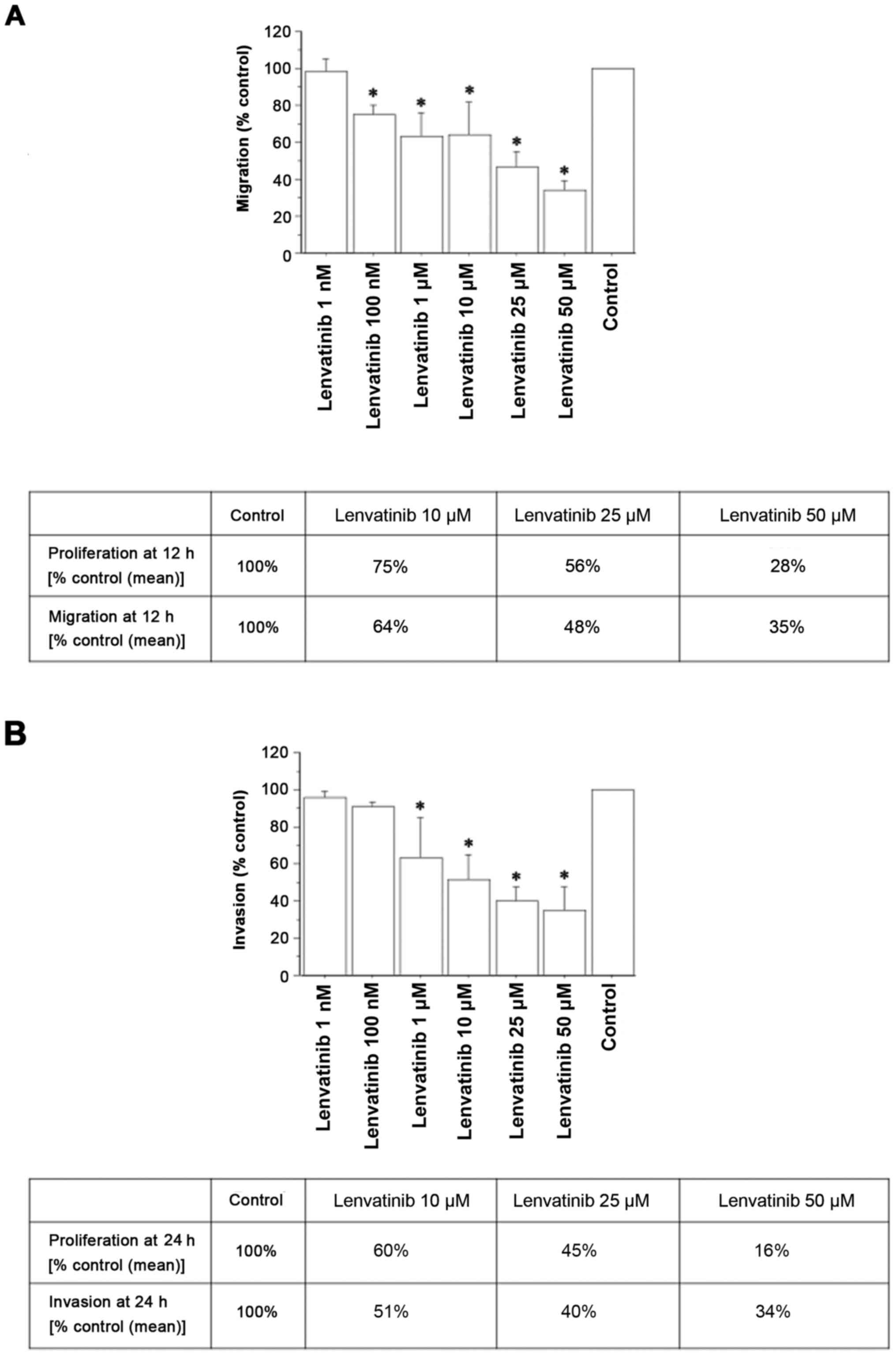
Migration and invasion tests
After reaching subconfluence, primary ATC cell
cultures were treated with increasing concentrations of lenvatinib.
Lenvatinib inhibited migration (Fig.
2A) and invasion (Fig. 2B), as
evaluated by the Transwell chamber (Corning Life Sciences).
Inhibition of EGFR
Lenvatinib significantly and dose-dependently
decreased the phosphorylated form of EGFR in ATC cell lysates
(Fig. 3A).
Inhibition of Akt or ERK1/2
phosphorylation
Phosphorylated/non-phosphorylated Akt or ERK1/2
proteins (evaluated by ELISA) in lenvatinib-treated samples were
significantly reduced in ATC cell cultures (Fig. 3B and C).
Lenvatinib reduces cyclin D1 protein
levels
Lenvatinib dose-dependently inhibited cyclin D1 gene
expression in ATC cell cultures (Fig.
3D; P<0.05). The intracellular cyclin D1 protein was
evaluated in cells exposed to lenvatinib or to vehicle. Lenvatinib
reduced cyclin D1 concentrations compared with vehicle-treated
cells.
In vitro studies in 8305C and AF
cells
Lenvatinib had a dose-dependent antiproliferative
activity in 8305C cells (IC50 of 6.3±2.2 µM) (Fig. 4A) and in AF cells (IC50
of 8.2±3.1 µM) (Fig. 4B).
Furthermore, lenvatinib increased apoptotic 8305C cells in a
dose-dependent manner. Following exposure to lenvatinib 10 µM, 15%
of cells were apoptotic and with lenvatinib 25 or 50 µM, 23.3 and
29.8% of cells were apoptotic, respectively (Fig. 4C; P<0.001, by ANOVA). Apoptotic
AF cells also increased in a dose-dependent manner. Following
exposure to lenvatinib 10 µM, 19.8% of cells were apoptotic and
with lenvatinib 25 or 50 µM, 25 and 30.8% of cells were apoptotic,
respectively (Fig. 4D; P<0.001,
by ANOVA).
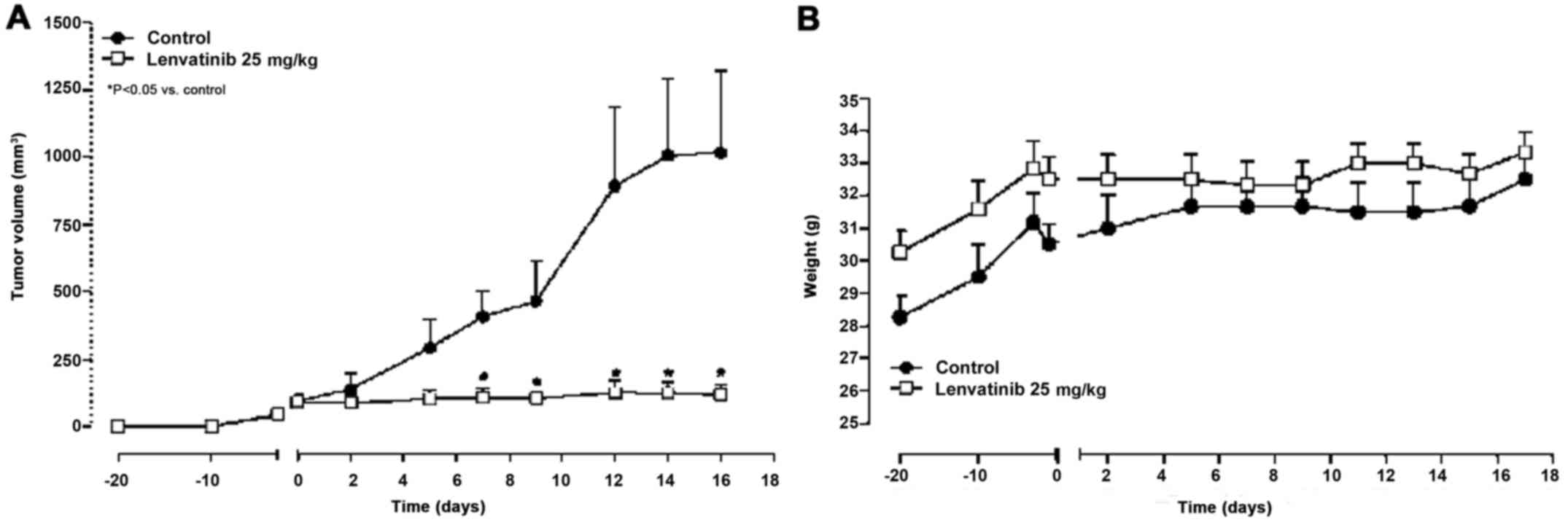
In vivo studies
Lenvatinib reduces AF tumor growth
with no weight loss
Twenty days after the subcutaneous
xenotransplantation of AF cells in CD nu/nu mice, tumor masses
reached an average volume of 100 mm3 and the treatment
started. Lenvatinib (25 mg/kg/day i.p.) significantly reduced tumor
growth, from day 7 after treatment started, compared with the
controls (Fig. 5A; e.g., at day 7,
107.3 mm3 vs. 408.1 mm3 in controls and at
day 16, 119.3 mm3 vs. 1016.1 mm3 in controls;
P<0.05). Notably, no loss of weight was observed throughout the
course of the experiment indicating that lenvatinib treatment was
well tolerated (Fig. 5B).
Lenvatinib reduces VEGF-A expression and microvessel
density in AF tumor tissues. Inoculation of AF cells led to the
formation of a tumor that was histologically consistent with ATC.
Lenvatinib significantly reduced VEGF-A and FVIII immunostaining. A
localized immunoreactivity for VEGF-A was identified in cells of
the control cancer mass which was reduced by lenvatinib (48±8 vs.
35±6; P<0.05), with a simultaneous reduction of microvessel
density (14±5 vs. controls 23±6; P<0.05).
Discussion
Research on the effects of TKIs for the treatment of
ATC is ongoing (32). In the
present study, we demonstrated that lenvatinib inhibited primary
ATC cell cultures proliferation in vitro, while also
increasing apoptosis and inhibiting migration and invasion. In
addition, lenvatinib inhibited the proliferation of 8305C and AF
cells in vitro, while also increasing apoptosis and reduced
AF cell tumor growth in CD nu/nu mice with no toxicity. These
results were consistent with previous studies that identified an
ability of lenvatinib to inhibit tumor growth of ATC cell lines
in vivo and to disrupt angiogenesis by decreasing vascular
permeability. An important antiangiogenic activity of lenvatinib in
8305C xenotransplants has also been reported (2,3). In
the present study, the antiproliferative effect of lenvatinib in
primary ATC cells was observed in all the samples, independently
from the absence or presence of V600EBRAF
mutation. This is probably due to lenvatinib being a multiple
signal transduction inhibitor with antiangiogenic effect.
The pharmacological and molecular inhibition of PI3K
or AKT isoforms can reduce in vitro growth and motility in
human TC cell lines (33,34). RAS-RAF-MAPK, ERK and PI3K pathways
are implicated in the carcinogenesis of TCs and mutations in these
genes are present in ATC (35). In
ATC, ERK and AKT proteins were phosphorylated and activated and
were thus considered as possible therapeutic targets. In the
present study, we demonstrated that lenvatinib inhibited ERK1/2 and
AKT phosphorylation in ATC cells. In addition, lenvatinib was
demonstrated to reduce EGFR phosphorylation, which is consistent
with the data reported by Di Desidero et al (36) and with our previous results on EGFR
phosphorylation inhibition by CLM3 in ATC cells (37).
A previous study has indicated the important role of
cyclin D1 in the regulation of cell cycle progression (38). Cyclin D1 expression was identified
by Lee et al (39) in 67% of
ATCs and by Wiseman et al (40) in 77% of ATCs. Lenvatinib is a dual
TKI, acting on EGFR and VEGFR-2, and is able to inhibit cell growth
by downregulating the expression of cyclin D1 and E (41). In the present study we demonstrated
that lenvatinib potentially downregulates cyclin D1 protein in the
ATC cells.
Lenvatinib exhibited a low-toxicity profile, since
it significantly inhibited AF cell growth in CD nu/nu mice with no
weight loss, unlike other compounds that cause various side-effects
in humans and animals (42).
However, further studies are required in order to elucidate
potential side-effects on the function of the kidney, liver and
other systems. Nevertheless, it may be hypothesized that the
antineoplastic activity of lenvatinib in ATC is the result of
multiple effects on tumor cells, namely: i) an antiproliferative
activity; ii) increased apoptosis; iii) inhibition of migration and
invasion; and iv) inhibition of cancer neovascularization.
Currently, novel therapeutic options for ATC are
being developed, although some limitations still exist in the
selective use of new molecules. For example, even if there are
potential targets in the tumor tissue, such as BRAF, tumor response
may only occur in a fraction of patients, and this could be due to
the activation of compensatory signal pathways, allowing cancer
cell proliferation. The effectiveness of the treatments could be
increased by testing the sensitivity of primary ATC cells from each
subject to different TKIs, as in vitro chemosensitivity
tests can predict in vivo effectiveness in 60% of cases
(43). In addition, a negative
chemosensitivity test in vitro is associated with a 90%
chance of ineffectiveness in vivo (43,44).
This is important in order to avoid the administration of inactive
chemotherapeutics to patients (21,22,30,32).
In the present study, we revealed for the first time
the antitumoral effect of lenvatinib, a multi-targeted kinase
inhibitor, in primary human ATC cell cultures obtained from
patients. These findings could open the way to the clinical use of
lenvatinib in the treatment of patients with ATC.
Acknowledgements
Not applicable.
References
|
1
|
Costa R, Carneiro BA, Chandra S, Pai SG,
Chae YK, Kaplan JB, Garrett HB, Agulnik M, Kopp PA and Giles FJ:
Spotlight on lenvatinib in the treatment of thyroid cancer: Patient
selection and perspectives. Drug Des Devel Ther. 10:873–884. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okamoto K, Kodama K, Takase K, Sugi NH,
Yamamoto Y, Iwata M and Tsuruoka A: Antitumor activities of the
targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against
RET gene fusion-driven tumor models. Cancer Lett. 340:97–103. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res. 2014:6387472014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cabanillas ME, Schlumberger M, Jarzab B,
Martins RG, Pacini F, Robinson B, McCaffrey JC, Shah MH, Bodenner
DL, Topliss D, et al: A phase 2 trial of lenvatinib (E7080) in
advanced, progressive, radioiodine-refractory, differentiated
thyroid cancer: A clinical outcomes and biomarker assessment.
Cancer. 121:2749–2756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schlumberger M, Jarzab B, Cabanillas ME,
Robinson B, Pacini F, Ball DW, McCaffrey J, Newbold K, Allison R,
Martins RG, et al: A phase II trial of the multitargeted tyrosine
kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid
cancer. Clin Cancer Res. 22:44–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schlumberger M, Tahara M, Wirth LJ,
Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff
AO, et al: Lenvatinib versus placebo in radioiodine-refractory
thyroid cancer. N Engl J Med. 372:621–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nair A, Lemery SJ, Yang J, Marathe A, Zhao
L, Zhao H, Jiang X, He K, Ladouceur G, Mitra AK, et al: FDA
approval summary: Lenvatinib for progressive,
radio-iodine-refractory differentiated thyroid cancer. Clin Cancer
Res. 21:5205–5208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Greene FL, Page DL, Fleming ID, Fritz AG,
Balch CM, Haller DG and Morrow M: ThyroidAJCC Cancer Staging
Manual. 6th. Springer-Verlag; New York: pp. 772002
|
|
9
|
Miccoli P, Materazzi G, Antonelli A,
Panicucci E, Frustaci G and Berti P: New trends in the treatment of
undifferentiated carcinomas of the thyroid. Langenbecks Arch Surg.
392:397–404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kebebew E: Anaplastic thyroid cancer:
Rare, fatal, and neglected. Surgery. 152:1088–1089. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hundahl SA, Fleming ID, Fremgen AM and
Menck HR: A National Cancer Data Base report on 53,856 cases of
thyroid carcinoma treated in the U.S., 1985–1995 [see commetns].
Cancer. 83:2638–2648. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kitamura Y, Shimizu K, Nagahama M, Sugino
K, Ozaki O, Mimura T, Ito K, Ito K and Tanaka S: Immediate causes
of death in thyroid carcinoma: Clinicopathological analysis of 161
fatal cases. J Clin Endocrinol Metab. 84:4043–4049. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Crevoisier R, Baudin E, Bachelot A,
Leboulleux S, Travagli JP, Caillou B and Schlumberger M: Combined
treatment of anaplastic thyroid carcinoma with surgery,
chemotherapy, and hyperfractionated accelerated external
radiotherapy. Int J Radiat Oncol Biol Phys. 60:1137–1143. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smallridge RC, Ain KB, Asa SL, Bible KC,
Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal
MS, et al: American Thyroid Association Anaplastic Thyroid Cancer
Guidelines Taskforce: American Thyroid Association guidelines for
management of patients with anaplastic thyroid cancer. Thyroid.
22:1104–1139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Antonelli A, Fallahi P, Ferrari SM,
Ruffilli I, Santini F, Minuto M, Galleri D and Miccoli P: New
targeted therapies for thyroid cancer. Curr Genomics. 12:626–631.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamazaki H, Shimizu S, Iwasaki H, Yoshida
T, Suganuma N, Yamanaka T, Kojima I, Masudo K, Toda S, Nakayama H,
et al: Efficacy and safety of lenvatinib for unresectable
anaplastic thyroid cancer. Gan To Kagaku Ryoho. 44:695–697.
2017.(In Japanese). PubMed/NCBI
|
|
17
|
Iñiguez-Ariza NM, Ryder MM, Hilger CR and
Bible KC: Salvage lenvatinib therapy in metastatic anaplastic
thyroid cancer. Thyroid. 27:923–927. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oishi K, Takabatake D and Shibuya Y:
Efficacy of lenvatinib in a patient with anaplastic thyroid cancer.
Endocrinol Diabetes Metab Case Rep 2017. pii: 16–0136.
2017.https://doi.org/10.1530/EDM-16-0136 View Article : Google Scholar
|
|
19
|
Fukuhara T, Donishi R, Koyama S, Miyake N,
Matsuda E, Fujiwara K, Kitano H and Takeuchi H: Significant
amelioration of tracheal stenosis following lenvatinib in a patient
who has anaplastic thyroid carcinoma with bronchomediastinal
infiltration: A Case Report. Case Rep Oncol. 10:175–181. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tahara M, Kiyota N, Yamazaki T, Chayahara
N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Y, et
al: Lenvatinib for Anaplastic Thyroid Cancer. Front Oncol.
7:252017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Antonelli A, Ferrari SM, Fallahi P, Berti
P, Materazzi G, Barani L, Marchetti I, Ferrannini E and Miccoli P:
Primary cell cultures from anaplastic thyroid cancer obtained by
fine-needle aspiration used for chemosensitivity tests. Clin
Endocrinol. 69:148–152. 2008. View Article : Google Scholar
|
|
22
|
Antonelli A, Ferrari SM, Fallahi P, Berti
P, Materazzi G, Marchetti I, Ugolini C, Basolo F, Miccoli P and
Ferrannini E: Evaluation of the sensitivity to chemotherapeutics or
thiazolidinediones of primary anaplastic thyroid cancer cells
obtained by fine-needle aspiration. Eur J Endocrinol. 159:283–291.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Antonelli A, Ferrari SM, Fallahi P, Berti
P, Materazzi G, Minuto M, Giannini R, Marchetti I, Barani L, Basolo
F, et al: Thiazolidinediones and antiblastics in primary human
anaplastic thyroid cancer cells. Clin Endocrinol. 70:946–953. 2009.
View Article : Google Scholar
|
|
24
|
Fiore L, Pollina LE, Fontanini G, Casalone
R, Berlingieri MT, Giannini R, Pacini F, Miccoli P, Toniolo A,
Fusco A, et al: Cytokine production by a new undifferentiated human
thyroid carcinoma cell line, FB-1. J Clin Endocrinol Metab.
82:4094–4100. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Agretti P, De Marco G, De Servi M,
Marcocci C, Vitti P, Pinchera A and Tonacchera M: Evidence for
protein and mRNA TSHr expression in fibroblasts from patients with
thyroid-associated ophthalmopathy (TAO) after adipocytic
differentiation. Eur J Endocrinol. 152:777–784. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Copland JA, Marlow LA, Williams SF, Grebe
SK, Gumz ML, Maples WJ, Silverman VE and Smallridge RC: Molecular
diagnosis of a BRAF papillary thyroid carcinoma with multiple
chromosome abnormalities and rare adrenal and hypothalamic
metastases. Thyroid. 16:1293–1302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Christensen L, Blichert-Toft M, Brandt M,
Lange M, Sneppen SB, Ravnsbaek J, Mollerup CL, Strange L, Jensen F,
Kirkegaard J, et al: Thyroperoxidase (TPO) immunostaining of the
solitary cold thyroid nodule. Clin Endocrinol. 53:161–169. 2000.
View Article : Google Scholar
|
|
28
|
Antonelli A, Ferrari SM, Fallahi P,
Frascerra S, Piaggi S, Gelmini S, Lupi C, Minuto M, Berti P,
Benvenga S, et al: Dysregulation of secretion of CXC
alpha-chemokine CXCL10 in papillary thyroid cancer: Modulation by
peroxisome proliferator-activated receptor-gamma agonists. Endocr
Relat Cancer. 16:1299–1311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Antonelli A, Bocci G, La Motta C, Ferrari
SM, Fallahi P, Fioravanti A, Sartini S, Minuto M, Piaggi S, Corti
A, et al: Novel pyrazolopyrimidine derivatives as tyrosine kinase
inhibitors with antitumoral activity in vitro and in vivo in
papillary dedifferentiated thyroid cancer. J Clin Endocrinol Metab.
96:E288–E296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Antonelli A, Bocci G, La Motta C, Ferrari
SM, Fallahi P, Ruffilli I, Di Domenicantonio A, Fioravanti A,
Sartini S, Minuto M, et al: CLM94, a novel cyclic amide with
anti-VEGFR-2 and antiangiogenic properties, is active against
primary anaplastic thyroid cancer in vitro and in vivo. J Clin
Endocrinol Metab. 97:E528–E536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bocci G, Fioravanti A, La Motta C, Orlandi
P, Canu B, Di Desidero T, Mugnaini L, Sartini S, Cosconati S, Frati
R, et al: Antiproliferative and proapoptotic activity of CLM3, a
novel multiple tyrosine kinase inhibitor, alone and in combination
with SN-38 on endothelial and cancer cells. Biochem Pharmacol.
81:1309–1316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Antonelli A, Fallahi P, Ulisse S, Ferrari
SM, Minuto M, Saraceno G, Santini F, Mazzi V, D'Armiento M and
Miccoli P: New targeted therapies for anaplastic thyroid cancer.
Anticancer Agents Med Chem. 12:87–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Z, Hou P, Ji M, Guan H, Studeman K,
Jensen K, Vasko V, El-Naggar AK and Xing M: Highly prevalent
genetic alterations in receptor tyrosine kinases and
phosphatidylinositol 3-kinase/akt and mitogen-activated protein
kinase pathways in anaplastic and follicular thyroid cancers. J
Clin Endocrinol Metab. 93:3106–3116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shinohara M, Chung YJ, Saji M and Ringel
MD: AKT in thyroid tumorigenesis and progression. Endocrinology.
148:942–947. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Santarpia L, El-Naggar AK, Cote GJ, Myers
JN and Sherman SI: Phosphatidylinositol 3-kinase/akt and
ras/raf-mitogen-activated protein kinase pathway mutations in
anaplastic thyroid cancer. J Clin Endocrinol Metab. 93:278–284.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Di Desidero T, Fioravanti A, Orlandi P,
Canu B, Giannini R, Borrelli N, Man S, Xu P, Fontanini G, Basolo F,
et al: Antiproliferative and proapoptotic activity of sunitinib on
endothelial and anaplastic thyroid cancer cells via inhibition of
Akt and ERK1/2 phosphorylation and by down-regulation of cyclin-D1.
J Clin Endocrinol Metab. 98:E1465–E1473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Antonelli A, Bocci G, Fallahi P, La Motta
C, Ferrari SM, Mancusi C, Fioravanti A, Di Desidero T, Sartini S,
Corti A, et al: CLM3, a multitarget tyrosine kinase inhibitor with
antiangiogenic properties, is active against primary anaplastic
thyroid cancer in vitro and in vivo. J Clin Endocrinol Metab.
99:E572–E581. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Klein EA and Assoian RK: Transcriptional
regulation of the cyclin D1 gene at a glance. J Cell Sci.
121:3853–3857. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee JJ, Au AY, Foukakis T, Barbaro M, Kiss
N, Clifton-Bligh R, Staaf J, Borg A, Delbridge L, Robinson BG, et
al: Array-CGH identifies cyclin D1 and UBCH10
amplicons in anaplastic thyroid carcinoma. Endocr Relat Cancer.
15:801–815. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wiseman SM, Masoudi H, Niblock P, Turbin
D, Rajput A, Hay J, Bugis S, Filipenko D, Huntsman D and Gilks B:
Anaplastic thyroid carcinoma: Expression profile of targets for
therapy offers new insights for disease treatment. Ann Surg Oncol.
14:719–729. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sarkar S, Mazumdar A, Dash R, Sarkar D,
Fisher PB and Mandal M: ZD6474, a dual tyrosine kinase inhibitor of
EGFR and VEGFR-2, inhibits MAPK/ERK and AKT/PI3-K and induces
apoptosis in breast cancer cells. Cancer Biol Ther. 9:592–603.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ye L, Santarpia L and Gagel RF: The
evolving field of tyrosine kinase inhibitors in the treatment of
endocrine tumors. Endocr Rev. 31:578–599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Blumenthal RD and Goldenberg DM: Methods
and goals for the use of in vitro and in vivo chemosensitivity
testing. Mol Biotechnol. 35:185–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Antonelli A, Ferri C, Ferrari SM,
Sebastiani M, Colaci M, Ruffilli I and Fallahi P: New targeted
molecular therapies for dedifferentiated thyroid cancer. J Oncol.
2010:9216822010. View Article : Google Scholar : PubMed/NCBI
|