Introduction
Bladder cancer (BC) is among the most common
malignant neoplasms of the urological and procreation system
worldwide, with estimated new cases that exceed 380,000 and
approximately 150,000 cancer-related deaths annually (1,2). The
occurrence of BC increases with age and is approximately 4-fold
more prevalent in males than in females (3). Over 90% of BCs originate from the
urothelial transitional epithelium, with 70% of patients presenting
as non-muscle-invasive BC (NMIBC), which shows a tendency to recur
but is generally not life threatening. Approximately 30% of
patients are diagnosed with muscle-invasive BC (MIBC) at disease
onset, with this diagnosis associated with high mortality due to
recurrence or distant metastases. Furthermore, approximately 20% of
NMIBC patients will undergo progression to MIBC, thereby worsening
their prognosis (4). Early BC
detection and intervention is important; therefore, a deeper
understanding of the molecular changes in BC during tumorigenesis
would help to develop a better therapeutic strategy for BC.
As a new regulatory molecule, non-coding RNA (ncRNA)
represents an emerging player in numerous pathophysiological
processes (5), including those
involving tumor development, apoptosis, and proliferation (6). Circular RNA (circRNA) is an endogenous
ncRNA that widely exists in the eukaryotic transcriptome (7,8).
CircRNAs are structured with a covalently closed loop via a
back-splicing process through multiple mechanisms, which is
different from linear RNAs harboring 5′ caps and 3′ tails (9,10).
CircRNAs were first identified as the result of
spliceosome-mediated splicing errors (11); however, with the development of
high-throughput sequencing and biotechnology, numerous functions of
circRNAs have been recently revealed, including their roles in
modulating gene expression, regulating alternative splicing,
functioning as microRNA (miRNA) sponges or sequestering proteins
(12–14).
Emerging evidence confirms that dysregulation of
circRNAs is linked to several human diseases, including cancers.
For instance, hsa_circ_0001445 has been revealed to be
overexpressed in hepatocellular carcinoma and to play an important
role in cell apoptosis, migration, and proliferation (15). Hsa_circ_0007835 has been revealed to
represent a functional oncogene in tumorigenesis in lung cancer
cells (16). In recent years, some
researchers have investigated the role of aberrantly expressed
circRNAs in the occurrence and development of BC (17,18).
However, the relevant in-depth research associated with the
pathogenic mechanism of circRNAs remains in its infancy in BC.
In the present study, circRNA-expression profiles
were screened and analyzed in three pairs of BC tissues and
adjacent non-neoplastic bladder (ANNB) tissues through circRNA
microarray, with the results identifying circRNAs with potential
roles in BC tumorigenesis. Based on the profile, the competing
endogenous RNA (ceRNA) networks in BC and the potential regulating
relationships between the novel circRNA hsa_circRNA_100876,
miR-136-5p and CBX4 were further investigated. Furthermore,
bioinformatics analyses combined with experimental confirmation
offered critical insight into the molecular signatures of circRNAs
in BC carcinogenesis.
Materials and methods
Acquisition of tissue samples
Human BC tissues were selected from 43 patients
(aged 50 to 68 years old) who received radical cystectomy between
Oct 2015 and Dec 2017 at our institution (Shengjing Hospital of
China Medical University, Shenyang, China) without preoperative
neoadjuvant chemotherapy or radiotherapy. Three pairs of BC and
matched ANNB tissues were used for circRNA microarray analysis,
with another 40 pairs for subsequent validation. All samples were
obtained within 5 min after resection and kept frozen in liquid
nitrogen at −80°C. All tissue samples were pathologically evaluated
by two well-experienced pathologists. The inclusion/exclusion
criteria were as follows: i) pathologically confirmed bladder
urothelial carcinoma; ii) no chemotherapy or radiotherapy before
surgery; iii) no history of other malignant tumors; iv) complete
clinicopathologic and follow-up data after surgery; and v) no
evidence of distant metastasis at the time of surgery. All study
participants provided their informed consent for inclusion in this
study, and the protocol of the study was ratified and approved by
the Research Ethics Committee of Shengjing Hospital of China
Medical University (approval no. 2017PS012J).
CircRNA microarray hybridization
A NanoDrop ND-1000 (Thermo Fisher Scientific, Inc.)
was used to quantify total RNA from each sample. Briefly, circRNAs
were enriched by eliminating linear RNAs with Rnase R (Epicentre
Biotechnologies), followed by amplification and transcription into
fluorescent circRNA using a random priming method (Arraystar Super
RNA labeling kit; Arraystar, Inc.). The labeled cRNAs were
hybridized onto the Arraystar Human circRNA Array V2 (8×15K;
Arraystar, Inc.). After washing the slides, the hybridized arrays
were washed by Arraystar Circular RNA microarray v 2.0. and then
fixed on the chip scanner for signal value detection. Then, 1 µg of
each labeled cRNA was fragmented by adding 5 µl 10X Blocking Agent
and 1 µl of 25X fragmentation buffer, then heated the mixture at
60°C for 30 min. Subsequently, 25 µl of 2X hybridization buffer was
added to dilute the labeled cRNA. Then, 50 µl of hybridization
solution was dispensed into the gasket slide and assembled to the
circRNA expression microarray slide. Finally, hybridized arrays
were scanned using an Agilent Scanner G2505C (Agilent Technologies,
Inc.).
Microarray data analysis
The feature extraction software (v11.0.1.1; Agilent
Technology, Inc.) was use to analyze the collected array images.
The limma package from the R software package (https://www.r-project.org/) was used for quantile
normalization and subsequent data processing. CircRNA exhibiting
fold change (FC) ≥2 and a P<0.05 were considered significant.
Volcano-plot (generated by R Software R-3.3.1 gplots) was performed
to identify differentially expressed circRNAs with statistical
significance between two groups and fold-change filtering was
performed to identify differentially expressed circRNAs between two
groups. Hierarchical clustering (produced by R software R-3.3.1
gplots, function heatmap2) was performed to display variable
circRNA-expression patterns among samples. Data conversion is based
on Z-Score. Arraystar miRNA target-prediction software utilizing
the TargetScan (http://www.targetscan.org/) (19) and miRanda (http://www.miranda.org) (20) databases was used to investigate
circRNA-miRNA relationships, with the top five predicted miRNAs for
each differentially expressed circRNA extracted for further
analysis. CircRNA-miRNA interactions were annotated in detail.
Reverse transcription-quantitative PCR
(RT-qPCR) validation
In order to be more representative of the chip as a
whole, 40 sets of BC and ANNB tissues were randomly selected from
the microarray for further verification by RT-qPCR analysis. Total
RNA was extracted from each frozen sample (the amount of tissue was
~100 mg) in accordance with the manufacturer's protocol (RNAiso
PLUS; Takara Bio, Inc.). The 1% agarose electrophoresis determined
the integrity of the isolated RNA, and PrimeScript RT reagent kit
with gDNA Eraser (Takara Bio, Inc.) was used to synthesize
first-strand cDNA. A spectrophotometer was used to measure the
optical density ratio at 260 and 280 nm (OD260/280=1.8–2.0) to
determine RNA concentration. Primer 5.0 software (Premier Biosoft)
was used to design specific primers, which are listed in Table SI. A StepOnePlus real-time PCR
system with SYBR-Green I (Takara Bio, Inc.) was used to conduct
RT-qPCR. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used
as an internal control. Each sample was tested in triplicate. The
thermocycling conditions used were as follows: 95°C for 30 sec,
followed by 40 cycles at 95°C for 5 sec and 60°C for 30 sec.
Relative mRNA expression levels were determined using the
2−ΔΔCq method (21).
Dual luciferase reporter assay
The mutant and wild-type sequences of
hsa-circRNA-100876 were cloned downstream of the firefly luciferase
gene pGL3 vector (Promega Corporation). Based on the instructions
of the manufacturer, Luciferase Reporter plasmid (Promega
Corporation) and miR-136-5p expression plasmid (pmirGLO) were
co-transfected instantaneously utilizing Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently,
pGL3-hsa_circRNA_100876 vectors and miR-136-5p mimics along with
negative control were co-transfected into 293 cells (Bena Culture
Collection). Then, 48 h later, Dual-Luciferase reporter gene assay
kit (Promega Corporation) was used to detect luciferase activity.
The sequences of miR-136-5p mimics were provided by RiboBio
Biotechnology Co., Ltd. and were as follows: forward,
5′-ACUCCAUUUGUUUUGAUGAUGGA-3′ and reverse,
5′-UCCAUCAUCAAAACAAAUGGAGU-3′. For each analysis, the
Renilla luciferase signal was standardized to the firefly
luciferase signal.
Cell culture and CCK-8 assay
The BC cell lines EJ-1 (EJ) and T24 used in the
present study were obtained from the Cell Bank of the Chinese
Academy of Sciences in Shanghai. In addition, both cells lines were
authenticated by STR profiles. Cells were grown at 37°C in a
humidified atmosphere of 5% CO2. All the cell lines were
cultured in DMEM medium (Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 1% penicillin-streptomycin and 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). Whether
hsa_circRNA_100876 is involved in BC cell proliferation ability was
measured using Cell Counting Kit-8 (CCK-8; Dojindo Molecular
technologies, Inc.) assay. Cells in each group were digested by
0.25% trypsin and seeded into 96-well plates (5,000 cells/well);
and three duplicate wells were set for each group of cells. CCK-8
(10 µl) reagent was added to each well and incubated at 37°C for
150 min after transfection for 24, 48, 72 and 96 h. All the
experiments were performed in triplicate. The absorbance was
detected at 450 nm using a microplate reader instrument (Sunrise™;
Tecan Group, Ltd.). The ratio of absorbance difference between the
experimental group and blank control group/24-h difference was the
cell proliferation rate. The small interfering RNA (siRNA)
targeting circRNA_100876 (si-circRNA_100876) was provided by
RiboBio Biotechnology Co., Ltd. and was as follows:
si-circRNA_100876, 5′-CTCCTACAATGTTGATATG-3′. si-NC (product no.
siN0000001-1-5) was also provided by RiboBio Biotechnology Co.,
Ltd.
Bioinformatics analysis
Gene Ontology (GO) enrichment analysis (22) was performed to determine functional
annotations of miRNA target genes, mainly including three
independent ontologies (molecular function, cellular component and
biological process). TopGO (https://bioconductor.org/packages/release/bioc/html/topGO.html;
version 2.32.0) was used to perform GO analysis of the
differentially expressed genes to infer their involvement in
molecular functions. Kyoto Encyclopedia of Genes and Genomes (KEGG)
enrichment analysis (23) was used
to clarify the interactions and functions among these
differentially expressed genes. The R script was used to calculate
the significance of differentially expressed genes and KEGG through
hypergeometric distribution, and Fisher's exact test was used to
calculate the P-value. Data downloads were based on the GO website
(http://geneontology.org) and KEGG website
(https://www.genome.jp/kegg/). The
threshold for the P-value was <0.05 and the count number >2.
The top 10 enriched GO items and KEGG pathways of the
differentially expressed mRNAs were ranked by enrichment score
[-log10 (P-value)].
Construction of a circRNA-miRNA-mRNA
regulatory network
The selected significantly expressed circRNAs,
predicted miRNAs and protein-coding mRNAs were used to construct a
circRNA-miRNA-mRNA regulatory network by a software based on
TargetScan and miRanda (http://www.miranda.org). The software that was used
considered the capacity and number of circRNA-microRNA binding
sites, as well as the binding capacity of microRNA and mRNA.
Western blotting
Total proteins (~100 mg of tissue) were extracted
from cell lysates with wash buffer (1X PBS, 0.1% SDS, 0.5%
nonidet-P-40 and 0.5% sodium deoxycholate) containing 1% (w/v)
protease inhibitor (Beyotime Institute of Biotechnology), and a BCA
protein assay kit (Beyotime Institute of Biotechnology) was used to
quantify the protein concentrations. Protein extract (80 µg) was
loaded onto 10% SDS-PAGE gels and then transferred to
polyvinylidene fluoride membranes (EMD Millipore). The membranes
were then incubated with Tris-buffered saline containing 0.05%
Tween-20 and 5% skim milk powder for 2 h at room temperature in the
presence of the rabbit anti-CBX4 monoclonal antibody at 4°C
overnight (dilution 1:1,000; cat. no. PA5-109482; Thermo Fisher
Scientific, Inc.), followed by incubation for 120 min at room
temperature with the corresponding secondary antibody
HRP-conjugated Affinipure goat anti-rabbit IgG (H+L) (dilution
1:2,000; cat. no. SA00001-2, ProteinTech Group, Inc). GAPDH was
used as an internal reference (1:10,000; cat. no. 10494-1-AP;
ProteinTech Group, Inc.), and an enhanced chemiluminescence kit was
used for visualization (Beyotime Institute of Biotechnology). The
relative expression of the target protein was quantified by ImageJ
software (version 1.48v; National Institutes of Health).
Immunohistochemical staining
The 40 pairs of paraffin sections from BC tissues
and ANNB tissues were prepared and analyzed. The sections (4-µm
thick) were fixed with 10% neutral buffer formalin at room
temperature for 12 h. The sections were randomly numbered and
double blinded. Immunohistochemistry was performed using an SP kit
(ZSGB-BIO; OriGene Technologies, Inc.). After rinsing with
phosphate-buffered saline (PBS), 3% H2O2 was
added at room temperature for 15 min. Then the sections were sealed
with goat serum at room temperature for 15 min (ZSGB-BIO; OriGene
Technologies, Inc), followed by antigen retrieval in a microwave
(total power 800 W, time 10 min). An appropriate dilution of
primary antibody (CBX4; 1:200; cat. no. sc-517216; Santa Cruz
Biotechnology, Inc.) was then added and the sections were incubated
overnight in wet box at 4°C. The sections were then washed and
incubated for 30 min at 37°C with a secondary antibody
[HRP-conjugated Affinipure goat anti-mouse IgG (H+L); cat. no.
SA00001-1; ProteinTech Group, Inc], rinsed with PBS and then
incubated with horseradish oxidase-labeled streptomycin avidin
working solution (ZSGB-BIO; OriGene Technologies, Inc.) for 30 min
at 37°C. Sections were then rinsed again in PBS and antibody
binding was developed using a DAB chromogenic kit (Beyotime
Institute of Biotechnology). Sections were then counterstained with
hematoxylin at room temperature for 90 sec. A light microscope
(original magnification, ×100; Nikon Corporation) was used to score
the results of immunostaining, by multiplying the score of staining
intensity of positive cells and the score of the percentage of
positive cells. The final score is the degree of staining
multiplied by the intensity.
Statistical analysis
Quantitative data are presented as the mean ± SEM.
One-way ANOVA analysis and paired Student's t-test were used to
assess significance among groups. Following one-way ANOVA, Tukey's
post hoc test was performed. SPSS software (v19.0; SPSS, Inc.) was
used to process all statistical analyses. A receiver operating
characteristic (ROC) curve was generated to evaluate its diagnostic
value and following calculation of a binomial exact-confidence
interval to determine the area under the ROC curve (AUC). The
associations between hsa_circRNA_100876 expression and prognosis of
BC patients were evaluated using Kaplan-Meier survival analysis and
log-rank tests. All tests were performed in triplicate. A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Overview of circRNA expression in
paired bladder tissues
We selected three pairs of primary BC and ANNB
tissues with similar clinicopathologic features to perform circRNA
microarray for screening dysregulated circRNAs in BC. With a
threshold of FC ≥2.0 and P<0.05, 512 differentially expressed
circRNAs were identified following scanning and normalization, with
340 circRNAs significantly upregulated and 172 significantly
downregulated. As revealed in Table
SII, the top 10 regulated circRNAs were presented, along with
other detailed information on P-value, FDR, strand, chromosome,
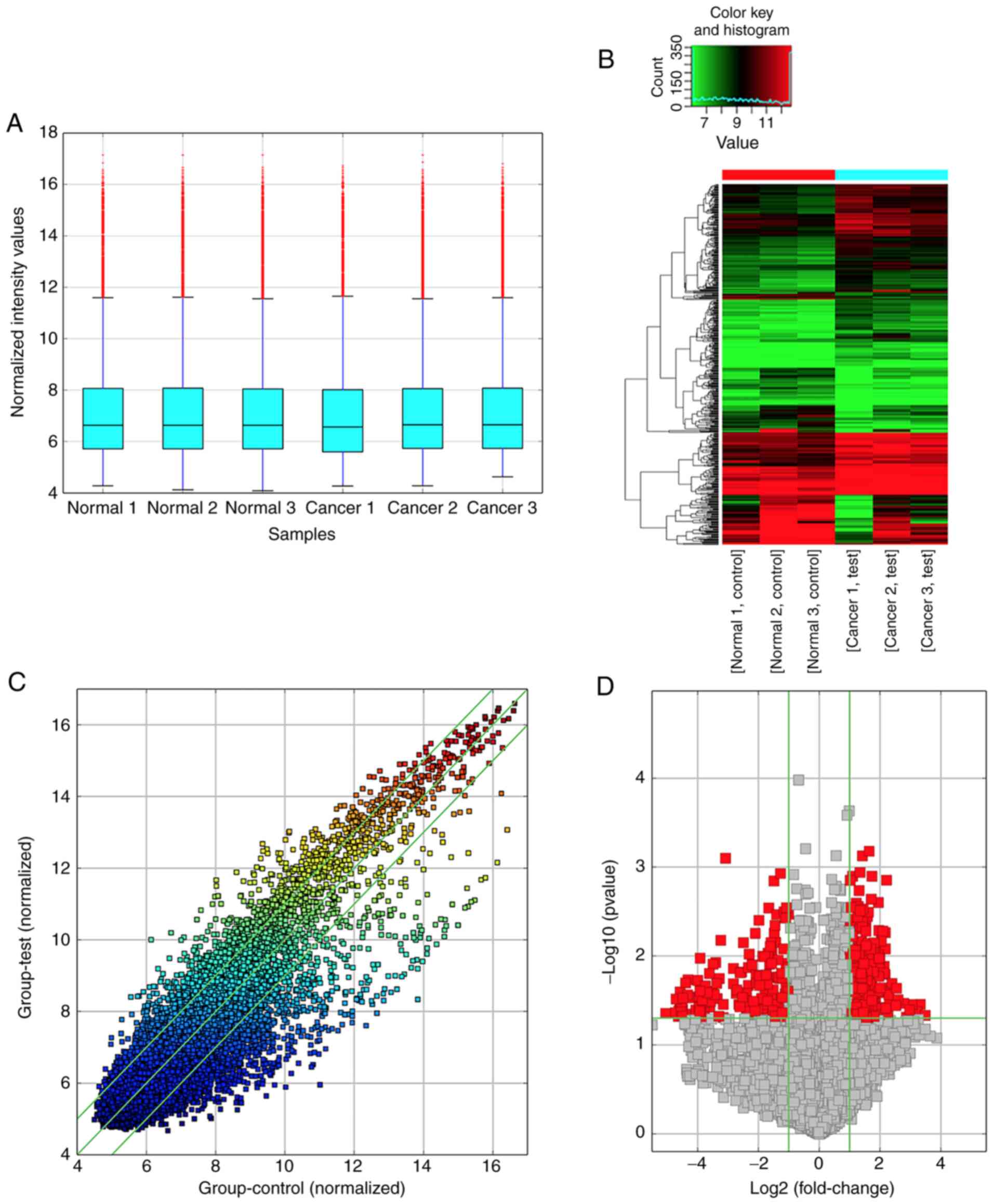
circRNA type and gene symbols. The box plot indicated that the
circRNA distribution among the six samples was nearly identical
after normalization (Fig. 1A), and
hierarchical clustering revealed clear variations in the expression
profiles between BC and ANNB tissues (Fig. 1B). Differentially expressed circRNAs
among samples were assessed by scatter-plot visualization (Fig. 1C) which revealed the variation of
circRNA expression between BC tissues and control groups, and
volcano map analysis (Fig. 1D)
which was applied to visualize differentially expressed
circRNAs.
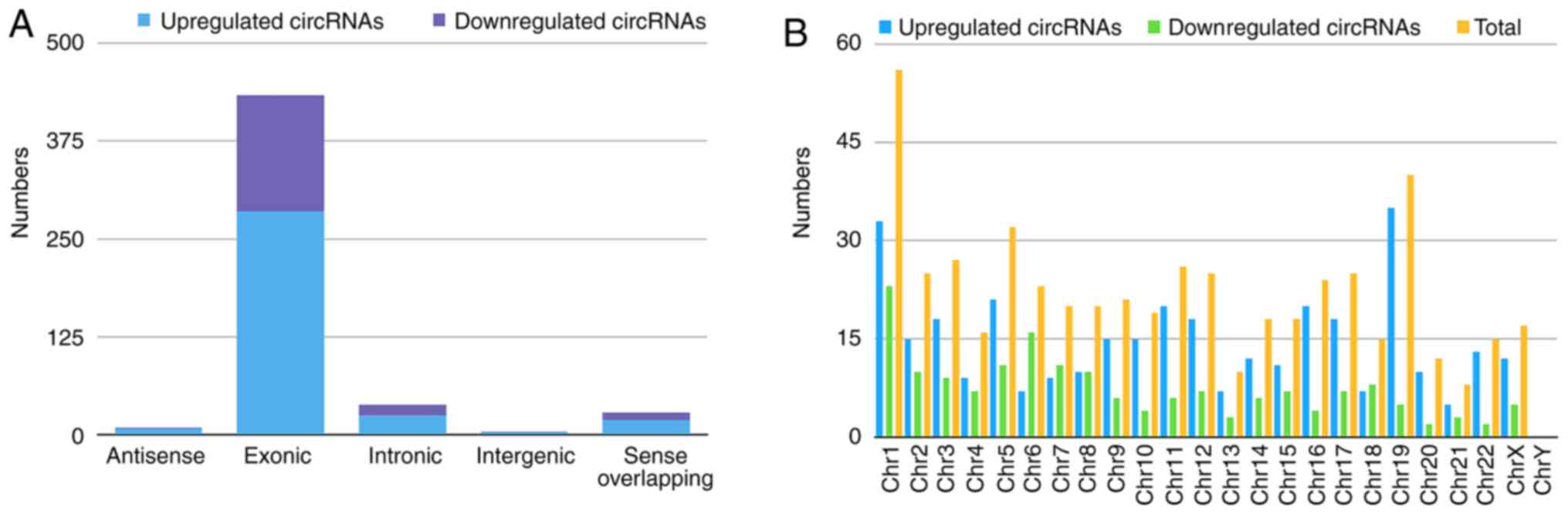
Classification of the dysregulated circRNAs is
presented in the bar diagram (Fig.
2A). The results identified 340 upregulated circRNAs comprising
285 exonic, 25 intronic, 19 sense-overlapping, eight antisense, and
three intergenic regions, whereas the 172 downregulated circRNAs
contained 148 exonic, 13 intronic, nine sense-overlapping, one
intergenic, and one antisense in BC-tissue samples. Chromosomal
distribution analysis indicated that most were located on
chromosome 1, while few located on chromosome 21 and the Y
chromosome (Fig. 2B).
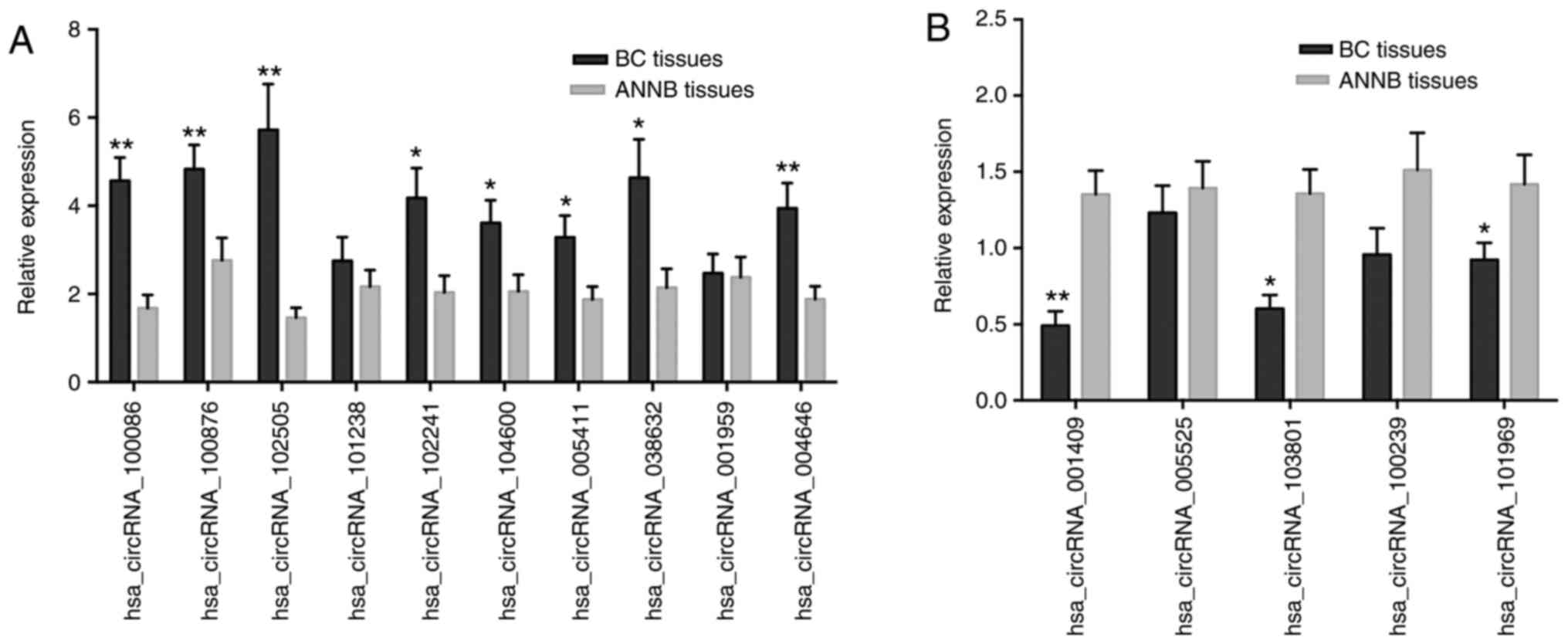
RT-qPCR validation of selected
identified dysregulated circRNAs
Fifteen circRNAs (ten upregulated and five
downregulated) were randomly selected from the microarray for
further verification by RT-qPCR analysis from 40 sets of BC and
ANNB tissues. Eleven of the 15 circRNAs were verified as
significantly differentially expressed in cancer tissues, with
eight upregulated and three downregulated (Fig. 3A and B). The results revealed
upregulation of hsa_circRNA_100086, _038632, _100876, _102241,
_004646, _102505, _104600, and _005411 expression in BC tissues
relative to their levels in ANNB tissues, whereas
hsa_circRNA_001409, _101969, and _103801 were downregulated. These
findings were consistent with microarray results.
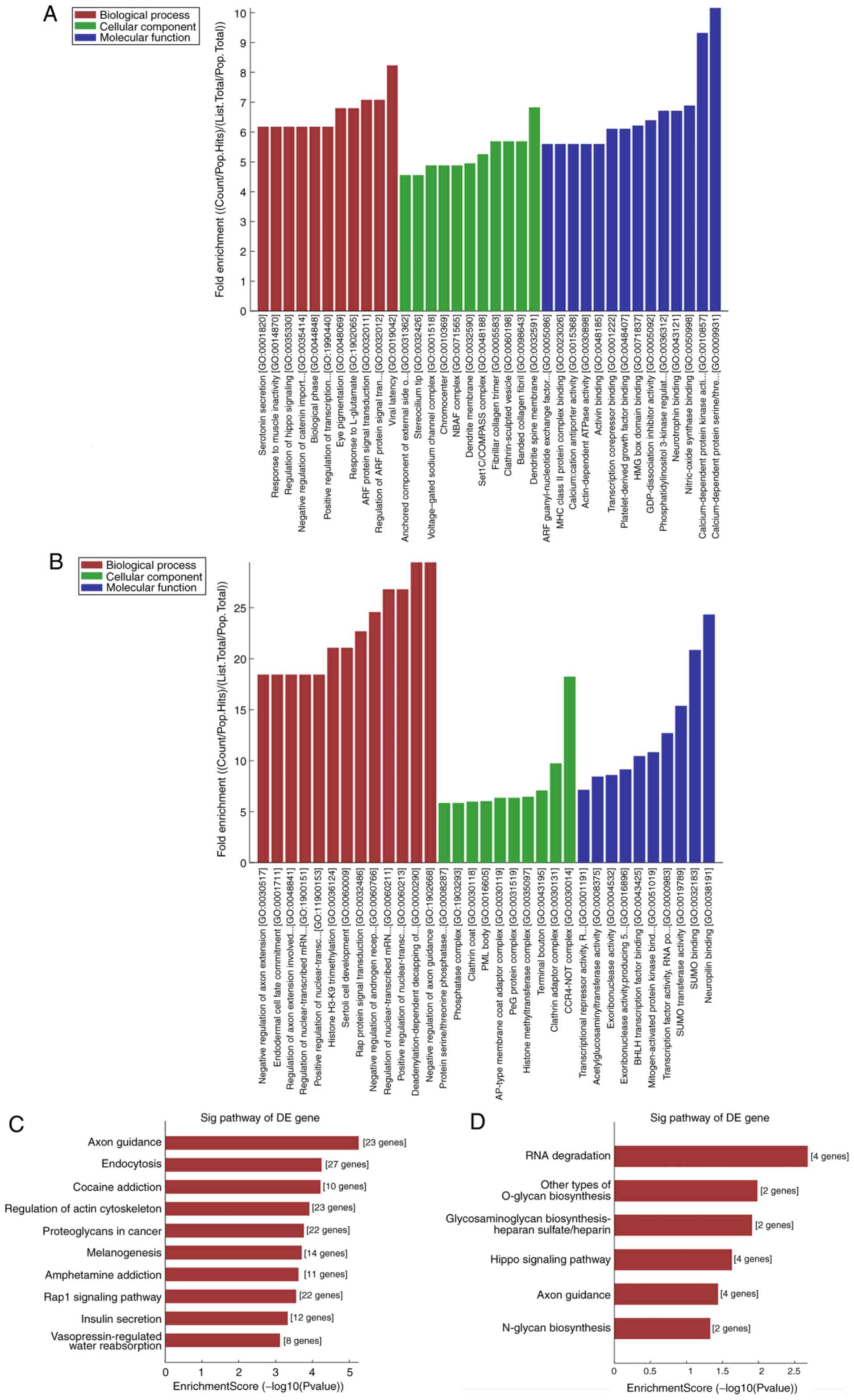
GO and KEGG pathway analyses of the
differentially expressed circRNAs
To investigate how circRNAs regulate the expression
of a target gene, nine circRNAs (7 of the nine circRNAs were
upregulated and 2 were downregulated) were randomly selected from
the validated circRNAs for GO and KEGG pathway analyses. GO
analysis annotated genes targeted by the nine differentially
expressed circRNAs in domains of molecular functions, biological
processes and cellular components. KEGG pathway analysis indicated
that several pathways involved in cancer were related to the
dysregulated circRNAs. The results were ranked by fold enrichment
[(Count/Pop.Hits)/ (List.Total/ Pop.Total)] and revealed enrichment
of multiple critical biological functions associated with BC
progression (results for hsa_circRNA_100876 and _038632 are
presented in Fig. 4A-D). In
addition, the supplementary results of GO and pathway analyses are
presented in the Figs. S1–S7, identifying multiple items associated
with cancer progression. For example, hsa_circRNA_102241 was
related to 51 pathways, of which the top 1 pathway was related with
‘proteoglycans in cancer’. As is known, some proteoglycans have
pro- and anti-angiogenic activities, whereas other proteoglycans
can also directly affect cancer growth by modulating key signaling
pathways.
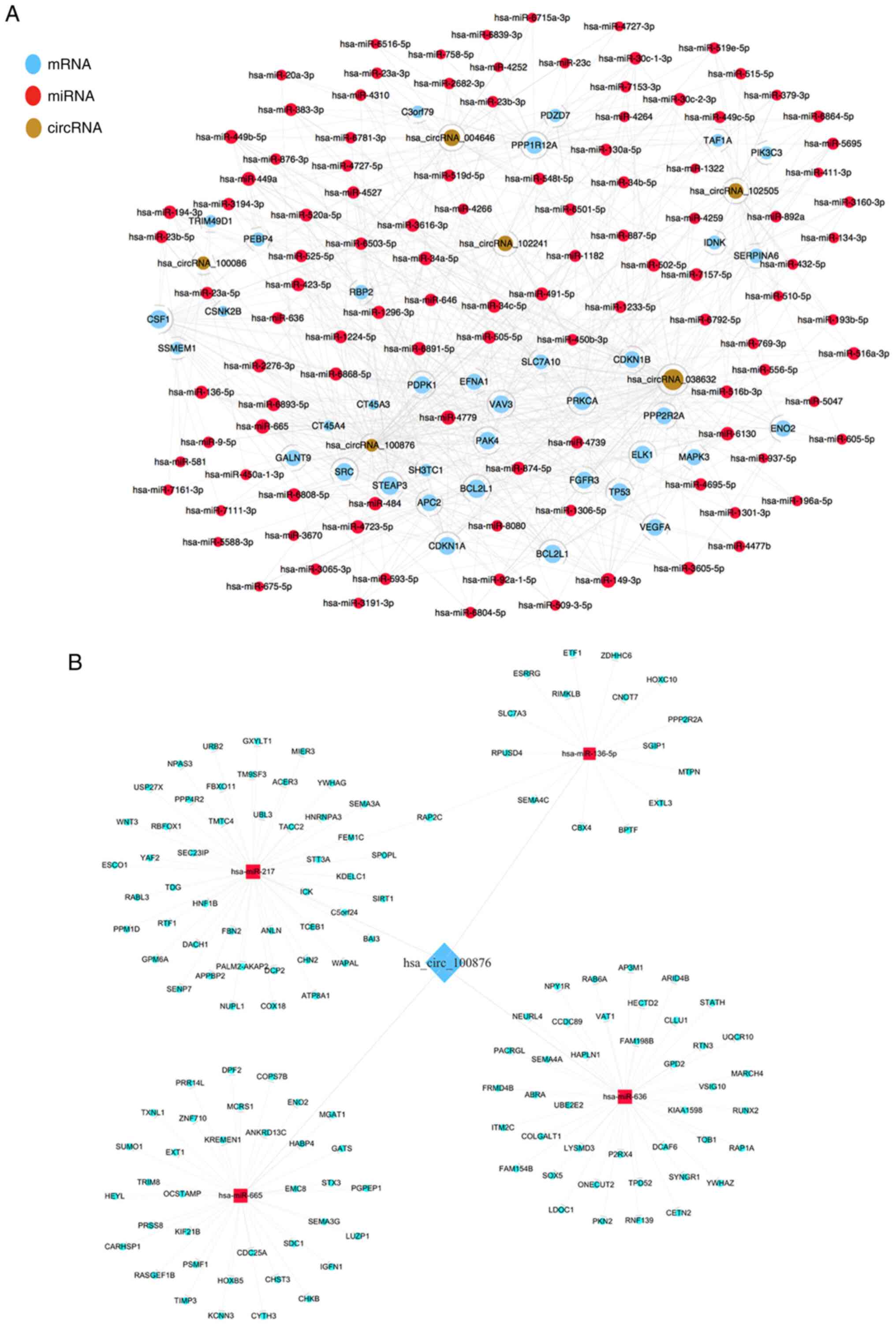
Construction an interaction network of
ceRNA
To visualize a circRNA-regulatory network,
interactions between the six confirmed differentially expressed
circRNAs and their association with miRNAs and target genes were
predicted, followed by construction of a unified
interaction-network model. The co-expression pattern of
circRNA-miRNA-mRNA is presented in Fig.
5A. These results indicated potential roles for the identified
circRNAs as endogenous RNAs capable of altering target gene
expression. In addition, a ceRNA network of
hsa_circRNA_100876-miRNA-mRNA is presented in Fig. 5B. A total of 4 miRNAs (miR-136-5p,
miR-217, miR-665 and miR-636) and corresponding target mRNAs were
predicted to have an interaction with hsa_circRNA_100876 in the
present study. For each of the four identified interacting miRNAs,
the intersection of predictions from TargetScan and miRanda were
used to find their targeting protein coding genes. A total of 140
miRNA-regulating protein coding genes were identified for
hsa_circRNA_100876. It was revealed that hsa_circRNA_100876 may be
highly correlated and co-expressed with mRNAs. This provides us
with another research strategy to explore the mechanism of
hsa_circRNA_100876 by identifying its associated mRNAs and
investigating whether it can play a role by regulating the
expression of certain associated mRNAs.
Clinicopathologic assessment of
hsa_circRNA_100876 expression in BC patients
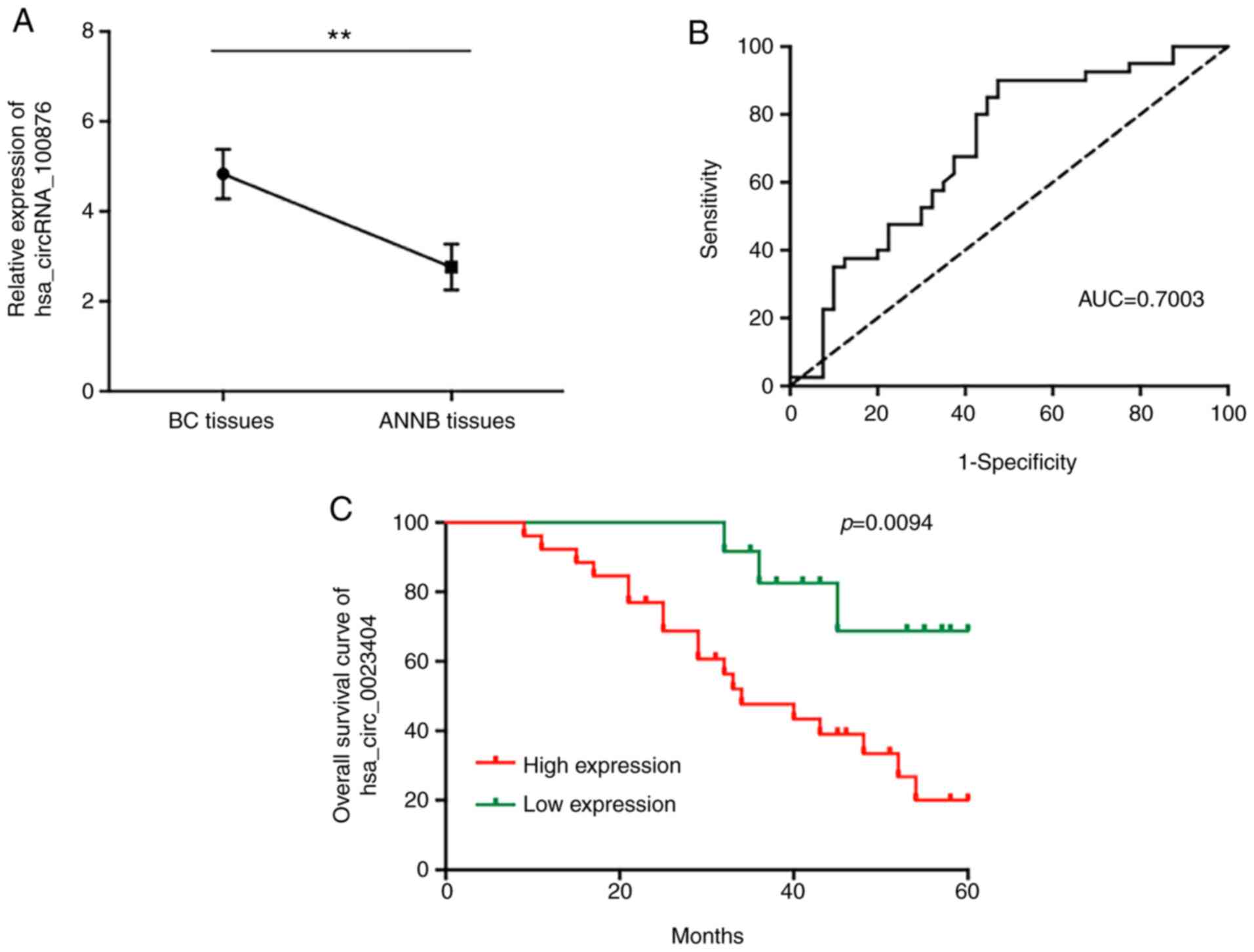
To determine possible roles of the dysregulated
circRNAs in BC carcinogenesis, hsa_circRNA_100876 was selected for
further study. The results revealed that hsa_circRNA_100876 was
located on chromosome 11 (71668272–71671937) and differentially
overexpressed in BC tissues relative to ANNB tissues (Fig. 6A). ROC curve analysis revealed an
AUC value of 0.7003 (Fig. 6B), with
a specificity of 52.5% and sensitivity of 90%, suggesting the
potential diagnostic efficacy of hsa_circRNA_100876 as a BC
biomarker.
To confirm the clinical value of these molecular
differences, analyses were performed to evaluate associations
between hsa_circRNA_100876 expression and clinicopathologic
features. As revealed in Table I,
increased levels of hsa_circRNA_100876 were significantly
associated with T stage and lymphatic metastasis in BC patients
(P<0.05), although the expression level was not related to sex,
age, histological grade, or recurrence. Furthermore, univariate
analysis indicated that the hsa_circRNA_100876 expression, T stage,
and lymphatic metastasis were significantly related to the overall
survival (OS) of patients (P<0.05), and multivariate analysis
revealed hsa_circRNA_100876 expression and lymphatic metastasis as
independent factors affecting BC patient prognosis (Table II). Moreover, Kaplan-Meier survival
analysis used to estimate the association between the
hsa_circRNA_100876 patient prognosis post-surgery indicated that
increased hsa_circRNA_100876 expression was correlated with
markedly shorter OS duration (Fig.
6C).
 | Table I.Relationship between the expression
levels of hsa_circRNA_100876 and clinicopathological features in
bladder cancer patients. |
Table I.
Relationship between the expression
levels of hsa_circRNA_100876 and clinicopathological features in
bladder cancer patients.
| Features | Group | Case | High
expression | Low expression | P-value |
|---|
| Age (years) | ≤60 | 14 | 8 | 6 | 0.336 |
|
| >60 | 26 | 18 | 8 |
|
| Sex | Male | 29 | 20 | 9 | 0.311 |
|
| Female | 11 | 6 | 5 |
|
| Histological
grade | Low grade | 13 | 8 | 5 | 0.509 |
|
| High grade | 27 | 18 | 9 |
|
| T Stage | T1-T2 | 11 | 4 | 7 | 0.026a |
|
| T3-T4 | 29 | 22 | 7 |
|
| Recurrence | Yes | 12 | 8 | 4 | 0.591 |
|
| No | 28 | 18 | 10 |
|
| Lymphatic
metastasis | Yes | 15 | 13 | 2 | 0.027a |
|
| No | 25 | 13 | 12 |
|
 | Table II.Univariate and multivariate analysis
for overall survival. |
Table II.
Univariate and multivariate analysis
for overall survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Parameters | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| T stage | 2.730 | 1.173–6.524 | 0.029a | 3.218 | 1.076–9.625 | 0.037a |
| Lymphatic
metastasis | 2.291 | 1.023–6.820 | 0.048a | 2.231 | 0.921–5.406 | 0.076 |
| Hsa_circRNA_100876
expression | 3.193 | 1.329–7.669 | 0.009b | 3.704 | 1.076–12.753 | 0.038a |
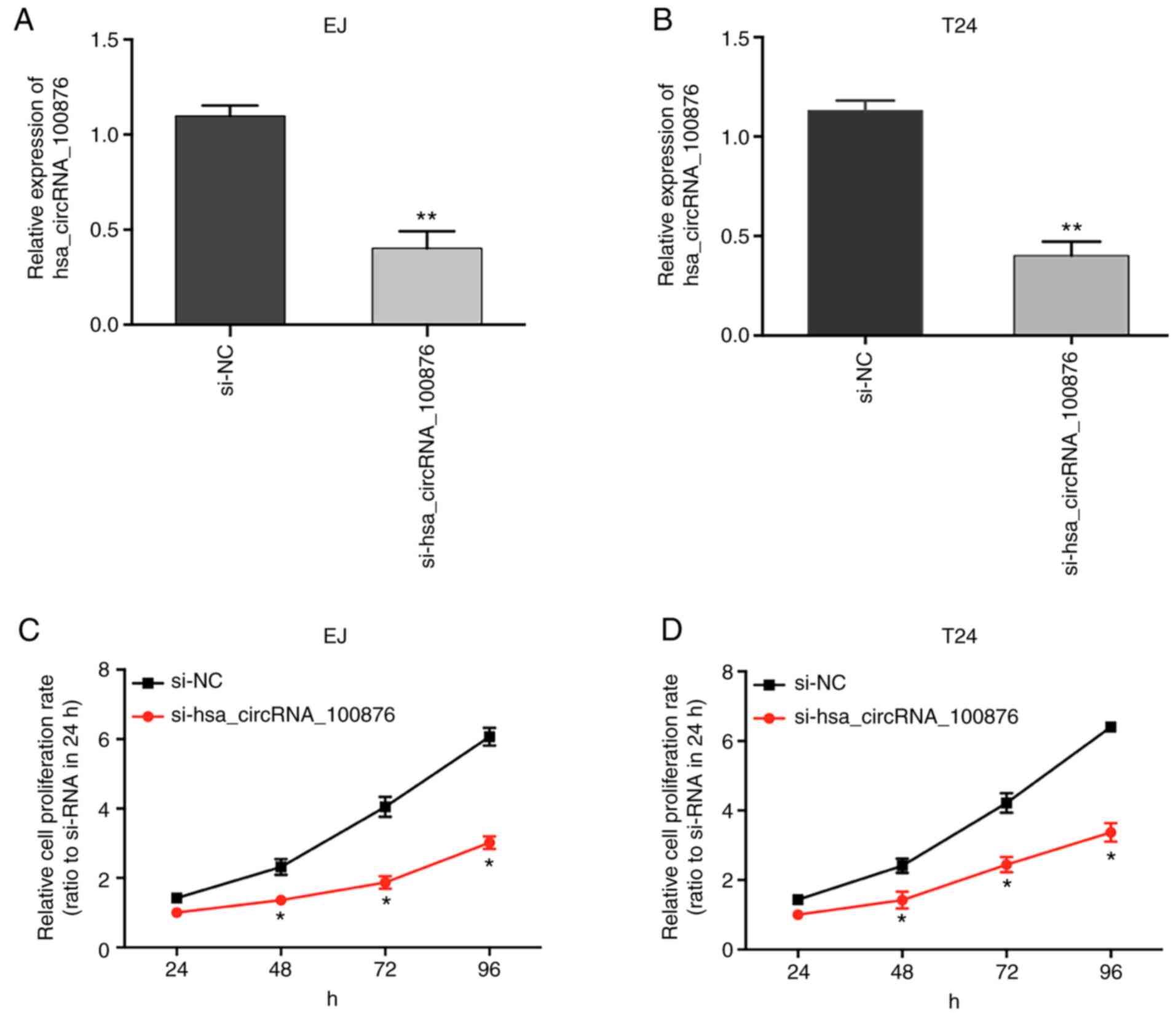
Hsa_circRNA_100876 inhibition
suppresses BC cell proliferation
To confirm the role of hsa_circRNA_100876 in cell
proliferation, specific siRNA targeting hsa_circRNA_100876 were
transfected into BC cells (EJ and T24), resulting in a significant
decrease in hsa_circRNA_100876 expression (Fig. 7A and B; P<0.05). The results of
CCK-8 assays revealed that, compared to the si-NC group, the
proliferation abilities of EJ and T24 cells transfected with
si-hsa_circRNA_100876 were significantly reduced (Fig. 7C and D; P<0.05), revealing that
knockdown of hsa_circRNA_100876 suppressed proliferation of BC
cells.
Overexpression of hsa_circRNA_100876
downregulates miR-136-5p and upregulates CBX4 levels
We constructed a network of circRNA-miRNA-mRNA
interactions to visualize potential relationships based on circRNA
microarray data. Prediction of miRNA targets revealed that
miR-136-5p shared binding sites with hsa_circRNA_100876 (Fig. 8A), and the binding sites were
further validated by luciferase reporter assay (Fig. 8C). Furthermore, the same
complementary binding sites were revealed between miR-136-5p and
CBX4 (Fig. 8A), which was further
validated through luciferase reporter assay (Fig. 8E). Moreover, RT-qPCR analysis
confirmed significant downregulation of miR-136-5p (Fig. 8B) in BC tissues relative to ANNB
tissues, along with its negative correlation with
hsa_circRNA_100876 expression (r=−0.7842, P<0.001) (Fig. 8F). Furthermore, it was observed that
CBX4 levels were aberrantly upregulated in BC tissues (Fig. 8D), revealing a possible positive
correlation with hsa_circRNA_100876 expression and a negative
correlation with miR-136-5p expression (r=0.7619 and −0.6404,
P<0.001, respectively) (Fig. 8G and
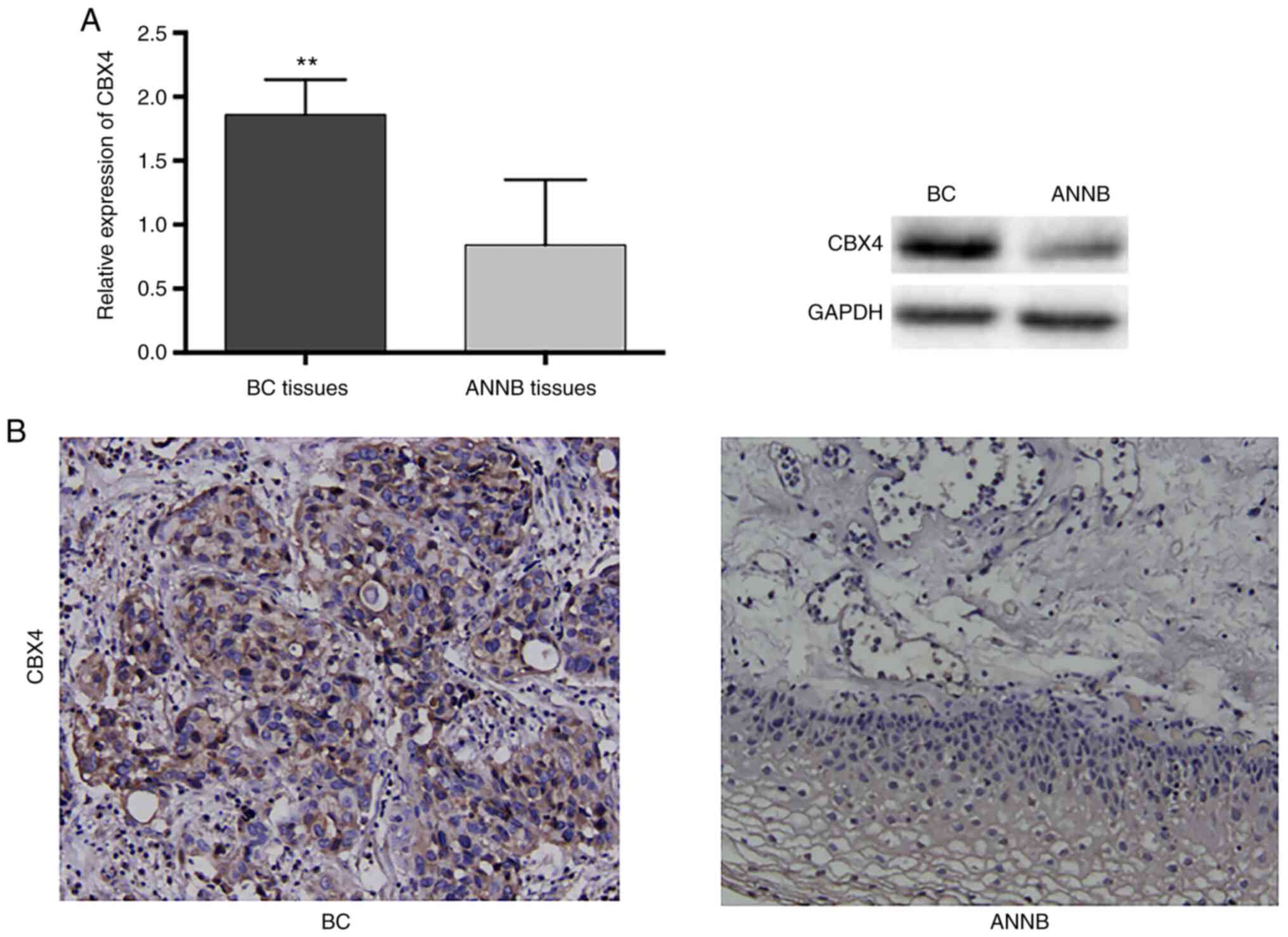
H). Western blot analysis indicated increased CBX4 levels in BC
tissues as compared with ANNB tissues, which was consistent with
the RNA levels (Fig. 9A).
Additionally, immunohistochemical staining results confirmed the
increased CBX4 levels in BC tissues and relatively low levels in
ANNB tissues (Fig. 9B).
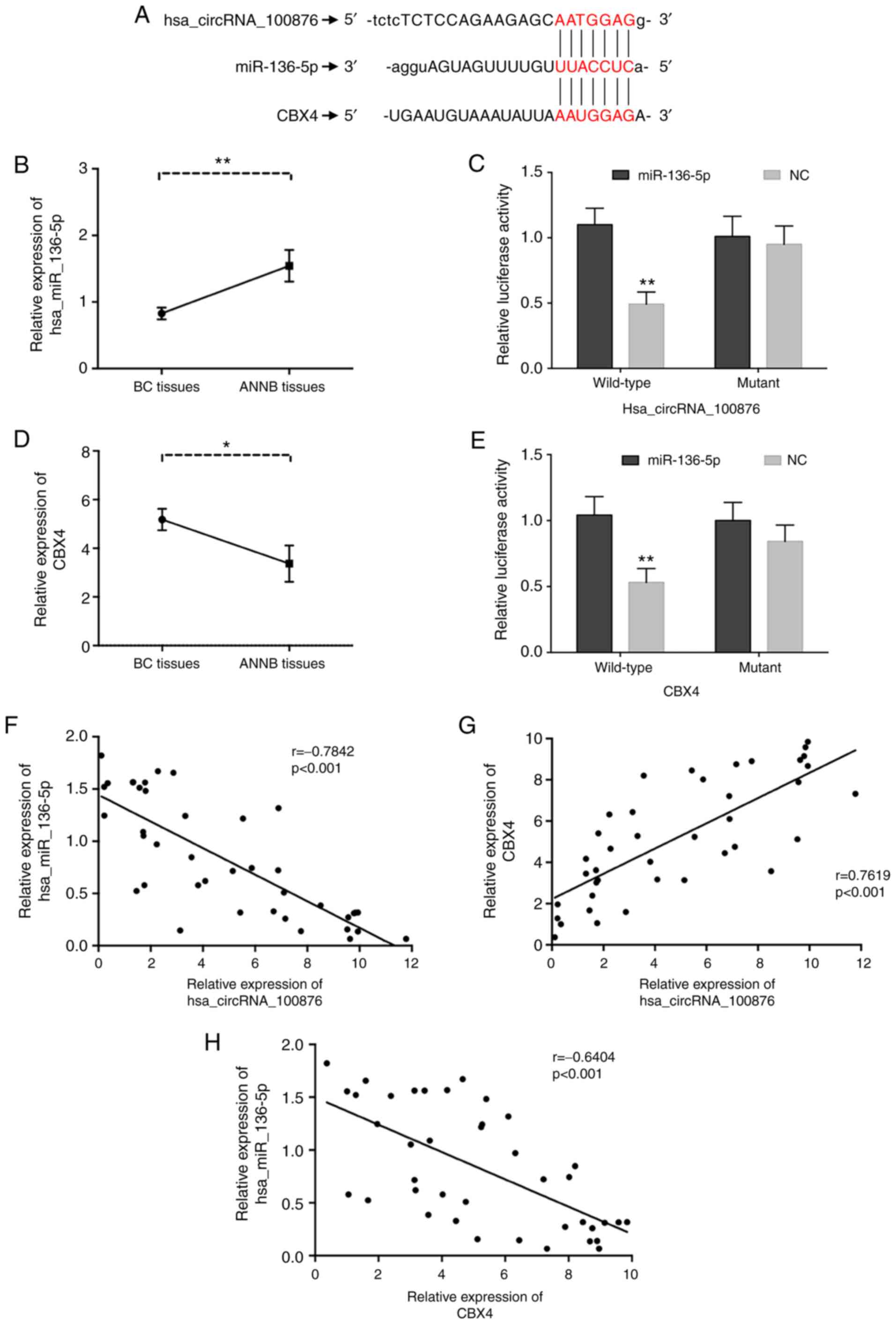 | Figure 8.Correlations of hsa_circRNA_100876,
miR-136-5p and CBX4. (A) The identical putative binding sites of
miR-136-5p on the hsa_circRNA_100876 and CBX4. (B) The relative
expression of miR-136-5p was validated in 40 pairs of BC tissues
and ANNB tissues by RT-qPCR. (C) A luciferase reporter assay
validated the binding of has_ circRNA_100876 and miR-136-5p. (D)
The relative expression of CBX4 was validated in 40 pairs of BC
tissues and ANNB tissues by RT-qPCR. (E) A luciferase reporter
assay validated the binding of miR-136-5p and CBX4. (F)
Hsa_circRNA_100876 expression was negatively correlated with
miR-136-5p expression in BC tissues (r=−0.7842, P<0.001). (G)
Hsa_circRNA_100876 expression was positively correlated with CBX4
expression in BC tissues (r=0.7619, P<0.001). (H) CBX4
expression was negatively correlated with miR-136-5p expression in
BC tissues (r=−0.6404, P<0.001). CBX4, chromobox 4; BC, bladder
cancer; ANNB tissues, adjacent non-neoplastic bladder tissues;
RT-qPCR, reverse transcription-quantitative PCR; NC, negative
control. *P<0.05, **P<0.01. |
Discussion
The molecular mechanisms associated with BC
progression continue to be the focus of laboratory and clinical
investigations, with recent findings identifying candidate miRNAs
and lncRNAs involved in BC (24–26).
As a new member of ncRNAs, circRNAs have attracted much attention
for their involvement with disease and miRNA regulation (27,28).
CircRNAs are abundant, conserved, stable, and cell-type specific
molecules involved in regulating cell function (29). Roles for circRNAs in diseases, such
as diabetes (30) and those
affecting the nervous (31) and
cardiovascular systems (32), and
tumorigenesis have received increased attention. For instance, a
recent study has reported that hsa-circ-0000190 may be considered
as a potential diagnostic biomarker for gastric cancer according to
its downregulation in cancer tissues (33). Circ-ITCH was reported to be
overexpressed in patients with lung cancer (34), and hsa_circRNA_100876 was associated
with lymph node metastasis and stage of lung cancer (35). Furthermore, six circRNAs were
identified to play important roles in carcinogenesis as ceRNA for
the regulation of the miRNA-mRNA network in colorectal cancer
(36). These findings indicate the
key roles of circRNAs as biomarkers in early diagnosis and
prognosis of cancers, as well as potential therapeutic targets.
Furthermore, circRNAs have been identified in urologic neoplasms
(37); however, their roles in BC
remain largely unknown.
To investigate the possible effects of circRNAs on
BC, 512 differentially expressed circRNAs from paired BC and ANNB
tissues were identified. RT-qPCR analysis of the dysregulated
circRNAs confirmed that 11 of the results were statistically
significant (P<0.05) and consistent with microarray results.
Reasons for the verification inconformity results compared with the
chip are: Firstly, the microarray chip technology itself has a
certain false positive rate, and the high cost of screening chips
leads to a limited number of cases for screening of circRNAs.
Additionally, different results may occur due to the inherent
differences among patients, such as the degree of pathological
differentiation and cancer stage and grade. Moreover, differences
in the standardization process between the microarray and RT-qPCR
could also lead to variations in the results.
The potential function of the differentially
expressed circRNAs was elucidated by GO and KEGG analyses. The
results indicated their relationship with the aberrant expression
of several mRNAs, as well as biological pathways alterations,
suggesting their potential roles in BC. GO analysis indicated that
hsa_circRNA_038632 was associated with ADP-ribosylation factors
(ARFs), which was associated with p53 degradation and
downregulation of p53 transcription. Certain oncogenes and
regulatory factors can regulate p53 function through ARFs, leading
to several cancers (38–41). Additionally, studies have reported
dysregulated ARF levels in BC along with abnormal promoter
methylation involved in BC development, suggesting it as a
potential prognostic marker of BC (42,43).
Moreover, GO analysis revealed that an hsa_circRNA_100876 target
gene was involved in Ras-related protein (RAP) signal transduction
and the CC chemokine receptor 4-negative regulator of transcription
(CCR4-NOT) complex. RAP plays an important part in the
mitogen-activated protein kinase (MAPK) pathway, which acts as a
central role in tumorigenesis by promoting cancer development and
progression, including that of BC (44). Additionally, the CCR4-NOT complex
plays extensive roles in gene regulation, such as transcription
regulation, mRNA attenuation and quality control, translation
inhibition, and protein ubiquitination (45). Therefore, these differentially
expressed circRNAs may exert their biological functions by
affecting the expression of target genes associated with pathways
involved in promoting BC development. KEGG analysis identified
multiple pathways associated with cancer progression. The results
revealed that hsa_circRNA_038632 was related to 61 pathways, 28 of
which are associated with cancers, including classic cancer
pathways involving p53, Wnt, and Ras signaling pathway. Among the
identified pathways related to malignancy, we discovered a
BC-specific pathway, with the corresponding mRNAs identified as
playing key roles in tumor aggressiveness (46,47).
Moreover, six pathways related to hsa_circRNA_100876 included four
malignancy-related pathways and the Hippo-signaling pathway was
recently confirmed as being involved in BC development (48). These findings suggested that the
identified circRNAs may affect the expression of target genes
associated with different BC-related pathways directly or
indirectly.
A previous study reported that circRNAs are enriched
with miRNA response elements (MREs), which could be regarded as
miRNA sponges to adsorb miRNA and eliminate their inhibitory
effects on target mRNA, thereby upregulating protein translation as
an important aspect of posttranscriptional regulation (13). The mechanisms associated with
circRNA functions as miRNA sponges related to BC have been reported
in several studies. Zhong et al (49) reported that circRNA MYLK reduced
miR-29a activity through sponge adsorption to advance the
progression of BC by measuring the vascular endothelial growth
factor (VEGF)/VEGF receptor-2 signaling pathway. Different from
traditional linear miRNA sponges with only one MRE, circRNA contain
several MREs. In a previous study, Li et al (50) reported that circ-ITCH competitively
adsorbed miR-17, miR-214 and miR-7, leading to an increased
expression of circ-ITCH and degraded phosphorylated Dvl2, thereby
hindering esophageal cancer tumor growth. Given that a single
circRNA could regulate numerous downstream genes via a common miRNA
target, our results suggested that these circRNAs may potentially
act as drivers of cancer through highly enriched signaling
pathways. CircRNAs may represent novel biomarkers for tumor
diagnosis and prognosis, as well as miRNA inhibitors involved in
antitumor therapy.
Identification of miRNA-binding sites via circRNA
microarray analysis allows investigation of their indirect effects
on several functional mRNAs, resulting in possible identification
of circRNA function(s). The microarray analysis identified five
miRNA-binding sites in circRNAs, and we anticipate that the
possible BC-specific biological function and mechanism of circRNAs
could be identified through these miRNAs or target mRNAs that share
the same MREs with these miRNAs. It was observed that as a tumor
suppressor, miRNA-136-5p played an important role in tumor
progression related to kidney (51), lung (52), and breast cancer (53). A recent study concerning miRNA
screening for BC revealed that miRNA-136-5p was among the top 10
downregulated miRNAs, but without large sample validation (54). In the present study, RT-qPCR
analysis of 40 paired tissue samples confirmed the downregulation
of miR-136-5p in BC, with the findings consistent with the
microarray results. Bioinformatics analysis subsequently identified
miR-136-5p-specific MREs present in both hsa_circRNA_100876 and
mRNA-CBX4, which was validated by luciferase assay. Therefore, it
was surmised that there may be important regulatory relationships
between hsa_circRNA_100876, miR-136-5p, and CBX4 resulting in
alteration of BC-related activity.
This study, to the best of our knowledge, is the
first study reporting significant upregulation of
hsa_circRNA_100876 levels in BC tissues. Additionally, it revealed
that upregulated expression of hsa_circRNA_100876 was positively
correlated with lymph node metastasis, T stage and significantly
shorter OS, suggesting that hsa_circRNA_100876 may play a critical
role in biological functions associated with the occurrence and
development of BC.
CBX proteins are members of the PcG family of
proteins, which participate in the malignant progression of
numerous human cancers. CBX4 is a unique protein with enzymatic
activity in the CBX family, and can act as small ubiquitin-like
modifier (SUMO) E3 ligase in SUMO modification, which plays a
significant role in regulation of transcription, DNA repair
structure, and alteration of chromatin (55). Abnormal SUMO modifications are
related to numerous diseases, including cancers (56). CBX4 regulates the levels of various
proteins via SUMO E3 activity, and its expression in malignant
tumors has become a research hotspot (57). Li et al (58) reported that CBX4 can upregulate
hypoxia-induced VEGF expression via SUMO modification of two lysine
sites in hypoxia-inducible-1a, thereby promoting angiogenesis in
hepatocellular carcinoma. In breast cancer, it was demonstrated
that CBX4 could reduce the cell proliferation induced by estrogen
via inhibiting estrogen-signal transduction by SUMO modification of
zinc finger protein 131 (59). In
the present study, GO analysis revealed that hsa_circRNA_100876 was
enriched in association with PcG complex, SUMO transfer activity,
and SUMO binding, with the corresponding genes universally
including CBX4. The results further confirmed the biological role
of CBX4 and revealed a potential relationship between
hsa_circRNA_100876 and CBX4. The subsequent correlation analysis
revealed a negative correlation between hsa_circRNA_100876 and CBX4
expression and that of miR-136-5p. Additionally, hsa_circRNA_100876
and CBX4 expression was positively correlated. These results
suggested that hsa_circRNA_100876 may act as sponge for miR-136-5p,
resulting in increased in CBX4 levels, thereby altering BC
progression.
Currently, the molecular mechanisms associated with
circRNAs in BC remain unclear. The present study demonstrated that
numerous circRNAs were dysregulated and these aberrantly expressed
circRNAs may serve key functions to the occurrence and development
of BC. The results provided novel insight into the complex
biological functions of circRNAs in the process of BC
carcinogenesis and circRNAs could serve as potential biomarkers for
BC. Furthermore, this is the first study reporting overexpression
of hsa_circRNA_100876 in BC and the potential role of CBX4 in BC.
The relationship between hsa_circRNA_100876, miR-136-5p and CBX4
may be a crucial molecular mechanism related to BC. A future study
will focus on elucidating the regulatory relationship between
hsa_circRNA_100876 and CBX4 in association with BC.
Supplementary Material
Supporting Data
Acknowledgements
The authors want to thank the Experimental Center of
Shengjing Hospital (Shenyang, China) for technical support.
Funding
The present study was supported by grants from the
Natural Science Foundation of Liaoning Province of China (grant no.
20170540988) and the Shenyang Science and Technology Program (grant
no. 17-231-1-57).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL contributed to the study design, data collection
and interpretation, and writing of the manuscript. SL and YZ
analyzed and interpreted the data and prepared the figures. XC and
SL designed the study. YZ and XC contributed to the revision and
editing of the manuscript and the submission of the manuscript for
publication. All authors reviewed and approved the final
manuscript
Ethics approval and consent to
participate
The use of clinical data was approved by the
Research Ethics Committee of Shengjing Hospital of China Medical
University (Shenyang, China). Informed consent was provided by all
subjects for inclusion in the study, and the research plan was
approved by the Research Ethics Committee of Shengjing Hospital of
China Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar
|
|
4
|
Kim WT, Kim YH, Jeong P, Seo SP, Kang HW,
Kim YJ, Yun SJ, Lee SC, Moon SK, Choi Y, et al: Urinary cell-free
nucleic acid IQGAP3: A new non-invasive diagnostic marker for
bladder cancer. Oncotarget. 9:14354–14365. 2018. View Article : Google Scholar
|
|
5
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar
|
|
6
|
Li PF, Chen SC, Xia T, Jiang XM, Shao YF,
Xiao BX and Guo JM: Non-coding RNAs and gastric cancer. World J
Gastroenterol. 20:5411–5419. 2014. View Article : Google Scholar
|
|
7
|
Qu S, Yang X, Li X, Wang J, Gao Y, Shang
R, Sun W, Dou K and Li H: Circular RNA: A new star of noncoding
RNAs. Cancer Lett. 365:141–148. 2015. View Article : Google Scholar
|
|
8
|
Greene J, Baird AM, Brady L, Lim M, Gray
SG, McDermott R and Finn SP: Circular RNAs: Biogenesis, Function
and Role in Human Diseases. Front Mol Biosci. 4:382017. View Article : Google Scholar
|
|
9
|
Chen LL and Yang L: Regulation of circRNA
biogenesis. RNA Biol. 12:381–388. 2015. View Article : Google Scholar
|
|
10
|
Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL
and Yang L: Complementary sequence-mediated exon circularization.
Cell. 159:134–147. 2014. View Article : Google Scholar
|
|
11
|
Cocquerelle C, Mascrez B, Hétuin D and
Bailleul B: Mis-splicing yields circular RNA molecules. FASEB J.
7:155–160. 1993. View Article : Google Scholar
|
|
12
|
Salzman J, Chen RE, Olsen MN, Wang PL and
Brown PO: Cell-type specific features of circular RNA expression.
PLoS Genet. 9:e10037772013. View Article : Google Scholar
|
|
13
|
Vidal AF, Sandoval GT, Magalhaes L, Santos
SE and Ribeiro-dos-Santos A: Circular RNAs as a new field in gene
regulation and their implications in translational research.
Epigenomics. 8:551–562. 2016. View
Article : Google Scholar
|
|
14
|
Xu S, Zhou L, Ponnusamy M, Zhang L, Dong
Y, Zhang Y, Wang Q, Liu J and Wang K: A comprehensive review of
circRNA: From purification and identification to disease marker
potential. PeerJ. 6:e55032018. View Article : Google Scholar
|
|
15
|
Zhang X, Zhou H, Jing W, Luo P, Qiu S, Liu
X, Zhu M, Liang C, Yu M and Tu J: The circular RNA hsa_circ_0001445
regulates the proliferation and migration of hepatocellular
carcinoma and may serve as a diagnostic biomarker. Dis Markers.
2018:30734672018. View Article : Google Scholar
|
|
16
|
Jiang MM, Mai ZT, Wan SZ, Chi YM, Zhang X,
Sun BH and Di QG: Microarray profiles reveal that circular RNA
hsa_circ_0007385 functions as an oncogene in non-small cell lung
cancer tumorigenesis. J Cancer Res Clin Oncol. 144:667–674. 2018.
View Article : Google Scholar
|
|
17
|
Zhong Z, Lv M and Chen J: Screening
differential circular RNA expression profiles reveals the
regulatory role of circTCF25-miR-103a-3p/miR-107-CDK6 pathway in
bladder carcinoma. Sci Rep. 6:309192016. View Article : Google Scholar
|
|
18
|
Cai D, Liu Z and Kong G: Molecular and
bioinformatics analyses identify 7 circular RNAs involved in
regulation of oncogenic transformation and cell proliferation in
human bladder cancer. Med Sci Monit. 24:1654–1661. 2018. View Article : Google Scholar
|
|
19
|
Enright AJ, John B, Gaul U, Tuschl T,
Sander C and Marks DS: MicroRNA targets in Drosophila. Genome Biol.
5:R12003. View Article : Google Scholar
|
|
20
|
Pasquinelli AE: MicroRNAs and their
targets: Recognition, regulation and an emerging reciprocal
relationship. Nat Rev Genet. 13:271–282. 2012. View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. Nat
Genet. 25:25–29. 2000. View
Article : Google Scholar
|
|
23
|
Gerlich M and Neumann S: KEGG: Kyoto
encyclopedia of genes and genomes. Nuclc Acids Res. 28:27–30. 2000.
View Article : Google Scholar
|
|
24
|
Shi Z, Kadeer A, Wang M, Wen B, Li M,
Huang J, Gao Y, Liu E, Liu D, Jia D and Liang C: The deregulation
of miR-133b is associated with poor prognosis in bladder cancer.
Pathol Res Pract. 215:354–357. 2019. View Article : Google Scholar
|
|
25
|
Yu QF, Liu P, Li ZY, Zhang CF, Chen SQ, Li
ZH, Zhang GY and Li JC: MiR-103/107 induces tumorigenicity in
bladder cancer cell by suppressing PTEN. Eur Rev Med Pharmacol Sci.
22:8616–8623. 2018.
|
|
26
|
Wang F, Zu Y, Zhu S, Yang Y, Huang W, Xie
H and Li G: Long noncoding RNA MAGI2-AS3 regulates CCDC19
expression by sponging miR-15b-5p and suppresses bladder cancer
progression. Biochem Biophys Res Commun. 507:231–235. 2018.
View Article : Google Scholar
|
|
27
|
Li Y, Wan B, Liu L, Zhou L and Zeng Q:
Circular RNA circMTO1 suppresses bladder cancer metastasis by
sponging miR-221 and inhibiting epithelial-to-mesenchymal
transition. Biochem Biophys Res Commun. 508:991–996. 2019.
View Article : Google Scholar
|
|
28
|
Sun M, Zhao W, Chen Z, Li M, Li S, Wu B
and Bu R: Circ_0058063 regulates CDK6 to promote bladder cancer
progression by sponging miR-145-5p. J Cell Physiol. 234:4812–4824.
2019. View Article : Google Scholar
|
|
29
|
Zhao X, Wang Y, Yu Q, Yu P, Zheng Q, Yang
X and Gao D: Circular RNAs in gastrointestinal cancer: Current
knowledge, biomarkers and targeted therapy (Review). Int J Mol Med.
46:1611–1632. 2020.
|
|
30
|
Zhang JR and Sun HJ: Roles of circular
RNAs in diabetic complications: From molecular mechanisms to
therapeutic potential. Gene. 763:1450662020. View Article : Google Scholar
|
|
31
|
Hua L, Huang L, Zhang X, Feng H and Shen
B: Knockdown of circular RNA CEP128 suppresses proliferation and
improves cytotoxic efficacy of temozolomide in glioma cells by
regulating miR-145-5p. Neuroreport. 30:1231–1238. 2019. View Article : Google Scholar
|
|
32
|
Gan J, Yuan J, Liu Y, Lu Z, Xue Y, Shi L
and Zeng H: Circular RNA_101237 mediates anoxia/reoxygenation
injury by targeting let-7a-5p/IGF2BP3 in cardiomyocytes. Int J Mol
Med. 45:451–460. 2020.
|
|
33
|
Chen S, Li T, Zhao Q, Xiao B and Guo J:
Using circular RNA hsa_circ_0000190 as a new biomarker in the
diagnosis of gastric cancer. Clin Chim Acta. 466:167–171. 2017.
View Article : Google Scholar
|
|
34
|
Xiao-Long M, Kun-Peng Z and Chun-Lin Z:
Circular RNA circ_HIPK3 is down-regulated and suppresses cell
proliferation, migration and invasion in osteosarcoma. J Cancer.
9:1856–1862. 2018. View Article : Google Scholar
|
|
35
|
Yao JT, Zhao SH, Liu QP, Lv MQ, Zhou DX,
Liao ZJ and Nan KJ: Over-expression of CircRNA_100876 in non-small
cell lung cancer and its prognostic value. Pathol Res Pract.
213:453–456. 2017. View Article : Google Scholar
|
|
36
|
Yuan W, Peng S, Wang J, Wei C, Ye Z, Wang
Y, Wang M, Xu H, Jiang S, Sun D, et al: Identification and
characterization of circRNAs as competing endogenous RNAs for
miRNA-mRNA in colorectal cancer. PeerJ. 7:e76022019. View Article : Google Scholar
|
|
37
|
Su Y, Du Z, Zhong G, Ya Y, Bi J, Shi J,
Chen L, Dong W and Lin T: circ5912 suppresses cancer progression
via inducing MET in bladder cancer. Aging (Albany NY).
11:10826–10838. 2019. View Article : Google Scholar
|
|
38
|
Chaar I, Amara S, Elamine OE, Khiari M,
Ounissi D, Khalfallah T, Ben Hmida A, Mzabi S and Bouraoui S:
Biological significance of promoter hypermethylation of p14/ARF
gene: Relationships to p53 mutational status in Tunisian population
with colorectal carcinoma. Tumour Biol. 35:1439–1449. 2014.
View Article : Google Scholar
|
|
39
|
Hsu HS and Wang YC, Tseng RC, Chang JW,
Chen JT, Shih CM, Chen CY and Wang YC: 5′ cytosine-phospho-guanine
island methylation is responsible for p14ARF inactivation and
inversely correlates with p53 overexpression in resected non-small
cell lung cancer. Clin Cancer Res. 10:4734–4741. 2004. View Article : Google Scholar
|
|
40
|
Iida S, Akiyama Y, Nakajima T, Ichikawa W,
Nihei Z, Sugihara K and Yuasa Y: Alterations and hypermethylation
of the p14(ARF) gene in gastric cancer. Int J Cancer. 87:654–658.
2000. View Article : Google Scholar
|
|
41
|
Ito T, Nishida N, Fukuda Y, Nishimura T,
Komeda T and Nakao K: Alteration of the p14(ARF) gene and p53
status in human hepatocellular carcinomas. J Gastroenterol.
39:355–361. 2004. View Article : Google Scholar
|
|
42
|
Domínguez G, Carballido J, Silva J, Silva
JM, García JM, Menéndez J, Provencio M, España P and Bonilla F:
p14ARF promoter hypermethylation in plasma DNA as an indicator of
disease recurrence in bladder cancer patients. Clin Cancer Res.
8:980–985. 2002.
|
|
43
|
Berggren P, Kumar R, Sakano S, Hemminki L,
Wada T, Steineck G, Adolfsson J, Larsson P, Norming U, Wijkström H
and Hemminki K: Detecting homozygous deletions in the
CDKN2A(p16(INK4a))/ARF(p14(ARF)) gene in urinary bladder cancer
using real-time quantitative PCR. Clin Cancer Res. 9:235–242.
2003.
|
|
44
|
Liang Z, Xie W, Wu R, Geng H, Zhao L, Xie
C, Li X, Zhu M, Zhu W, Zhu J, et al: Inhibition of tobacco
smoke-induced bladder MAPK activation and epithelial-mesenchymal
transition in mice by curcumin. Int J Clin Exp Pathol. 8:4503–4513.
2015.
|
|
45
|
Miller JE and Reese JC: Ccr4-Not complex:
The control freak of eukaryotic cells. Crit Rev Biochem Mol Biol.
47:315–333. 2012. View Article : Google Scholar
|
|
46
|
Noel N, Couteau J, Maillet G, Gobet F,
D'Aloisio F, Minier C and Pfister C: TP53 and FGFR3 gene mutation
assessment in urine: Pilot study for bladder cancer diagnosis.
Anticancer Res. 35:4915–4921. 2015.
|
|
47
|
Knowles MA: Role of FGFR3 in urothelial
cell carcinoma: Biomarker and potential therapeutic target. World J
Urol. 25:581–593. 2007. View Article : Google Scholar
|
|
48
|
Dong L, Lin F, Wu W, Liu Y and Huang W:
Verteporfin inhibits YAP-induced bladder cancer cell growth and
invasion via Hippo signaling pathway. Int J Med Sci. 15:645–652.
2018. View Article : Google Scholar
|
|
49
|
Zhong Z, Huang M, Lv M, He Y, Duan C,
Zhang L and Chen J: Circular RNA MYLK as a competing endogenous RNA
promotes bladder cancer progression through modulating VEGFA/VEGFR2
signaling pathway. Cancer Lett. 403:305–317. 2017. View Article : Google Scholar
|
|
50
|
Li F, Zhang L, Li W, Deng J, Zheng J, An
M, Lu J and Zhou Y: Circular RNA ITCH has inhibitory effect on ESCC
by suppressing the Wnt/β-catenin pathway. Oncotarget. 6:6001–6013.
2015. View Article : Google Scholar
|
|
51
|
Chen P, Zhao L, Pan X, Jin L, Lin C, Xu W,
Xu J, Guan X, Wu X, Wang Y, et al: Tumor suppressor microRNA-136-5p
regulates the cellular function of renal cell carcinoma. Oncol
Lett. 15:5995–6002. 2018.
|
|
52
|
Shen S, Yue H, Li Y, Qin J, Li K, Liu Y
and Wang J: Upregulation of miR-136 in human non-small cell lung
cancer cells promotes Erk1/2 activation by targeting PPP2R2A.
Tumour Biol. 35:631–640. 2014. View Article : Google Scholar
|
|
53
|
Yan M, Li X, Tong D, Han C, Zhao R, He Y
and Jin X: miR-136 suppresses tumor invasion and metastasis by
targeting RASAL2 in triple-negative breast cancer. Oncol Rep.
36:65–71. 2016. View Article : Google Scholar
|
|
54
|
Scelfo A, Piunti A and Pasini D: The
controversial role of the Polycomb group proteins in transcription
and cancer: How much do we not understand Polycomb proteins? FEBS
J. 282:1703–1722. 2015. View Article : Google Scholar
|
|
55
|
Haindl M, Harasim T, Eick D and Muller S:
The nucleolar SUMO-specific protease SENP3 reverses SUMO
modification of nucleophosmin and is required for rRNA processing.
EMBO Rep. 9:273–279. 2008. View Article : Google Scholar
|
|
56
|
Eifler K and Vertegaal AC: Mapping the
SUMOylated landscape. FEBS J. 282:3669–3680. 2015. View Article : Google Scholar
|
|
57
|
Ma RG, Zhang Y, Sun TT and Cheng B:
Epigenetic regulation by polycomb group complexes: Focus on roles
of CBX proteins. J Zhejiang Univ Sci B. 15:412–428. 2014.
View Article : Google Scholar
|
|
58
|
Li J, Xu Y, Long XD, Wang W, Jiao HK, Mei
Z, Yin QQ, Ma LN, Zhou AW, Wang LS, et al: Cbx4 governs HIF-1α to
potentiate angiogenesis of hepatocellular carcinoma by its SUMO E3
ligase activity. Cancer Cell. 25:118–131. 2014. View Article : Google Scholar
|
|
59
|
Fukagawa A, Ishii H, Miyazawa K and Saitoh
M: δEF1 associates with DNMT1 and maintains DNA methylation of the
E-cadherin promoter in breast cancer cells. Cancer Med. 4:125–135.
2015. View Article : Google Scholar
|