Introduction
The incidence of human oral squamous cell carcinoma
(OSCC) is increasing rapidly over the years. On average, 5% of all
cancers diagnosed annually in the Western countries are OSCC,
whereas in the Asian countries, especially in India, Taiwan, and
the Philippines, the incidence rate of OSCC is ~10–50% due to the
popularity of tobacco, betel nut, and alcohol consumption in these
regions (1,2). Surgery combined with chemotherapy or
radiotherapy has improved the survival rate. However, metastatic
OSCC, which accounts for almost 80% of all OSCC patients, is the
major cause of death among cancer patients. Metastatic OSCC, which
invades through cervical lymph nodes, presents a difficult
challenge for surgical therapy and often results in recurrence and
death. Therefore, identifying reliable biomarkers would allow early
diagnosis of the disease, and hence improve the prognosis for the
patients.
Podocalyxin-like 1 (also known as gp135, PCLP and
PODXL) is a cell surface glycoprotein belonging to the CD34 family
including CD34, podocalyxin-like 1 (PODXL) and endoglycan (PODXL2).
CD34 is predominantly expressed in hematopoietic stem cells and
vascular endothelial cells (3,4).
Podocalyxin-like 1 (PODXL) was initially identified in kidney
glomerular epithelial cells (5),
and was also found in hematopoietic progenitor cells and vascular
endothelial cells (6). The protein
domains of CD34 and PODXL are similar, which include an
O-glycosylated and sialylated enriched extracellular domain,
a transmembrane domain, and a short cytoplasmic domain for docking
of proteins with the PDZ domain, are similar in structures
(7). Proteins with a PDZ domain,
such as Ezrin-binding protein (EBP) and the
Na+/H+ exchanger regulatory factor (NHERF),
often participate in cytoskeletal rearrangement, implying that
PODXL may have a function in cellular morphogenesis. The cellular
function of PODXL was reported to support the structure of the
podocyte basal surface and the formation of a preapical domain
during polarization (8). The
binding of PODXL to Ezrin or NHERF induces activation of small
GTPase RhoA and Rac1, and facilitates actin reorganization
(9–11). Dissociation of PODXL from actin
results in loss of glomerular foot formation and podocyte
integrity, and the absence of PODXL leads to perinatal lethality
(12). In tumor cells, abnormal
expression of PODXL was reported to produce anti-adhesion and
characteristics of aggressiveness in a variety of cancers.
Overexpression of PODXL is associated with lymphatic invasion of
breast cancer (13) and poor
prognosis in colorectal, bladder and brain tumors (14–16).
Despite the critical role it plays in cancer metastasis, the exact
mechanism of PODXL in OSCC is still unclear.
Epigenetic regulation, including DNA methylation and
histone modification, plays important roles in gene modulation. DNA
methylation of promoter CpG islands regulates transcriptional
activation and repression. Dysregulation of the DNA methylation
status alters the transcription activity of oncogenes and tumor
suppressor genes, which results in abnormalities in cellular
behavior, and eventually contributes to neoplastic formation
(17). Aberrant changes in DNA
methylation were reported with malignant progression in OSCC
(18). Promoter hypermethylation
of ALK in OSCC was correlated with node-negative metastasis
(19). A global methylation
analysis of OSCC revealed an aberrant methylation status enriched
in genes often found in the WNT and mitogen-activated protein
kinase (MAPK) signaling pathway (20), yet the DNA methylation of PODXL and
its expression in association with tumor aggressiveness in OSCC are
still unclear. In the present study, we investigated the role of
PODXL in contributing to tumor metastasis in human OSCC. We found
that PODXL expression was associated with tumor aggressiveness and
invasiveness. PODXL regulates the phosphorylation of focal adhesion
kinase (FAK) and paxillin, and the formation of filopodia and
invadopodia. PODXL expression was associated with the DNA
methylation status. Modulation of the extracellular matrix (ECM)
and pro-metastatic gene expression levels by PODXL contribute to
tumor metastasis.
Materials and methods
Cell culture
Human OSCC lines (FaDu and SAS) were purchased from
American Type Culture Collection (ATCC; Manassas, VA, USA). SAS
cells were grown in Dulbecco’s modified Eagle’s medium (DMEM), and
FaDu cells were grown in RPMI-1640. All culture media were
supplemented with 5% fetal bovine serum (FBS, Gibco, Grand Island,
NY, USA) and penicillin/streptomycin (Gibco), and were grown at
37°C in a 5% CO2 atmosphere.
Lentivirus production
Small hairpin RNA vectors for PODXL silencing
(5′-GTCGTCAAAGAAATCACTATT-3′) were obtained from the National RNAi
Core Facility (Academia Sinica, Taiwan). To generate stable
PODXL-knockdown cell lines, HEK293T packaging cells were
co-transfected with a packaging plasmid (pCMV-ΔR8.91), and envelope
(pMDG) and hairpin pLKO-RNAi vectors using a PolyJET Transfection
kit (SignaGen Laboratories, Ijamsville, MD, USA). At 48-h
post-transfection, virus-containing supernatants were collected,
mixed with fresh media containing polybrene (8 μg/ml), and
incubated with target cells for another 48 h. Transduced cells were
selected with puromycin (2 μg/ml) for 7 days.
Transwell migration and invasion
assays
Cells (105) were seeded in a Transwell
insert (8-μm filters, Corning, New York, NY, USA) coated with or
without Matrigel (BD Biosciences, La Jolla, CA, USA) for 8 (for the
migration assay) and 24 h (for the invasion assay). After
incubation, cells were fixed with 4% paraformaldehyde for 10 min.
Cells that had not invaded were removed with a cotton swab; invaded
cells were stained with 4′,6-diamino-2-phenylindole (DAPI), imaged
under an inverted fluorescent microscope (Zeiss), and quantified
using ImageJ software.
Time-lapse migration assay
Cells (2×104) were seeded in a chamber
with complete growth media, and monitored under an inverted light
microscope (Zeiss HAL100 reflected-light microscope) with a
temperature and CO2 control system. Images were captured
every 10 min for 6 h. The migration distance was defined as the
movement of the cell center per unit time, as measured by MetaMorph
software.
Cell proliferation assay
Differences in the proliferation of SAS/LKO and
SAS/shPODXL cells were evaluated by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay. Briefly, 2,000 cells were seeded in a 96-well plate with
complete media for 1–5 days, and cells were incubated in 50 μl of
0.5 mg/ml MTT for 3 h. DMSO was added to dissolve the crystals,
which were measured on a microplate reader at 540-nm absorbance.
Data are presented as the percentage of growth on the day after
seeding. To test the effect of PODXL on colony formation, 2000
SAS/LKO and SAS/shPODXL cells were seeded in 6-well plates and
incubated for 7 days. Surviving colonies were stained with crystal
violet after methanol fixation. Visible colonies (≥50 cells) were
counted.
Western blotting
Cells were lysed in RIPA buffer (150 mM NaCl, 1%
Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecylsulfate
(SDS), and 50 mM Tris-HCl at pH 7.4) containing a protease and
phosphatase inhibitor cocktail (Roche, Basel, Switzerland). For
immunoprecipitation, cell lysates were precleared with
agarose-protein G for 1 h and incubated overnight at 4°C with the
appropriate protein G-conjugated primary antibodies. Beads were
washed three times with RIPA buffer and boiled in sample buffer (50
mM Tris-HCl at pH 6.8, 2% SDS, 0.1% bromophenol blue, and 10%
glycerol). Equal amounts of proteins were separated on
SDS-polyacrylamide gel electrophoresis (PAGE) and then transferred
to polyvinylidene difluoride membranes (Millipore, Bedford, MA,
USA). Membranes were blocked with 1% bovine serum albumin
(BSA)/TBST and incubated overnight with specific primary antibodies
against PODXL (1:500, Santa Cruz Biotechnology, Santa Cruz, CA,
USA), FAK (1:2000, Millipore), phospho-FAK (1:2000, Millipore),
paxillin (1:2000, Millipore), phospho-paxillin (1:500, Millipore),
or α-tubulin (1:5000, Sigma, St. Louis, MO, USA). Membranes were
then incubated with the appropriate horseradish peroxidase
(HRP)-conjugated secondary antibodies (1:10000, Jackson
Immunoresearch) for 1 h at room temperature, and proteins were
detected using an ECL kit (Millipore, Temecula, CA, USA).
Immunofluorescence
Cells were grown onto cover slide and fixed with 4%
paraformaldehyde for 10 min, followed by blocking with 3% bovine
serum albumin (BSA) for 1 h. The cover slide was incubated with
FITC-conjugated cortactin (Merck Millipore) and
rhodamine-conjugated phalloidin (Invitrogen) for 1 h at room
temperature. The fluorescent images were observed under a confocal
microscope (TCS-SP5, Leica).
Immunohistochemical (IHC) analysis of
PODXL protein
A paraffin-embedded human colon cancer tissue
microarray (US Biomax Inc.) was immersed in xylene for 30 min and
then rehydrated in graded ethanol. The slide was immersed with 0.01
M sodium citric buffer (pH 6.0) for antigen retrieval, followed by
soaking with 3% hydrogen peroxide and blocking with normal horse
serum (ABC kit, Vector). The slide was incubated with anti-PODXL
antibody (10 μg/ml, Sigma) for 1 h at room temperature. After
washing three times with PBST, the Super Enhancer reagent (Super
Sensitive Polymer HRP detection system, BioGenex) was added and
incubated for 20 min. DAB Chromogen was then added for 3 min, and
the reaction was stopped by PBS washing. The slide was stained by
hematoxylin for 3 min and mounted with a xylene-based mounting
solution.
Detection of PODXL methylation
The methylation status in PODXL was analyzed by a
methylation-specific polymerase chain reaction (MSP) assay using an
EZ DNA Methylation-Direct assay kit (Zymo Research). Briefly, the
genomic DNA of SAS and FaDu cells was extracted and modified by
sodium bisulfate treatment, which converted all of the unmethylated
cytosines to uracils while leaving methylated cytosines unchanged.
Bisulfate-converted DNA was subjected to subsequent PCR
amplification. The specific MSP primers for PODXL (methylated,
PODXL-M: 5′-TCGTCGGGTTTAT TTAGAAGTATTC-3′ and 5′-TATATATACGCGAAAA
CCAAAACG-3′) and (unmethylated, PODXL-U: 5′-TGT
TGGGTTTATTTAGAAGTATTTGG-3′ and 5′-TATATATA CACAAAAACCAAAACAA-3′)
were designed from the MethPrimer database. Methylated and
unmethylated genomic DNAs (Zymo Research) were used as an
experimental control. To analyze the methylated CpG site in the
PODXL promoter region, specific PCR primers designed from the
MethPimer database were used for amplification, and PCR products
were cloned into the pGEM-T vector for further sequencing analysis.
Four clones were sequenced to assess the level of methylation of
each CpG site.
Real-time PCR
Total RNA was extracted using an RNeasy Plus Mini
kit (Qiagen) and reverse-transcribed using SuperScrip III reverse
transcriptase (Invitrogen). A quantitative PCR was performed using
the resulting cDNA, LightCycler 480 SYBR Green I Master Mix (Roche)
and a LightCycler 480 System (Roche). Results are expressed as
multiples of change relative to the control sample, using the
method of ΔΔCT. GAPDH or 18SrRNA was used
as internal control for normalization. Primer sequences are listed
in Table I.
 | Table IList of oligonucleotides for
real-time PCR assay. |
Table I
List of oligonucleotides for
real-time PCR assay.
| Gene symbol | Name | Sequence
(5′→3′) |
|---|
| PODXL | Podocalyxin-like
1 | F:
aaggccaggggttcacat
R: agcctcgcatccctctaact |
| LOX | Lysyl oxidase | F:
tgggaatggcacagttgtc
R: aaacttgctttgtggccttc |
| LOXL4 | Lysyl oxidase-like
4 | F:
ccagcttctgtctggaggac
R: aagttggcacatgcgtagc |
| COL17A1 | Collagen type XVII,
α1 | F:
gctggagatctggattacaatga
R: ccttgcagtaggccctga |
| COL13A1 | Collagen type XIII,
α1 | F:
taagcagcatgccagcag
R: cagtcgcactgaattgagga |
| ITGB3 | Integrin β3 | F:
cgctaaatttgaggaagaacg
R: gaaggtagacgtggcctcttt |
| CDH1 | E-cadherin | F:
ggaactatgaaaagtgggcttg
R: aaattgccaggctcaatgac |
| CDH3 | P-cadherin | F:
caccacccaccctgagag
R: tttggcctcaaaatccaaac |
| CDH11 | OB-cadherin | F:
aaacagcctggctcaacatc
R: cttcctgatgccgattgtg |
| CREM | cAMP responsive
element modulator | F:
tgatggcacacagcagttct
R: atgtcaccagtggcagctt |
| MMP1 | Matrix
metalloproteinase 1 | F:
gctaacctttgatgctataactacga
R: tttgtgcgcatgtagaatctg |
| IL1B | Interleukin 1β | F:
tacctgtcctgcgtgttgaa
R: tctttgggtaatttttgggatct |
| IL1A | Interleukin 1α | F:
acaaaaggcgaagaagactga
R: ggaactttggccatcttgac |
| IL8 | Interleukin 8 | F:
agacagcagagcacacaagc
R: atggttccttccggtggt |
| IL24 | Interleukin 24 | F:
gaagaattgaggctgcttgg
R: gagggcagaagggtctgg |
| TIMP1 | Tissue inhibitor of
metalloproteinase 1 | F:
gggcttcaccaagacctaca
R: tgcaggggatggataaaca |
| CCNA2 | Cyclin A2 | F:
ggtactgaagtccgggaacc
R: gaagatccttaaggggtgcaa |
| ODC1 | Ornithine
decarboxylase 1 | F:
aaaacatgggcgcttacact
R: tggaattgctgcatgagttg |
| CCNE2 | Cyclin E2 | F:
gccattgattcattagagttcca
R: ctgtcccactccaaacctg |
| GAPDH |
Glyceraldehyde-3-phosphate
dehydrogenase | F:
cttcaccaccatggaggaggc
R: ggcatggactgtggtcatgag |
| 18s
rRNA | | F:
gcaattattccccatgaacg
R: gggacttaatcaacgcaagc |
cDNA microarray and bioinformatic
analysis
Total RNA was extracted using an RNeasy Plus Mini
kit (Qiagen), and RNA quality was analyzed on an Agilent 2100
Bioanalyzer (RNA6000 nanochip). A human cDNA microarray was
analyzed according to the protocol of the Agilent 2 color system
(Agilent G4845A, 4×44K). Experiments were performed and analyzed by
the Microarray Core Facility of the Institute of Molecular Biology,
and the Core Facility of the Institute of Cellular Organismic and
Biology, Academia Sinica. Genetic networks were analyzed using
Pathway Studio 7 and Sub-network Enrichment Analysis (SNEA)
software (Ariadne Genomics, Rockville, MD, USA). Individual cancer
datasets were downloaded from Oncomine (Compendia Bioscience).
p-values are given for the medium-rank analysis.
Statistical analysis
The data were derived from at least three
independent experiments. Values are expressed as the mean ±
standard error of the mean (SEM). Significant differences were
determined using a two-tailed, unpaired t-test, unless otherwise
specified. A p-value of <0.05 or <0.01 was considered
significant.
Results
PODXL is overexpressed in cancer cells
and is associated with tumor aggressiveness in OSCC
In order to investigate the association of PODXL
with cancer, we used the Oncomine Cancer Profiling database to
analyze expression levels of PODXL in cancerous vs. normal tissues.
Five types of cancer were selected, and five array datasets were
analyzed in each cancer type including colon (21–25),
gastric (26–28), liver (29–32),
esophageal (33–37), and head and neck cancers (38–42).
Results showed that PODXL was overexpressed in all types of
cancerous tissues, compared to normal ones (Fig. 1), suggesting that PODXL expression
may be associated with cancer. Assessment of the PODXL messenger
RNA level by real-time PCR analyses showed similar results in colon
cancer specimens (Fig. 2A).
Moreover, results of the immunohistochemical assay revealed that
the PODXL protein level was upregulated in lymph node metastatic
colon tumor tissues, compared to matched primary tumor sites (n=33,
p=0.022) (Fig. 2B).
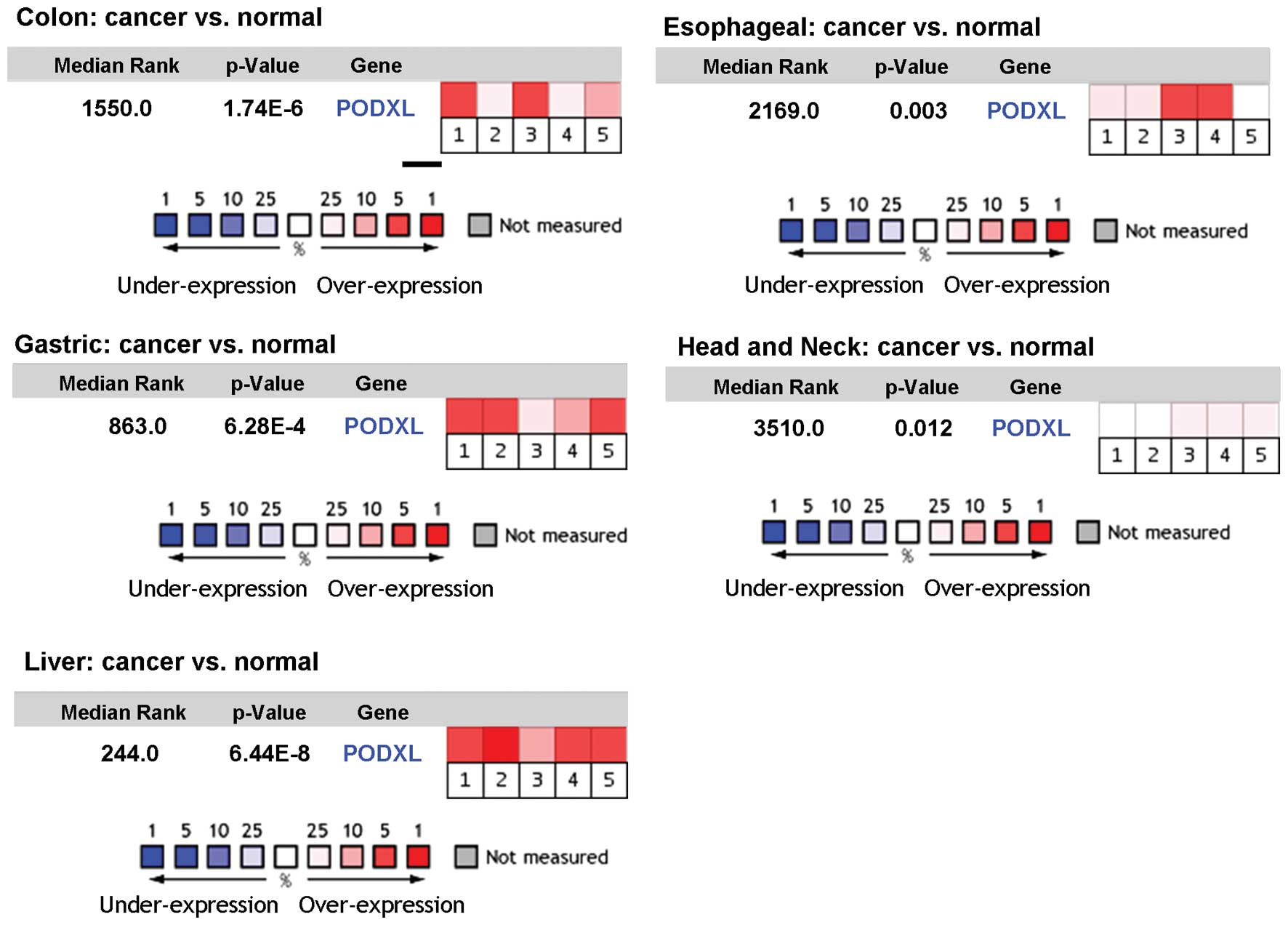 | Figure 1Comparison of PODXL expression in
cancerous vs. normal tissues of different cancer types. The
Oncomine Cancer Profiling Database was used to assess the
expression level of PODXL in cancer vs. normal tissues. Each box
represents a dataset, which includes colon [1, Hong (21); 2, Kaiser (22); 3, Ki et al (23); 4, Sabates-Bellver (24); 5, Skrzypczak (25)], gastric [1, Cho (26); 2, D’Errico (27); 3, TCGA_dataset; 4, TCGA_dataset; 5,
Wang (28)], liver [1, Chen
(29); 2, Mas (30); 3, Roessler (31); 4, Roessler (31); 5, Wurmbach (32)], esophageal [1, Hao (34); 2, Hu (35); 3, Kim (36); 4, Kimchi (37); 5, Su (33)], head and neck [1, Cromer (38); 2, Ginos (39); 3, Schlingemann (40); 4, Sengupta (41); 5, Toruner (42)] (see refs. 21–42).
The p-value is given for the medium-rank analysis. |
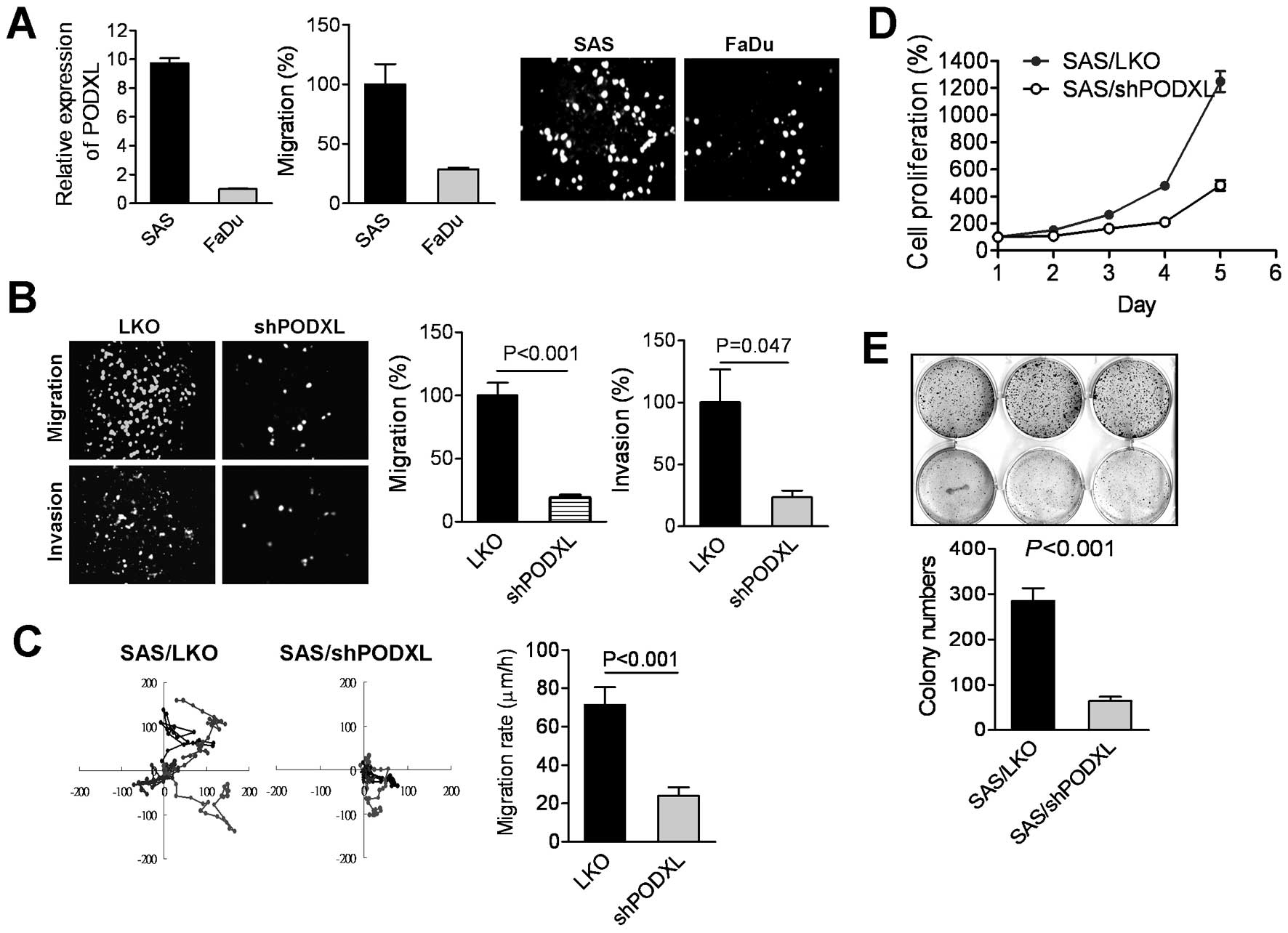
We further examined the correlation of PODXL
expression with tumor migration in two human oral squamous cancer
cell lines, SAS and FaDu. Results of real-time PCR analysis showed
a higher expression level of PODXL in SAS cells that exhibited
potential migratory ability compared to FaDu cells (Fig. 3A), suggesting that PODXL expression
might be associated with tumor motility. We used shRNA to silence
PODXL mRNA in SAS cells, and results of the Transwell analyses
showed that the knockdown of PODXL significantly diminished tumor
migration and invasion (Fig. 3B).
Moreover, time-lapse photographic observations revealed that
suppression of PODXL impacted the cell migratory velocity (Fig. 3C). We further found that inhibition
of PODXL effectively reduced tumor proliferation and colony
formation in SAS cells (Fig. 3D and
E), indicating that PODXL expression was associated with tumor
aggressiveness.
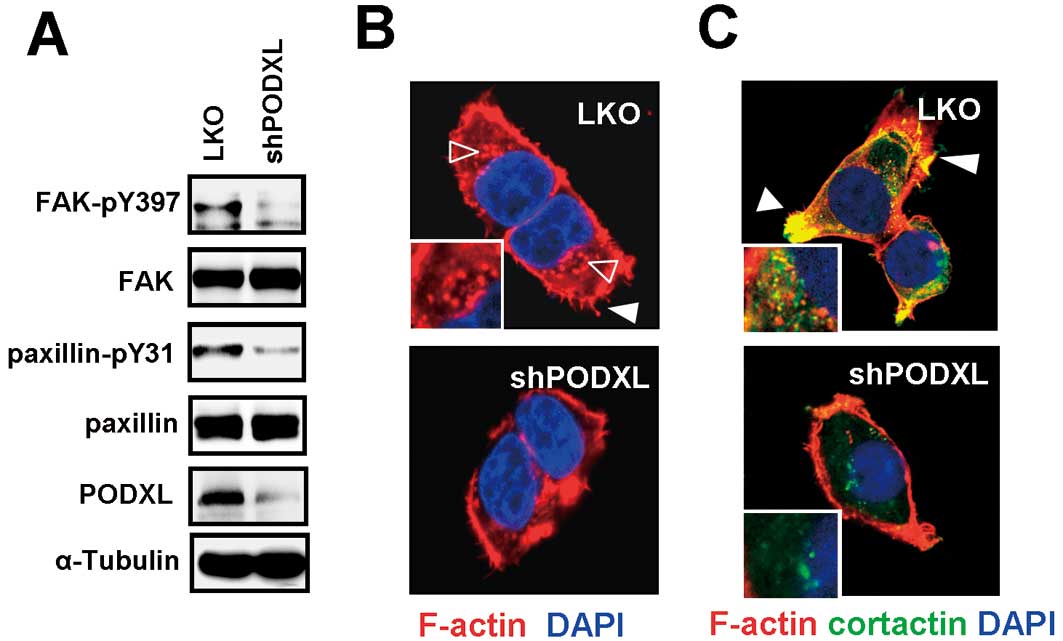
Suppression of PODXL inhibited FAK
activation and filopodia formation
Given that the activation of focal adhesion kinase
(FAK) participates in promoting cell migration and invasion, we
next assessed whether PODXL regulated FAK signaling. Results showed
that the phosphorylation of FAK and its downstream molecule,
paxillin, was diminished in PODXL-knockdown SAS cells (Fig. 4A). FAK and paxillin play important
roles in modulating F-actin reorganization, which results in
enhanced cell polarity. We next examined F-actin expression using
phalloidin staining, and the results showed that suppression of
PODXL reduced the expression levels of filopodia at the membrane
edge (closed arrow) and podosome-like structures in the perinuclear
region (open arrows), compared to mock-transduced cells (Fig. 4B). To further analyze the effect of
PODXL on invadopodia formation, immunofluorescent co-staining with
F-actin and cortactin, which were used to identify structures of
invadopodia, were performed. Results showed that knockdown of PODXL
effectively reduced F-actin and cortactin colocalization. In
mock-transduced cells, most of the cortactin and F-actin had
co-localized at the membrane edge, indicating the invasive front.
Some of the colocalization was observed in the perinuclear region,
which indicated the invadopodia precursor (43). However, suppression of PODXL
significantly inhibited the colocalization of cortactin and F-actin
at both the membrane edge and in perinuclear regions (Fig. 4C), suggesting that inhibition of
PODXL impacted focal kinase activation and invadopodia
formation.
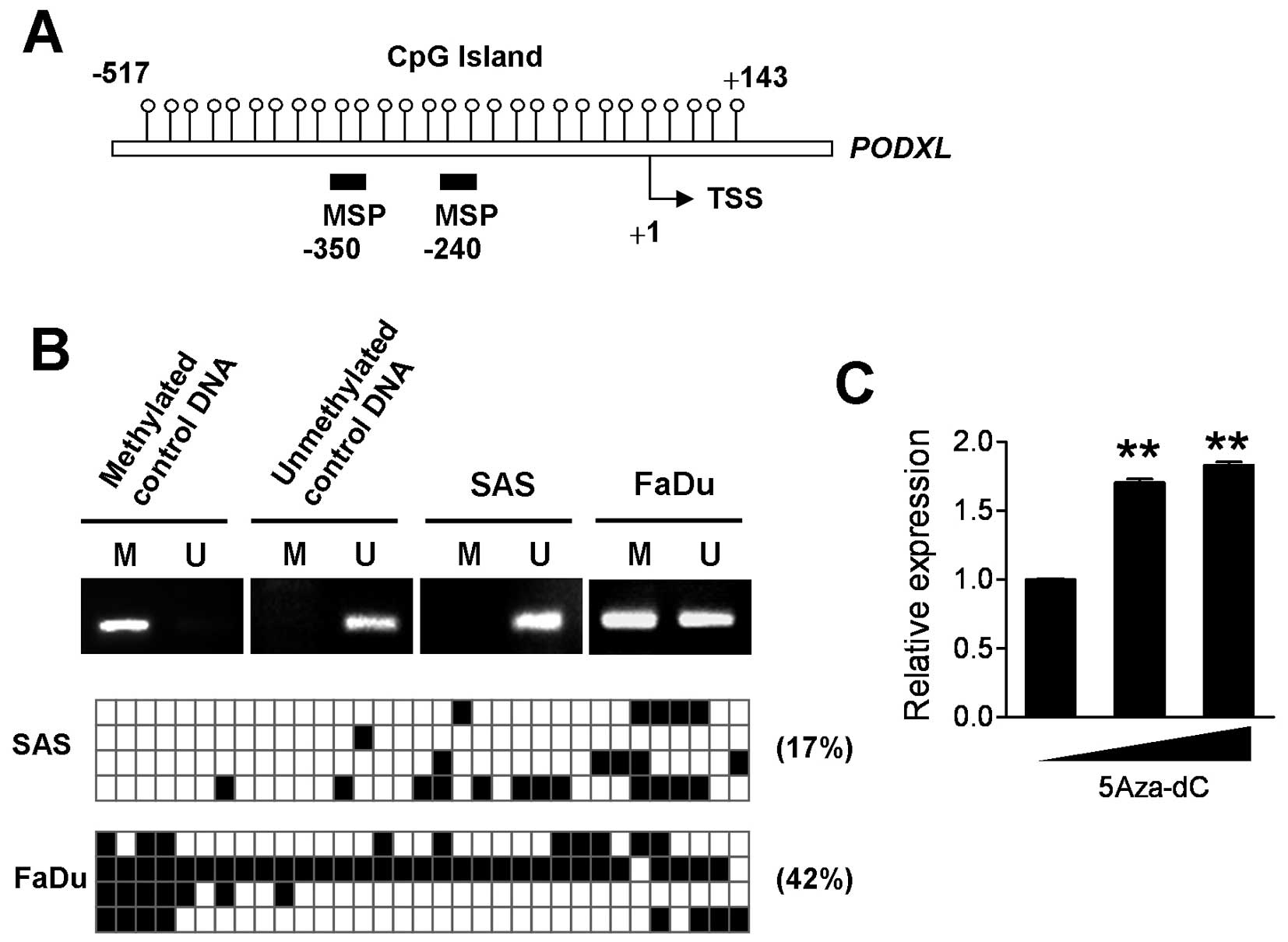
Expression of PODXL is regulated by DNA
methylation
Epigenetic regulation including histone modification
and DNA methylation often participates in modulating gene
transcription. We next assessed whether the PODXL expression was
associated with methylation of its DNA. The MethPrimer database was
used to predict a CpG island located from downstream 143 to
upstream 517 of the PODXL gene, which was related to the
transcriptional start site (Fig.
5A). Data of the methylate-specific PCR (MSP) assay showed that
PODXL displayed totally unmethylated expression in SAS cells,
whereas it showed only partially unmethylated status in FaDu cells
(Fig. 5B upper panel). Totally
unmethylated and methylated genomic DNA was used as experimental
controls. Further analysis of the precise methylated level of each
CpG site by a bisulfate sequencing assay showed that PODXL DNA in
SAS cells was 17% methylated, whereas 42% methylation of the CpG
island of PODXL was detected in FaDu cells (Fig. 5B lower panel). These data were
consistent with findings of the real-time PCR and MSP analyses.
Moreover, treatment of FaDu cells with 5-aza-deoxycytidine
(5-aza-dC) for 72 h, a DNA methyltransferase inhibitor, significant
increased the PODXL mRNA level (Fig.
5C), indicating that DNA methylation may participate in
regulating PODXL expression.
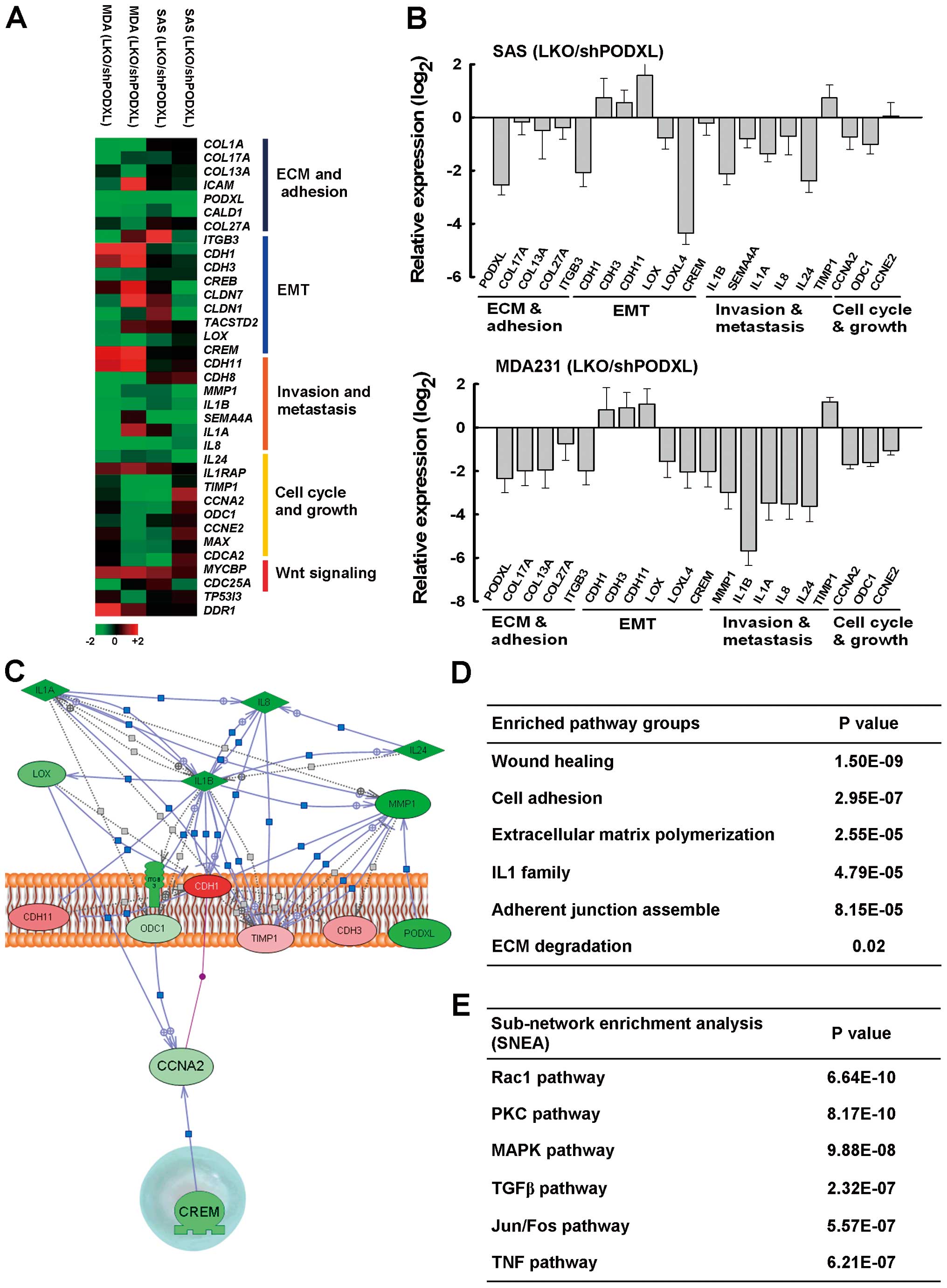
PODXL modulates ECM and pro-metastatic
gene expression levels
To further explore PODXL-mediated gene expression
levels, the cDNA of two biological repeats from PODXL-knockdown SAS
and MDA-MB-231 cells was subjected to microarray analyses to
examine gene changes after silencing of PODXL. We found that PODXL
suppression markedly attenuated genes specifically associated with
ECM organization (COL13, COL17 and ITGB3), cell
adhesion and the EMT (CDH1, CDH3, LOX and LOX4), and
pro-metastasis cytokines (interleukin (IL)1β, IL8 and
IL24) (Fig. 6A). The
expression levels of these genes were further confirmed by
real-time PCR analysis (Fig. 6B).
Cellular functional grouping by a Gene Set Enrichment Analysis
(GSEA) illustrated that suppression of PODXL significantly affected
wound-healing, cell adhesion, ECM polymerization and degradation,
and adherent junction assembly (Fig.
6C and D). We further analyzed the hit entities affected by
PODXL and linked to the tentative intracellular kinase cascade
underlying PODXL using sub-network enrichment analysis (SNEA)
algorithm software. The hit entities selected by the microarray
data were analyzed by an SNEA algorithm, and the results showed
that the pivotal effectors involved in the downstream signaling of
PODXL responsible for regulating gene expression, might include
Rac1, protein kinase C (PKC), mitogen-activated protein kinase
(MAPK), transforming growth factor (TGF)-β, and activator protein
(AP)-1 pathways (Fig. 6E). These
data indicated that PODXL plays a crucial role in promoting tumor
metastasis.
Discussion
Overexpression of PODXL was demonstrated to be an
independent factor in the poor prognoses of several cancer types
such as breast, colon, bladder and brain tumors. However, the role
of PODXL in OSCC and its underlying mechanism have not yet been
delineated. In the present study, we found that elevation of PODXL
correlated with the migratory and invasive abilities of OSCC.
Suppression of PODXL significantly diminished oral cancer cell
aggressiveness. PODXL silencing inhibited FAK and paxillin
activation, and suppressed F-actin and cortactin colocalization.
Gene expression profile and molecular pathway analyses revealed
that PODXL regulates genes associated with the EMT, ECM
polymerization, cell adhesion and metastatic cytokine expression
levels. These data suggest that PODXL might play a crucial role in
promoting tumor metastases in OSCC.
FAK and its downstream molecule, paxillin, play
critical roles in signaling transduction by integrins and the ECM.
Activation of FAK and paxillin promotes actin cytoskeletal
rearrangement at the leading edge of lamella structures during cell
migration. In addition, activation of cortactin participates in
actin nucleation, and cortactin colocalization to sites of actin
assembly in lamellipodia promotes dynamic cell spreading and
motility (44). Colocalization of
cortactin with F-actin in the cytoplasm indicates the formation of
the invadopodia precursor (45),
which further enhances ECM degradation for tumor invasion (46).
Our data showed that knockdown of PODXL inhibited
activation of FAK and paxillin, and suppressed colocalization of
cortactin with actin both at the membrane edge and in the
cytoplasm. Phalloidin staining data also showed that knockdown of
PODXL reduced the formation of filopodia and podosome-like punctae,
indicating that PODXL is crucial for cell mobility. Moreover, a
gene analytical profile revealed that suppression of PODXL
significantly affected a cohort of genes associated with ECM
organization (COL13, COL17 and ITGB3), cell adhesion
and the EMT (CDH1, CDH3, LOX and LOX4), and
pro-metastasis cytokines (IL1β, IL8 and IL24). The
induction of matrix metalloproteinase (MMP)-9 by PODXL was also
identified in MCF7 breast cancer cells (9), suggesting that PODXL predominantly
regulates tumor invasiveness. In addition to regulating
intracellular signaling by PODXL, the extracellular domain of PODXL
is enriched in sialofucosylated oligosaccharides, which both serve
as O-linked glycans to bind E- and L-selectin (47,48),
and facilitate interactions of circulating tumor cells and the
vasculature during tumor metastasis. Together, the evidence
highlights the importance of PODXL in tumor invasiveness and
metastases.
Aberrant DNA methylation was demonstrated to be
associated with cancer formation. Deciphering abnormalities in DNA
methylation would be conducive to understanding the early onset of
neoplastic progression. A global analysis of DNA methylation in
oral cancer revealed that increased DNA hypermethylation was
detected in dysplasia, compared to normal tissues (20). Both DNA hypomethylation and
hypermethylation were changed more frequently in oral carcinoma
in situ (OIC/OSCC), suggesting that epigenetic deregulation
is more prevalent in OSCC progression. The most commonly reported
changes in OSCC are the hypermethylation of E-cadherin (CDH1),
PTEN, and p16 (CDKN2A) (49–51).
Very few studies delineated DNA hypomethylation in OSCC. We found
that the PODXL promoter region from −517 to +143 exhibited abundant
CpG dinucleotides. Highly invasive SAS cells showed hypomethylation
in the PODXL promoter region, whereas lowly invasive FaDu cells
showed half methylation. Treatment of low PODXL-expressing cells
with 5-aza-dC, a DNA methyltransferase inhibitor, increased the
PODXL transcription level. A previous study reported that
transcriptional regulation of PODXL is supported by the Sp1
transcription factor (52) and
that binding of Sp1 to DNA interferes with DNA methylation
(53). However, it is still
unclear whether the hypomethylation of PODXL DNA is regulated by
Sp1 binding or by other passive DNA demethylation processes.
Nevertheless, our data suggest that hypomethylation of the PODXL
promoter is associated with cell invasiveness and can be used as a
diagnostic biomarker for OSCC.
In conclusion, our results showed that elevation of
PODXL is associated with tumor aggressiveness through
FAK/paxillin/cortactin signaling induction and metastatic gene
expression level promotion in OSCC, suggesting its clinical value
as a prognostic biomarker and as a therapeutic target for managing
metastatic OSCC.
Acknowledgements
We thank the Core Facility of the Institute of
Cellular and Organismic Biology, Microarray Core Facility of the
Institute of Molecular Biology, and the RNAi Core, Academia Sinica
for their technical support. This study was supported by grants
from Academia Sinica and the National Science Council
(NSC101-2321-B-001-021 and NSC102-2325-B-001-010 to H.-C.W.) and
(NSC102-2320-B-038-005 to C.-W.L.).
References
|
1
|
Chen YJ, Chang JT, Liao CT, et al: Head
and neck cancer in the betel quid chewing area: recent advances in
molecular carcinogenesis. Cancer Sci. 99:1507–1514. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zygogianni AG, Kyrgias G, Karakitsos P, et
al: Oral squamous cell cancer: early detection and the role of
alcohol and smoking. Head Neck Oncol. 3:22011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fina L, Molgaard HV, Robertson D, et al:
Expression of the CD34 gene in vascular endothelial cells. Blood.
75:2417–2426. 1990.PubMed/NCBI
|
|
4
|
Labastie MC, Cortes F, Romeo PH, Dulac C
and Peault B: Molecular identity of hematopoietic precursor cells
emerging in the human embryo. Blood. 92:3624–3635. 1998.PubMed/NCBI
|
|
5
|
Kerjaschki D, Sharkey DJ and Farquhar MG:
Identification and characterization of podocalyxin - the major
sialoprotein of the renal glomerular epithelial cell. J Cell Biol.
98:1591–1596. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doyonnas R, Nielsen JS, Chelliah S, et al:
Podocalyxin is a CD34-related marker of murine hematopoietic stem
cells and embryonic erythroid cells. Blood. 105:4170–4178. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nielsen JS and McNagny KM: The role of
podocalyxin in health and disease. J Am Soc Nephrol. 20:1669–1676.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Economou CG, Kitsiou PV, Tzinia AK, et al:
Enhanced podocalyxin expression alters the structure of podocyte
basal surface. J Cell Sci. 117:3281–3294. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sizemore S, Cicek M, Sizemore N, Ng KP and
Casey G: Podocalyxin increases the aggressive phenotype of breast
and prostate cancer cells in vitro through its interaction with
ezrin. Cancer Res. 67:6183–6191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsu YH, Lin WL, Hou YT, et al: Podocalyxin
EBP50 ezrin molecular complex enhances the metastatic potential of
renal cell carcinoma through recruiting Rac1 guanine nucleotide
exchange factor ARHGEF7. Am J Pathol. 176:3050–3061. 2010.
View Article : Google Scholar
|
|
11
|
Schmieder S, Nagai M, Orlando RA, Takeda T
and Farquhar MG: Podocalyxin activates RhoA and induces actin
reorganization through NHERF1 and Ezrin in MDCK cells. J Am Soc
Nephrol. 15:2289–2298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doyonnas R, Kershaw DB, Duhme C, et al:
Anuria, omphalocele, and perinatal lethality in mice lacking the
CD34-related protein podocalyxin. J Exp Med. 194:13–27. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Forse CL, Yilmaz YE, Pinnaduwage D, et al:
Elevated expression of podocalyxin is associated with lymphatic
invasion, basal-like phenotype, and clinical outcome in axillary
lymph node-negative breast cancer. Breast Cancer Res Treat.
137:709–719. 2013. View Article : Google Scholar
|
|
14
|
Binder ZA, Siu IM, Eberhart CG, et al:
Podocalyxin-like protein is expressed in glioblastoma multiforme
stem-like cells and is associated with poor outcome. PLoS One.
8:e759452013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boman K, Larsson AH, Segersten U, et al:
Membranous expression of podocalyxin-like protein is an independent
factor of poor prognosis in urothelial bladder cancer. Br J Cancer.
108:2321–2328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Larsson A, Johansson ME, Wangefjord S, et
al: Overexpression of podocalyxin-like protein is an independent
factor of poor prognosis in colorectal cancer. Br J Cancer.
105:666–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen QW, Zhu XY, Li YY and Meng ZQ:
Epigenetic regulation and cancer (Review). Oncol Rep. 31:523–532.
2014.
|
|
18
|
Jithesh PV, Risk JM, Schache AG, et al:
The epigenetic landscape of oral squamous cell carcinoma. Br J
Cancer. 108:370–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang TT, Gonzales CB, Gu F, et al:
Epigenetic deregulation of the anaplastic lymphoma kinase gene
modulates mesenchymal characteristics of oral squamous cell
carcinomas. Carcinogenesis. 34:1717–1727. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Towle R, Truong D, Hogg K, Robinson WP,
Poh CF and Garnis C: Global analysis of DNA methylation changes
during progression of oral cancer. Oral Oncol. 49:1033–1042. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong Y, Downey T, Eu KW, Koh PK and Cheah
PY: A ‘metastasis-prone’ signature for early-stage mismatch-repair
proficient sporadic colorectal cancer patients and its implications
for possible therapeutics. Clin Exp Metastasis. 27:83–90. 2010.
|
|
22
|
Kaiser S, Park YK, Franklin JL, et al:
Transcriptional recapitulation and subversion of embryonic colon
development by mouse colon tumor models and human colon cancer.
Genome Biol. 8:R1312007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ki DH, Jeung HC, Park CH, et al: Whole
genome analysis for liver metastasis gene signatures in colorectal
cancer. Int J Cancer. 121:2005–2012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sabates-Bellver J, Van der Flier LG, de
Palo M, et al: Transcriptome profile of human colorectal adenomas.
Mol Cancer Res. 5:1263–1275. 2007. View Article : Google Scholar
|
|
25
|
Skrzypczak M, Goryca K, Rubel T, et al:
Modeling oncogenic signaling in colon tumors by multidirectional
analyses of microarray data directed for maximization of analytical
reliability. PLoS One. 5:pii: e13091. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cho JY, Lim JY, Cheong JH, et al: Gene
expression signature-based prognostic risk score in gastric cancer.
Clin Cancer Res. 17:1850–1857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
D’Errico M, de Rinaldis E, Blasi MF, et
al: Genome-wide expression profile of sporadic gastric cancers with
microsatellite instability. Eur J Cancer. 45:461–469.
2009.PubMed/NCBI
|
|
28
|
Wang Q, Wen YG, Li DP, et al: Upregulated
INHBA expression is associated with poor survival in gastric
cancer. Med Oncol. 29:77–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X, Cheung ST, So S, et al: Gene
expression patterns in human liver cancers. Mol Biol Cell.
13:1929–1939. 2002. View Article : Google Scholar PubMed/NCBI
|
|
30
|
Mas VR, Maluf DG, Archer KJ, et al: Genes
involved in viral carcinogenesis and tumor initiation in hepatitis
C virus-induced hepatocellular carcinoma. Mol Med. 15:85–94.
2009.PubMed/NCBI
|
|
31
|
Roessler S, Jia HL, Budhu A, et al: A
unique metastasis gene signature enables prediction of tumor
relapse in early-stage hepatocellular carcinoma patients. Cancer
Res. 70:10202–10212. 2010. View Article : Google Scholar
|
|
32
|
Wurmbach E, Chen YB, Khitrov G, et al:
Genome-wide molecular profiles of HCV-induced dysplasia and
hepatocellular carcinoma. Hepatology. 45:938–947. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Su H, Hu N, Yang HH, et al: Global gene
expression profiling and validation in esophageal squamous cell
carcinoma and its association with clinical phenotypes. Clin Cancer
Res. 17:2955–2966. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hao Y, Triadafilopoulos G, Sahbaie P,
Young HS, Omary MB and Lowe AW: Gene expression profiling reveals
stromal genes expressed in common between Barrett’s esophagus and
adenocarcinoma. Gastroenterology. 131:925–933. 2006.PubMed/NCBI
|
|
35
|
Hu N, Clifford RJ, Yang HH, et al: Genome
wide analysis of DNA copy number neutral loss of heterozygosity
(CNNLOH) and its relation to gene expression in esophageal squamous
cell carcinoma. BMC Genomics. 11:5762010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim SM, Park YY, Park ES, et al:
Prognostic biomarkers for esophageal adenocarcinoma identified by
analysis of tumor transcriptome. PLoS One. 5:e150742010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kimchi ET, Posner MC, Park JO, et al:
Progression of Barrett’s metaplasia to adenocarcinoma is associated
with the suppression of the transcriptional programs of epidermal
differentiation. Cancer Res. 65:3146–3154. 2005.
|
|
38
|
Cromer A, Carles A, Millon R, et al:
Identification of genes associated with tumorigenesis and
metastatic potential of hypopharyngeal cancer by microarray
analysis. Oncogene. 23:2484–2498. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ginos MA, Page GP, Michalowicz BS, et al:
Identification of a gene expression signature associated with
recurrent disease in squamous cell carcinoma of the head and neck.
Cancer Res. 64:55–63. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schlingemann J, Habtemichael N, Ittrich C,
et al: Patient-based cross-platform comparison of oligonucleotide
microarray expression profiles. Lab Invest. 85:1024–1039. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sengupta S, den Boon JA, Chen IH, et al:
Genome-wide expression profiling reveals EBV-associated inhibition
of MHC class I expression in nasopharyngeal carcinoma. Cancer Res.
66:7999–8006. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Toruner GA, Ulger C, Alkan M, et al:
Association between gene expression profile and tumor invasion in
oral squamous cell carcinoma. Cancer Genet Cytogenet. 154:27–35.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Murphy DA and Courtneidge SA: The ‘ins’
and ‘outs’ of podosomes and invadopodia: characteristics, formation
and function. Nat Rev Mol Cell Biol. 12:413–426. 2011.
|
|
44
|
Sung BH, Zhu X, Kaverina I and Weaver AM:
Cortactin controls cell motility and lamellipodial dynamics by
regulating ECM secretion. Curr Biol. 21:1460–1469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Oser M, Yamaguchi H, Mader CC, et al:
Cortactin regulates cofilin and N-WASp activities to control the
stages of invadopodium assembly and maturation. J Cell Biol.
186:571–587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Clark ES, Whigham AS, Yarbrough WG and
Weaver AM: Cortactin is an essential regulator of matrix
metalloproteinase secretion and extracellular matrix degradation in
invadopodia. Cancer Res. 67:4227–4235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dallas MR, Chen SH, Streppel MM, Sharma S,
Maitra A and Konstantopoulos K: Sialofucosylated podocalyxin is a
functional E- and L-selectin ligand expressed by metastatic
pancreatic cancer cells. Am J Physiol Cell Physiol. 303:C616–C624.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Thomas SN, Schnaar RL and Konstantopoulos
K: Podocalyxin-like protein is an E-/L-selectin ligand on colon
carcinoma cells: comparative biochemical properties of selectin
ligands in host and tumor cells. Am J Physiol Cell Physiol.
296:C505–C513. 2009. View Article : Google Scholar
|
|
49
|
Su PF, Huang WL, Wu HT, Wu CH, Liu TY and
Kao SY: p16(INK4A) promoter hypermethylation is associated with
invasiveness and prognosis of oral squamous cell carcinoma in an
age-dependent manner. Oral Oncol. 46:734–739. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Alyasiri NS, Ali A, Kazim Z, et al:
Aberrant promoter methylation of PTEN gene among Indian patients
with oral squamous cell carcinoma. Int J Biol Markers. 28:298–302.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Viswanathan M, Tsuchida N and Shanmugam G:
Promoter hypermethylation profile of tumor-associated genes p16,
p15, hMLH1, MGMT and E-cadherin in oral squamous cell carcinoma.
Int J Cancer. 105:41–46. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Butta N, Larrucea S, Alonso S, et al: Role
of transcription factor Sp1 and CpG methylation on the regulation
of the human podocalyxin gene promoter. BMC Mol Biol. 7:172006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cao YX, Jean JC and Williams MC: Cytosine
methylation of an Sp1 site contributes to organ-specific and
cell-specific regulation of expression of the lung epithelial gene
t1alpha. Biochem J. 350:883–890. 2000. View Article : Google Scholar : PubMed/NCBI
|