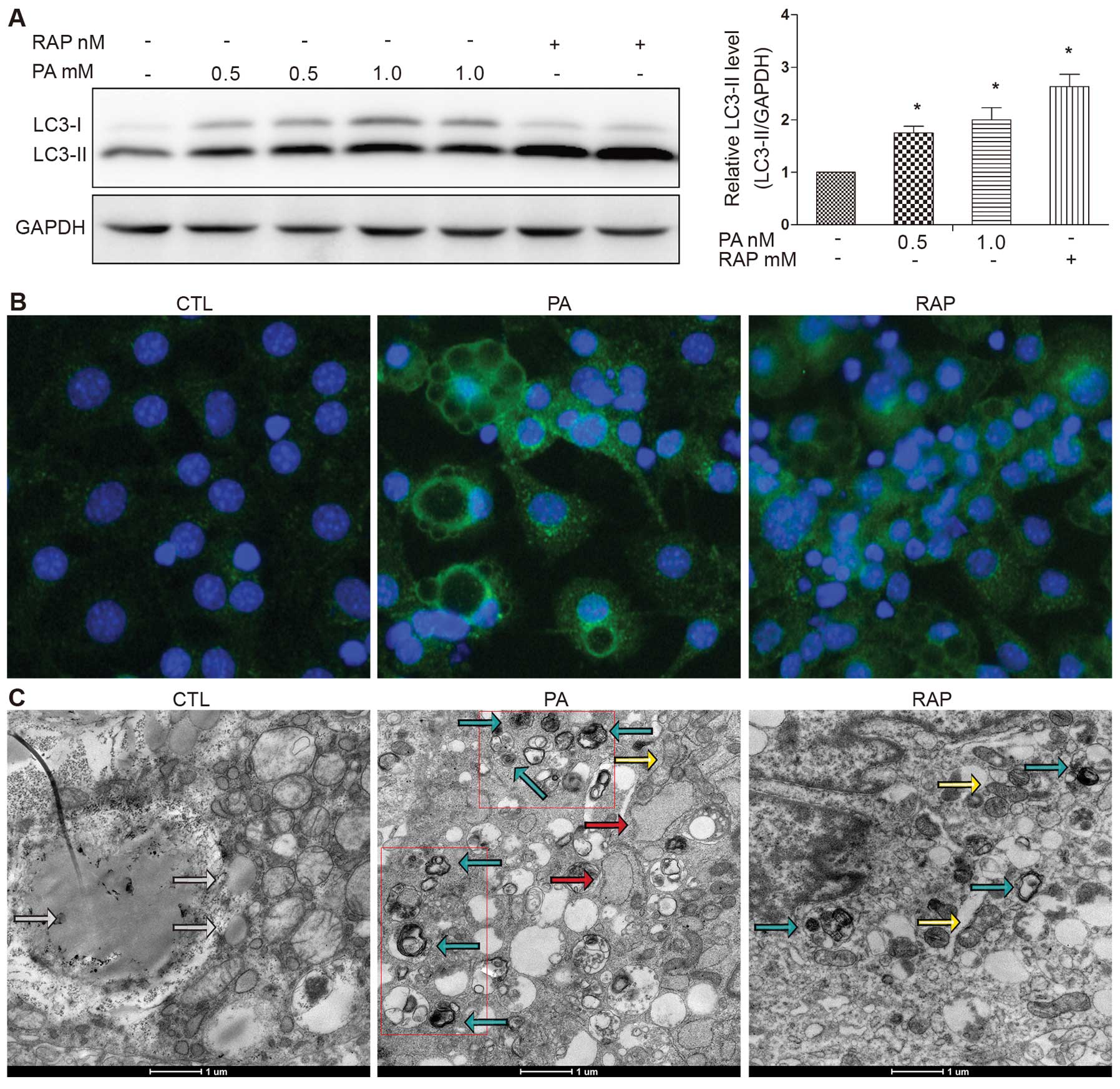
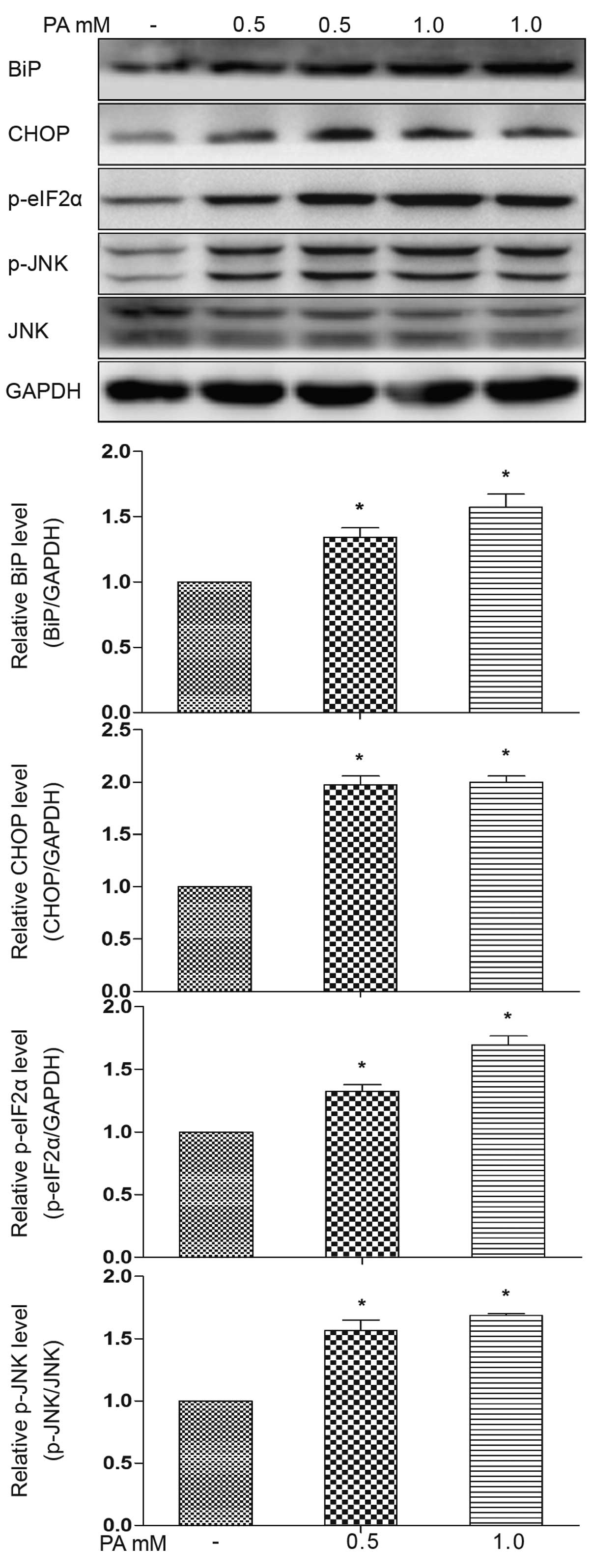
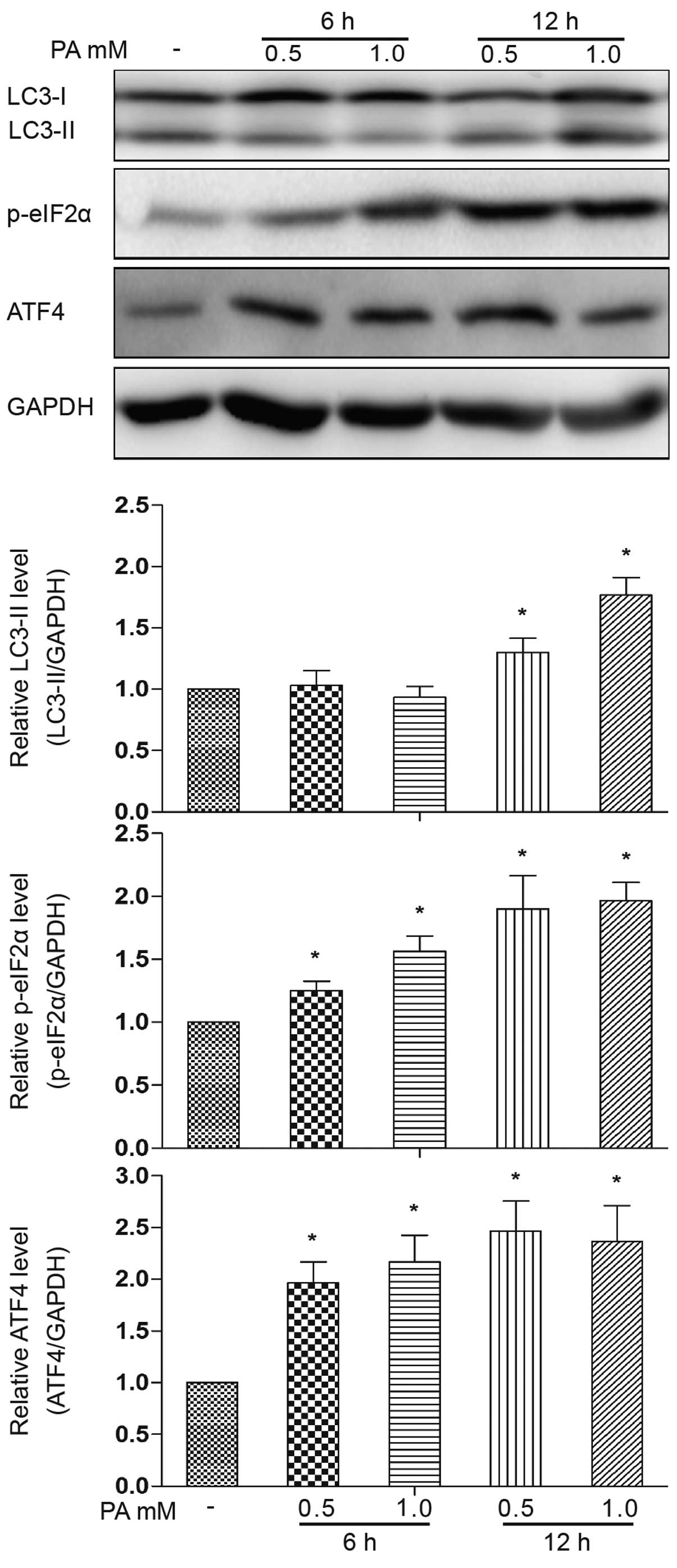
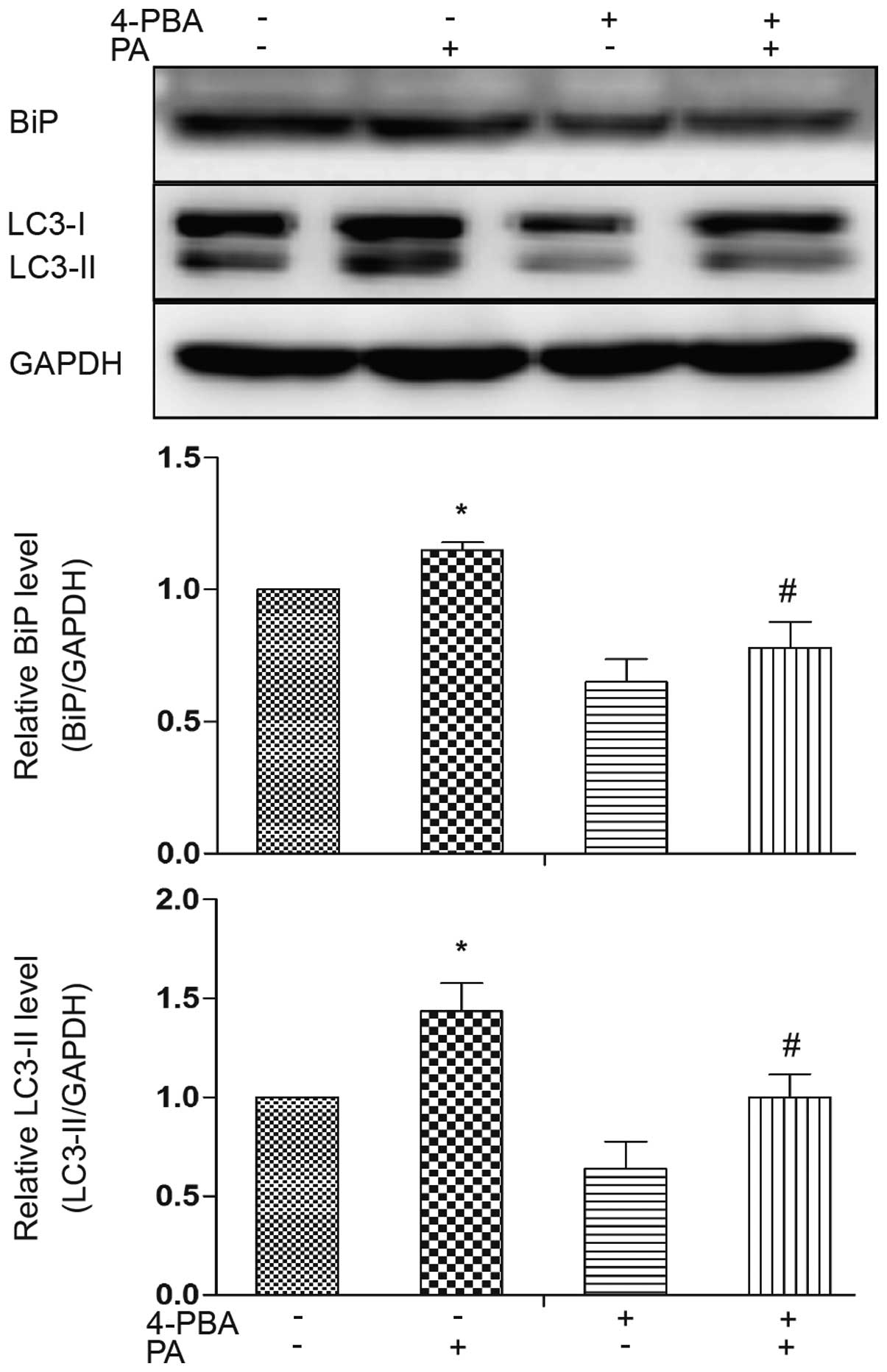
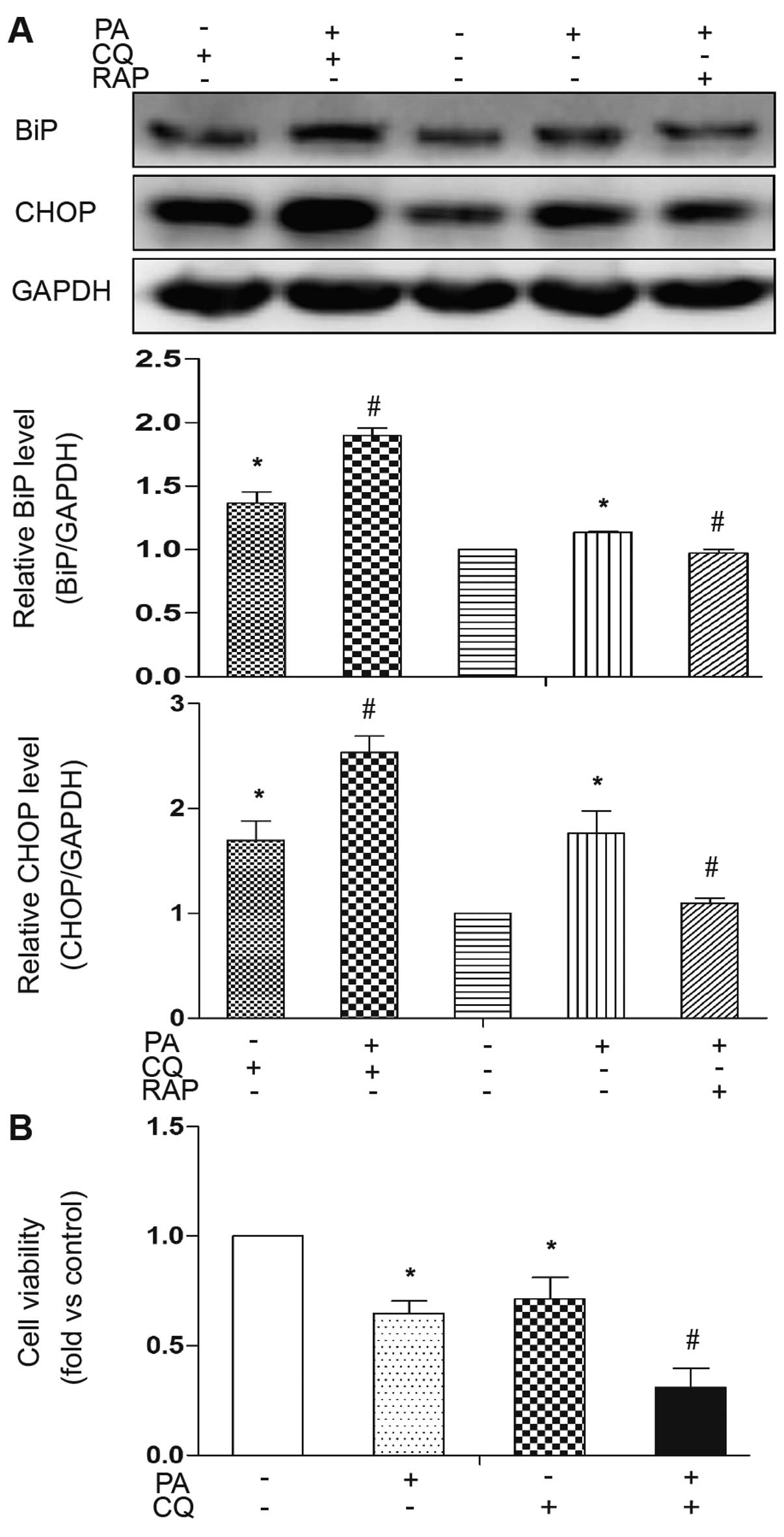
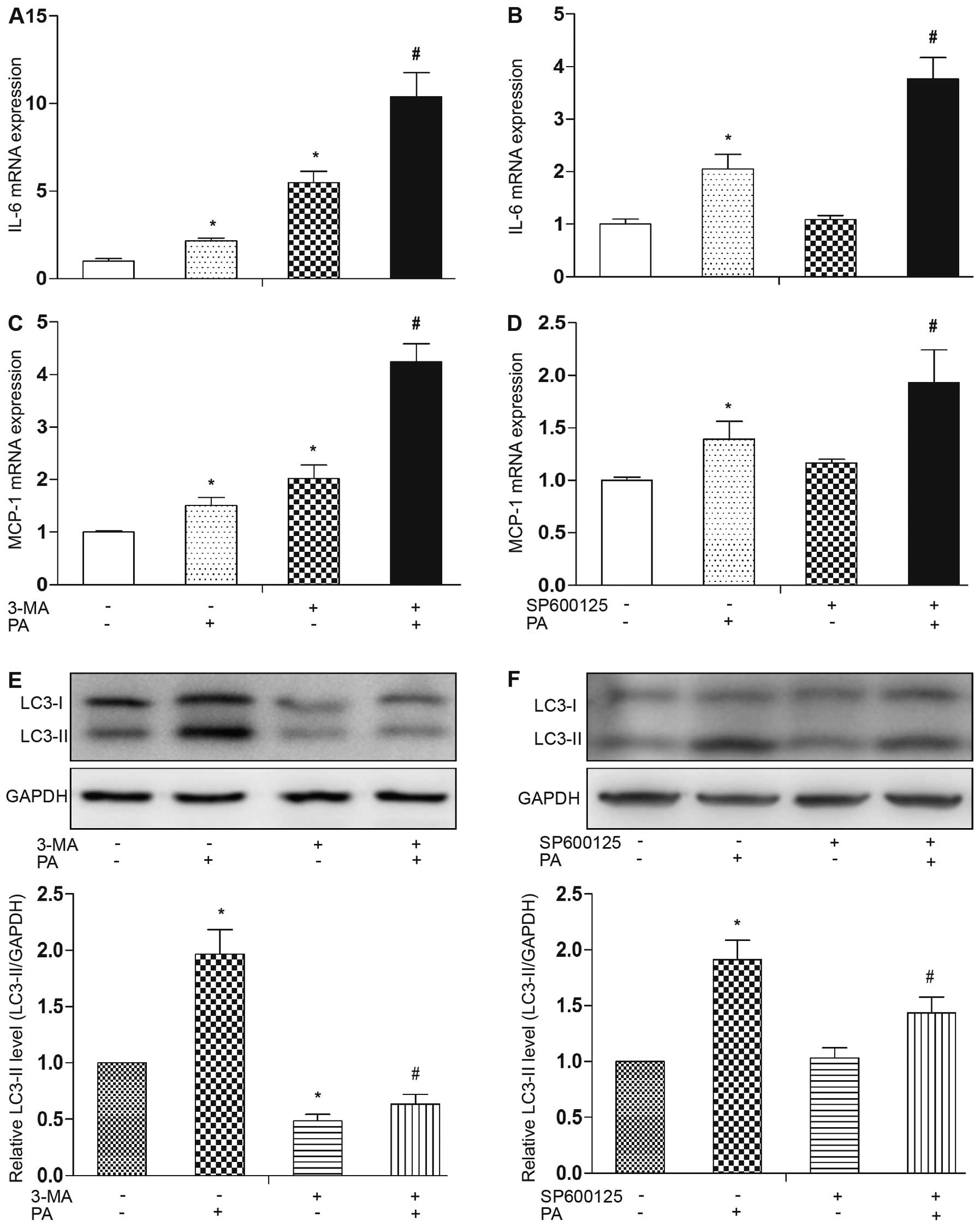
|
1
|
Harvey AE, Lashinger LM and Hursting SD:
The growing challenge of obesity and cancer: an inflammatory issue.
Ann NY Acad Sci. 1229:45–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
González-Chávez A, Elizondo-Argueta S,
Gutiérrez-Reyes G and Leon-Pedroza JI: Pathophysiological
implications between chronic inflammation and the development of
diabetes and obesity. Cir Cir. 79:209–216. 2011.PubMed/NCBI
|
|
3
|
Wang Z and Nakayama T: Inflammation, a
link between obesity and cardiovascular disease. Mediators Inflamm.
2010:5359182010.PubMed/NCBI
|
|
4
|
Takahashi K, Yamaguchi S, Shimoyama T, et
al: JNK- and IkappaB-dependent pathways regulate MCP-1 but not
adiponectin release from artificially hypertrophied 3T3-L1
adipocytes preloaded with palmitate in vitro. Am J Physiol
Endocrinol Metab. 294:E898–E909. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuballa P, Nolte WM, Castoreno AB and
Xavier RJ: Autophagy and the immune system. Annu Rev Immunol.
30:611–646. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin JJ, Li YB, Wang Y, et al: The role of
autophagy in endoplasmic reticulum stress-induced pancreatic beta
cell death. Autophagy. 8:158–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang L, Li P, Fu S, Calay ES and
Hotamisligil GS: Defective hepatic autophagy in obesity promotes ER
stress and causes insulin resistance. Cell Metab. 11:467–478. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saitoh T, Fujita N, Jang MH, et al: Loss
of the autophagy protein Atg16L1 enhances endotoxin-induced
IL-1beta production. Nature. 456:264–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hotamisligil GS: Endoplasmic reticulum
stress and the inflammatory basis of metabolic disease. Cell.
140:900–917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ost A, Svensson K, Ruishalme I, et al:
Attenuated mTOR signaling and enhanced autophagy in adipocytes from
obese patients with type 2 diabetes. Mol Med. 16:235–246. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshizaki T, Kusunoki C, Kondo M, et al:
Autophagy regulates inflammation in adipocytes. Biochem Biophys Res
Commun. 417:352–357. 2012. View Article : Google Scholar
|
|
13
|
Hummasti S and Hotamisligil GS:
Endoplasmic reticulum stress and inflammation in obesity and
diabetes. Circ Res. 107:579–591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Listenberger LL, Ory DS and Schaffer JE:
Palmitate-induced apoptosis can occur through a
ceramide-independent pathway. J Biol Chem. 276:14890–14895. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizushima N, Yoshimori T and Levine B:
Methods in mammalian autophagy research. Cell. 140:313–26. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rubinsztein DC, Gestwicki JE, Murphy LO
and Klionsky DJ: Potential therapeutic applications of autophagy.
Nat Rev Drug Discov. 6:304–312. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ravikumar B, Vacher C, Berger Z, et al:
Inhibition of mTOR induces autophagy and reduces toxicity of
polyglutamine expansions in fly and mouse models of Huntington
disease. Nat Genet. 36:585–595. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar
|
|
19
|
Klionsky DJ, Abdalla FC, Abeliovich H, et
al: Guidelines for the use and interpretation of assays for
monitoring autophagy. Autophagy. 8:445–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ogata M, Hino S, Saito A, et al: Autophagy
is activated for cell survival after endoplasmic reticulum stress.
Mol Cell Biol. 26:9220–9231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Olefsky JM and Glass CK: Macrophages,
inflammation, and insulin resistance. Annu Rev Physiol. 72:219–246.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Zhou B, Xu L, et al: The reciprocal
interaction between autophagic dysfunction and ER stress in adipose
insulin resistance. Cell Cycle. 13:565–579. 2014. View Article : Google Scholar
|
|
23
|
Zhou L and Liu F: Autophagy: roles in
obesity-induced ER stress and adiponectin downregulation in
adipocytes. Autophagy. 6:1196–1197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martinez J, Verbist K, Wang R and Green
DR: The relationship between metabolism and the autophagy machinery
during the innate immune response. Cell Metab. 17:895–900. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schäffler A and Schölmerich J: Innate
immunity and adipose tissue biology. Trends Immunol. 31:228–235.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yin J, Peng Y, Wu J, Wang Y and Yao L:
Toll-like receptor 2/4 links to free fatty acid-induced
inflammation and β-cell dysfunction. J Leukoc Biol. 95:47–52. 2014.
View Article : Google Scholar
|
|
27
|
González-Rodríguez A, Mayoral R, Agra N,
et al: Impaired autophagic flux is associated with increased
endoplasmic reticulum stress during the development of NAFLD. Cell
Death Dis. 5:e11792014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Levine B and Klionsky DJ: Development by
self-digestion: molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ishida Y and Nagata K: Autophagy
eliminates a specific species of misfolded procollagen and plays a
protective role in cell survival against ER stress. Autophagy.
5:1217–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qin L, Wang Z, Tao L and Wang Y: ER stress
negatively regulates AKT/TSC/mTOR pathway to enhance autophagy.
Autophagy. 6:239–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jansen HJ, van Essen P, Koenen T, et al:
Autophagy activity is up-regulated in adipose tissue of obese
individuals and modulates proinflammatory cytokine expression.
Endocrinology. 153:5866–5874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen ML, Yi L, Jin X, et al: Resveratrol
attenuates vascular endothelial inflammation by inducing autophagy
through the cAMP signaling pathway. Autophagy. 9:2033–2045. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harris J, Hartman M, Roche C, et al:
Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for
degradation. J Biol Chem. 286:9587–9597. 2011. View Article : Google Scholar : PubMed/NCBI
|