Introduction
The infection with hepatitis B virus (HBV) is a
major health concern worldwide. It is estimated that ~350 million
individuals are carriers of the hepatitis B surface S protein (HBs)
and over one million patients eventually succumb to HBV-related
chronic liver diseases annually (1–2).
Persistent HBV infection confers a high risk of chronic hepatitis,
liver cirrhosis and hepatocellular carcinoma (HCC) (3). Three forms of viral particles may be
detected in the serum of HBV-infected patients: 42-nm diameter
mature virion particles, 22-nm diameter spherical particles and
22-nm diameter filamentous particles (4–5).
Subviral particles (22 nm), composed of HBs, are unique in that do
not contain viral DNA and usually exceed the numbers of virions by
≥1,000-fold in the patient serum (5). A number of individuals reportedly
reached a state of non-replicative infection following persistent
anti-virus therapy; however, numerous HBs particles were still
detected in their serum and the prolonged immunological response to
infection may result in the development of fibrosis in the majority
of the patients and eventually lead to the development of
cirrhosis, liver failure, or HCC in ~40% of the patients (6). During this process, HBs may play an
important role. However, the mechanism underlying the hepatic
fibrogenesis induced by HBs has not yet been fully elucidated.
It was demonstrated that the progression of hepatic
fibrosis requires sustained inflammation, leading to the activation
of the hepatic stellate cells (HSCs) into a fibrogenic and
proliferative cell type, such as the fibroblast (7). Regardless of the underlying disease,
HSCs, the key fibrogenic cells, have been established as the main
extracellular matrix (ECM)-producing cells in liver injury
(8).
We hypothesized that HBs contributes to the
regulation of HSCs activation and ECM deposition during the process
of hepatic fibrogenesis. The proliferative activity and the
expression of collagen type I (Col I) and α-smooth muscle actin
(α-SMA) in HSCs were evaluated.
Materials and methods
Cell lines and cell culture
LX-2, a strain of human hepatic stellate cell line,
was obtained from Professor Friedman SL. HepG2, a type of human HCC
cell line, was purchased from the Insitute of Biochemistry and Cell
Biology of the Chinese Academy of Sciences (Shanghai, China). The
LX-2 and HepG2 cells were cultured in Dulbecco’s modified Eagle’s
medium (Gibco-BRL, Grand Island, NY, USA) supplemented with 100
U/ml penicillin G, 100 μg/ml streptomycin and 10% fetal bovine
serum (Gibco-BRL) in an incubator with 95% humidity and 5% carbon
dioxide at 37°C.
Essential reagents
Recombinant HBs (no. 10-251-40733)was purchased from
GenWay Biotech Inc. (San Diego, CA, USA). ELISA kits for Col I
(CSB-E08082h; detection range, 1.56–100 ng/ml; sensitivity, 0.39
ng/ml), α-SMA (CSB-E09343h; detection range, 3.12–200 ng/ml;
sensitivity, 0.78 ng/ml) and transforming growth factor-β1 (TGF-β1)
(CSB-E04725h; detection range, 0.78–50 ng/ml; sensitivity, 0.39
ng/ml) were purchased from Cusabio Biotech Co., Ltd. (Wuhan,
China).
MTT assay
The LX-2 cell proliferation was determined by the
MTT assay. The cells were cultured at a density of 2×103
cells/well in flat-bottomed 96-well microplates. After 24 h, the
experimental cultures were divided into 6 groups, followed by the
addition of 0.5–50 ng/ml recombinant HBs per well. A total of 6
parallel cells were set for each group. After a 24- or 48-h
incubation at 37°C, cell viability was determined by the MTT assay.
The cells were incubated with 0.5 % MTT for 4 h. Upon removal of
the supernatant, 150 μl dimethyl sulfoxide was added and shaken for
5 min until the crystals were dissolved. The optical density value
at 492 nm (OD492) was measured by ELISA. The negative
control well was used as zero point of absorbance. All the
experiments were independently performed in triplicate.
ELISA
Col I, α-SMA and TGF-β1 were measured by the
standard sandwich ELISA according to the instructions provided by
the manufacturer. A total of 6 parallel cells were set for each
group. The absorbance was measured at 450 nm using a microplate
reader (model 680; Bio-Rad, Hercules, CA, USA).
Statistical analysis
The results are expressed as means ± standard error
(SE). The statistical analysis was performed with an analysis of
variance and P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of HBs on LX-2 cell
proliferation
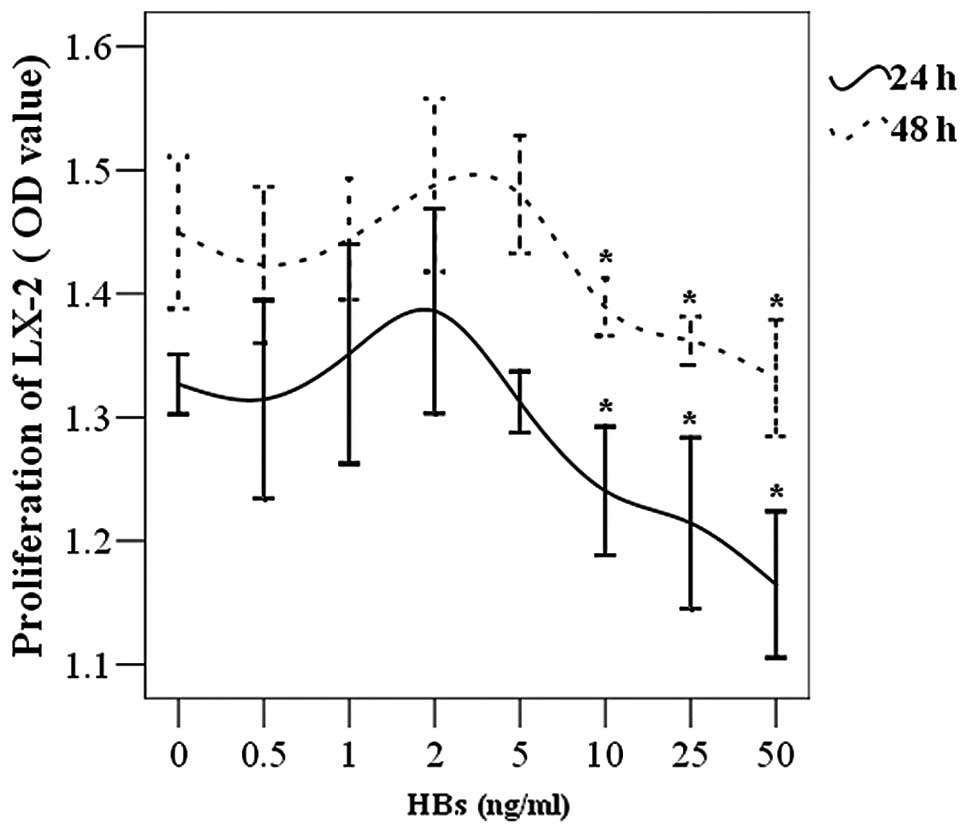
The LX-2 cells were placed in 96-well plates and
incubated with various concentrations of HBs. It was demonstrated
that high concentrations of HBs (10–50 ng/ml) inhibited the
proliferation of LX-2 cells. This inhibitory effect was gradually
enhanced with increasing concentration of HBs. Low concentrations
of HBs (0.5–5 ng/ml) did not affect cell proliferation (Fig. 1). Although the OD value was
increased, no significant difference was observed in LX-2 cell
survival when the HBs concentration reached a plateau of 1–5
ng/ml.
Effect of HBs on the secretion of α-SMA
and Col I in LX-2 cells and co-culture system of HepG2 and LX-2
cells
As described above, the proliferation of LX-2 cells
was significantly inhibited by HBs at concentrations ≥10 ng/ml.
Therefore, the concentration of 10 ng/ml of HBs was employed in
subsequent experiments. Col I is the major content of ECM and α-SMA
is an indicator of HSCs transforming into fibroblasts. Col I and
α-SMA were used to evaluate the role of HBs in the fibrogenetic
process. The cells (2×103 cells/well) were cultured for
24 h and incubated with HBs (10 ng/ml) for 48 h. The ELISA results
demonstrated that the changes in Col I and α-SMA were no different
between the HBs treatment and control groups (Col I: 28.61±3.25 vs.
26.30±3.69 ng/ml, t=0.47, P=0.648 and α-SMA: 25.08±5.33 vs.
24.48±2.62 ng/ml, t=0.101, P=0.962, respectively).
A receptor of HBs exists in hepatocytes, although it
has not been definitively determined. We investigated whether the
expression of Col I and α-SMA in LX-2 cell supernatants was
affected by hepatocytes. HepG2 (2×103 cells/well) and
LX-2 cells (2×103 cells/well) were co-cultured for 24 h,
incubated with HBs (10 ng/ml) for 24 h and the supernatants were
collected for ELISA. The ELISA demonstrated that the Col I levels
were significantly increased following HBs treatment (48.51±3.51
vs. 28.23±2.55 ng/ml, t=4.674, P=0.001), whereas there was no
obvious change in α-SMA levels (30.66±2.69 vs. 23.42±3.86 ng/ml,
t=1.538, P=0.155). Likewise, the HepG2 cells (2×103
cells/well) were cultured alone with HBs for 24 h to eliminate the
secretion of Col I and α-SMA. The ELISA demonstrated that Col I was
increased in the HepG2 cell supernatants (37.63±3.43 vs. 18.49±3.58
ng/ml, t=3.856, P=0.003), although α-SMA was not (20.70±2.38 vs.
18.46±1.48 ng/ml, t=0.799, P=0.443). However, Col I was
signficantly lower in the supernatant of HepG2 cells stimulated by
HBs than that in the supernatant of the co-culture system
(37.63±3.43 vs. 48.51±3.51 ng/ml, t=3.132, P=0.03) (Fig. 2).
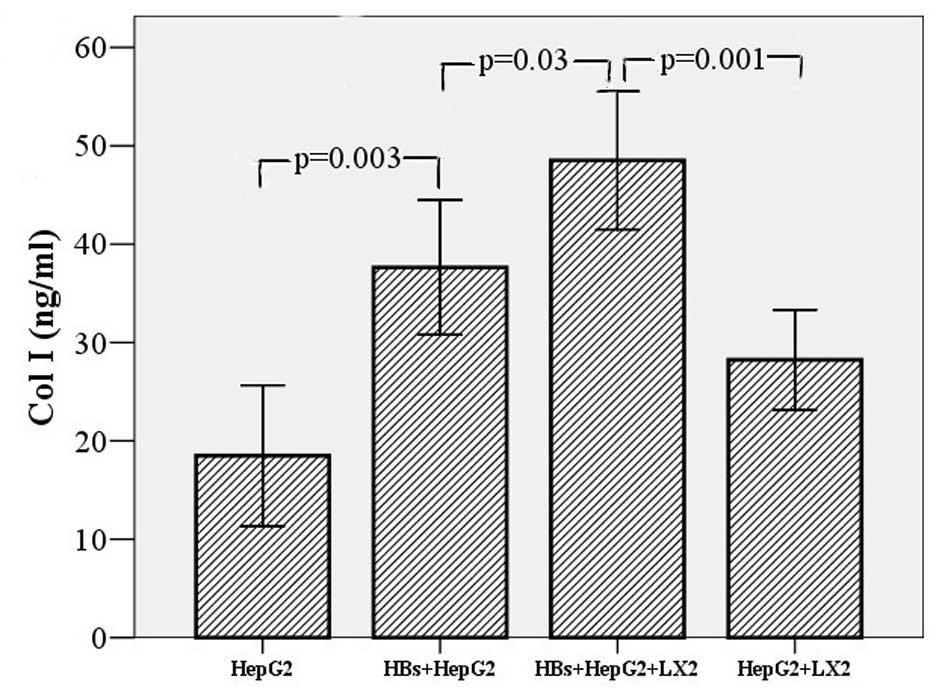 | Figure 2Effect of hepatitis B surface S
protein (HBs) on secretion of α-smooth muscle actin (α-SMA) and
collagen type I (Col I) in LX-2 cells and the co-culture system of
HepG2 and LX-2 cells. To detect the effect of HBs on the expression
of Col I and α-SMA in LX-2 cells, the cells (2×103
cells/well) were cultured for 24 h, then incubated with HBs (10
ng/ml) for 48 h. The change in Col I and α-SMA levels was no
different between the HBs treatment and the control groups (data
not shown): Col I, 28.61±3.25 vs. 26.30±3.69 ng/ml, t=0.47, P=0.648
and α-SMA, 25.08±5.33 vs. 24.48±2.62 ng/ml, t=0.101, P=0.962,
respectively. HepG2 (2×103 cells/well) and LX-2 cells
(2×103 cells/well) were co-cultured for 24 h, then
incubated with HBs (10 ng/ml) for 24 h. Col I was found to be
significantly increased following HBs treatment (48.51±3.51 vs.
28.23±2.55 ng/ml, t=4.674, P=0.001), whereas there was no obvious
change in α-SMA (data not shown; 30.66±2.69 vs. 23.42±3.86 ng/ml,
t=1.538, P=0.155). The HepG2 cells (2×103 cells/well)
were cultured alone with HBs for 24 h to eliminate the secretion of
Col I and α-SMA. The results demonstrated that Col I was increased
in HepG2 cell supernatants (37.63±3.43 vs. 18.49±3.58 ng/ml,
t=3.856, P=0.003), although α-SMA was not (20.70±2.38 vs.
18.46±1.48 ng/ml, t=0.799, P=0.443). However, Col I was
significantly lower in the supernatant of HepG2 cells stimulated by
HBs than in the supernatant of the co-culture system (37.63±3.43
vs. 48.51±3.51 ng/ml, t=3.132, P=0.03), whereas the data did not
reveal similar results for α-SMA. Error bars, ±2.00 SE. |
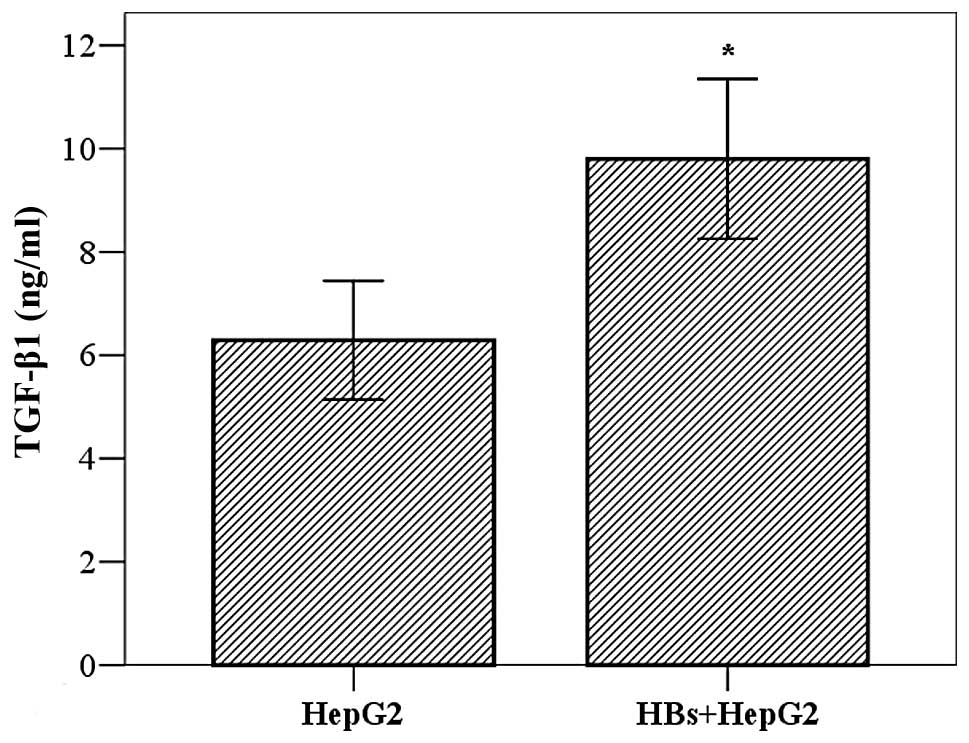
Effect of HBs on secretion of TGF-β1 in
HepG2 cells
TGF-β1 is considered to be the major cytokine
affecting HSCs. To elucidate the mechanism underlying the effect of
HepG2 cells on LX-2 cells, we investigated whether HBs promoted
TGF-β1 secretion from HepG2 cells. The cells (2×103
cells/well) were cultured for 24 h, incubated with HBs (10 ng/ml)
for 24 h and the TGF-β1 in the cell supernatants was measured by
ELISA. The results demonstrated that the TGF-β1 levels were higher
in the HBs treatment group compared to those in the control group
(9.80±1.89 vs. 6.49±1.41 ng/ml, t=3.635, P=0.005) (Fig. 3).
Discussion
All chronic liver diseases may cause liver fibrosis
through a similar pathway; however, different causes of liver
injury may employ various mechanisms in this process. Among the
major causes of chronic liver disease, hepatitis B confers a
particularly high risk of fibrosis progression (9). Among the proteins encoded by HBV, it
was demonstrated that proteins E and X may activate HSCs and
directly promote the expression of collagens (10–15).
However, whether HBs leads to fibrosis has not been established.
Although HBs was identified as the neutralizing antigen of HBV and
has been used as the major component of the preventive vaccine for
viral hepatitis B, the persistence of HBs in the serum of patients
has been recognized as a high-risk factor for the development of
liver cirrhosis and HCC (16–17).
One-fourth of the hepatitis B surface antigen-positive patients
will eventually develop complications, such as cirrhosis or HCC,
which constitute major causes of liver disease-related mortality
(18).
It was previously demonstrated that hepatocytes may
be a harbor of refuge for hepatitis C virus (HCV) replication and
the hepatocyte medium is stimulated by the HCV envelope protein,
promoting HSC activity and production of Col I (19). HBs, similar to the envelope protein,
exerts an effect similar to that of the HCV envelope protein.
However, the number of available studies on HBs-related liver
fibrosis is limited. We first investigated the effect of HBs on the
proliferation of HSCs, which are considered to play a central role
in hepatic fibrogenesis. There is a 98.7% similarity in gene
expression between LX-2 cells and primary HSCs (20); therefore, LX-2 cells were used as
substitutes of HSCs in our experiments. No effect on LX-2 cell
proliferation was observed by low concentrations of HBs (0.5–5
ng/ml), whereas the proliferation was inhibited by high
concentrations (10–50 ng/ml). However, Liu et al(15) reported that HBs (1.25–20 μg/ml)
inhibited the proliferation of LX-2 cells, whereas low
concentrations of HBs (0.04–0.62 μg/ml) promoted LX-2 cell
proliferation. This difference may be attributed to the use of
recombinant HBs. In the experiments conducted in that study, the
injection vaccine protecting against hepatitis B was used as
recombinant HBs and its constitution and purity may have affected
the experimental results.
The secretion and expression of Col I and α-SMA at
the protein level is an indicator of fibrosis and transformation of
HSCs into fibroblasts, respectively (21). We demonstrated that the expression
of Col I and α-SMA in LX-2 cell supernatants was not increased
following treatment with HBs. Therefore, it was suggested that HBs
is not a direct activator of LX-2 cells during the fibrogenetic
process.
It was previously demonstrated that ethanol induces
TGF-α expression in hepatocytes, leading to the stimulation of
collagen synthesis by HSCs (22).
Furthermore, toxic iron overload was shown to modulate HSCs
proliferation and gene expression by rat hepatocytes (23). Accordingly, we hypothesized that HBs
binds to its receptor on hepatocytes and sequentially stimulates
the activation of LX-2 cells. Therefore, LX-2 and HepG2 cells were
co-cultured in HBs conditioned medium and we observed that the Col
I levels were significantly higher compared to those in the control
group; however, there was no significant difference regarding the
production of α-SMA between the HBs treatment and the control
groups, although the value was higher in the former. We
hypothesized that HSCs increased the release and expression of Col
I prior to their transformation into fibroblasts. In addition, it
was demonstrated that HBs stimulated the enhanced expression of Col
I, but not α-SMA, in HepG2 cells. However, the production of Col I
was lower in HepG2 cells compared to that in the co-cultured
system. Therefore, we concluded that HBs promotes Col I expression
in HSCs by virtue of hepatocytes.
The activation of HSCs is triggered by adjacent
hepatocytes, Kupffer cells and liver sinus endothelial cells, by a
paracrine secretion pathway. TGF-β1, one of the most important cell
factors secreted by the above-mentioned cells, significantly
promotes collagen expression (8,24).
Therefore, TGF-β1 was measured in the HepG2 cell supernatants. As
was expected, HBs promoted TGF-β1 expression in HepG2 cells. We
concluded that the increased secretion of latent TGF-β1 by
hepatocytes is a potential factor affecting the fibrogenic behavior
of HSCs. The HBs level is a reflection of the transcriptional
activity of covalently closed circular DNA (cccDNA), is an
important marker of active hepatitis B infection and may also
predict clinical and treatment outcomes. Higher HBs levels indicate
a higher risk of cirrhosis (25).
The HBs quantification has been used for monitoring natural history
and treatment outcome (26). The
HBs concentration that inhibited HSCs promoted the expression of
Col I in our study and proved the role of HBs in clinical cases. In
addition, the presence of peritumoral activated HSCs in HBV-related
HCC was recently demonstrated (27). HepG2 HCC cells activated HSCs
through HBs in our study, in accordance with the above-mentioned
findings. The inhibition of HSCs proliferation is considered to be
the most important strategy for anti-fibrotic therapy (28). Our results indicated that inhibiting
the HBs receptor expression may be a target for the treatment of
the liver fibrosis.
There were certain limitation to our study. Although
primary human cells are difficult to obtain, the use of human
primary liver cells and primary HSCs may validate our conclusions.
In summary, our data suggest that the HBs of HBV is crucial in
liver fibrogenesis. Through HBs stimulation, the hepatocytes
exhibit increased expression of TGF-β1 and promote Col I production
in adjacent HSCs. This may be a novel explanation for the
fibrogenetic mechanism induced by HBV-related proteins. However,
further investigations of the role of HBs in fibrogenesis are
required.
Acknowledgements
This study was supported by a grant from the Guangxi
Natural Science Foundation (no. 2011GXNSFB217009). The authors
would like to thank Professor Scott L. Friedman (Mount Sinai School
of Medicine, New York, NY, USA) for kindly donating the LX-2
cells.
References
|
1
|
Ocama P, Opio CK and Lee WM: Hepatitis B
virus infection: current status. Am J Med. 118:14132005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lavanchy D: Hepatitis B virus
epidemiology, disease burden, treatment, and current and emerging
prevention and control measures. J Viral Hepat. 11:97–107. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kao JH and Chen DS: Global control of
hepatitis B virus infection. Lancet Infect Dis. 2:395–403. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee WM: Hepatitis B virus infection. N
Engl J Med. 337:1733–1745. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ganem D and Prince AM: Hepatitis B virus
infection - natural history and clinical consequences. N Engl J
Med. 350:1118–1129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wright TL: Introduction to chronic
hepatitis B infection. Am J Gastroenterol. 101(Suppl 1): S1–S6.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eng FJ and Friedman SL: Fibrogenesis I.
New insights into hepatic stellate cell activation: the simple
becomes complex. Am J Physiol Gastrointest Liver Physiol.
279:G7–G11. 2000.PubMed/NCBI
|
|
8
|
Friedman SL: Molecular regulation of
hepatic fibrosis, an integrated cellular response to tissue injury.
J Biol Chem. 275:2247–2250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poynard T, Mathurin P, Lai CL, et al: A
comparison of fibrosis progression in chronic liver diseases. J
Hepatol. 38:257–265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Norton PA, Reis HM, Prince S, et al:
Activation of fibronectin gene expression by hepatitis B virus x
antigen. J Viral Hepat. 11:332–341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo GH, Tan DM, Zhu PA and Liu F:
Hepatitis B virus X protein promotes proliferation and upregulates
TGF-beta1 and CTGF in human hepatic stellate cell line, LX-2.
Hepatobiliary Pancreat Dis Int. 8:59–64. 2009.PubMed/NCBI
|
|
12
|
Martin-Vilchez S, Sanz-Cameno P,
Rodriguez-Munoz Y, et al: The hepatitis B virus X protein induces
paracrine activation of human hepatic stellate cells. Hepatology.
47:1872–1883. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zan Y, Zhang Y and Tien P: Hepatitis B
virus e antigen induces activation of rat hepatic stellate cells.
Biochem Biophys Res Commun. 435:391–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen HY, Wang XZ and Chen ZX: Expression
of the hepatitis B virus X gene in liver cells promotes the
proliferation and migration of co-cultured hepatic stellate cells.
World Chin J Digestol. 20:721–728. 2012.(In Chinese).
|
|
15
|
Liu X, Zhu ST, You H, Cong M, Liu TH, Wang
BE and Jia JD: Hepatitis B virus infects hepatic stellate cells and
affects their proliferation and expression of collagen type I. Chin
Med J (Engl). 122:1455–1461. 2009.PubMed/NCBI
|
|
16
|
Beasley RP, Shiao IS, Wu TC and Hwang LY:
Hepatoma in an HBsAg carrier - seven years after perinatal
infection. J Pediatr. 101:83–84. 1982.PubMed/NCBI
|
|
17
|
Lupberger J and Hildt E: Hepatitis B
virus-induced oncogenesis. World J Gastroenterol. 13:74–81. 2007.
View Article : Google Scholar
|
|
18
|
Chevaliez S: Is HBsAg quantification
ready, for prime time? Clin Res Hepatol Gastroenterol. Aug
7–2013.(Epub ahead of print). View Article : Google Scholar
|
|
19
|
Mazzocca A, Sciammetta SC, Carloni V, et
al: Binding of hepatitis C virus envelope protein E2 to CD81
up-regulates matrix metalloproteinase-2 in human hepatic stellate
cells. J Biol Chem. 280:11329–11339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu L, Hui AY, Albanis E, et al: Human
hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis
of hepatic fibrosis. Gut. 54:142–151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gabele E, Brenner DA and Rippe RA: Liver
fibrosis: signals leading to the amplification of the fibrogenic
hepatic stellate cell. Front Biosci. 8:d69–d77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kato J, Sato Y, Inui N, et al: Ethanol
induces transforming growth factor-alpha expression in hepatocytes,
leading to stimulation of collagen synthesis by hepatic stellate
cells. Alcohol Clin Exp Res. 27(Suppl 8): 58S–63S. 2003. View Article : Google Scholar
|
|
23
|
Parkes JG and Templeton DM: Modulation of
stellate cell proliferation and gene expression by rat hepatocytes:
effect of toxic iron overload. Toxicol Lett. 144:225–233. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeisberg M, Yang C, Martino M, et al:
Fibroblasts derive from hepatocytes in liver fibrosis via
epithelial to mesenchymal transition. J Biol Chem. 282:23337–23347.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tseng TC, Liu CJ, Yang HC, et al: Serum
hepatitis B surface antigen levels help predict disease progression
in patients with low hepatitis B virus loads. Hepatology.
57:441–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martinot-Peignoux M, Lapalus M, Asselah T
and Marcellin P: The role of HBsAg quantification for monitoring
natural history and treatment outcome. Liver Int. 33(Suppl 1):
125–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liao R, Wu H, Yi Y, et al: Clinical
significance and gene expression study of human hepatic stellate
cells in HBV related-hepatocellular carcinoma. J Exp Clin Cancer
Res. 32:222013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Greupink R, Bakker HI, Bouma W, et al: The
antiproliferative drug doxorubicin inhibits liver fibrosis in bile
duct-ligated rats and can be selectively delivered to hepatic
stellate cells in vivo. J Pharmacol Exp Ther. 317:514–521. 2006.
View Article : Google Scholar : PubMed/NCBI
|