Introduction
In 2014, the American Cancer Society reported that
cancer was the second leading cause of death in the USA, estimating
that >1,665,540 new cancer cases would be diagnosed that year
and 585,720 cancer deaths would occur in the USA. To develop
effective treatments, scientists have isolated anticancer agents
from natural materials and identified a number of promising drug
candidates (1).
In the search for potential therapies, we screened
509 natural products and found 14 compounds that demonstrate
anticancer properties. One of these natural products was
6,7-di-O-acetylsinococuline (FK-3000), which was isolated from
Stephania delavayi Diels. A literature search of the
pharmaceutical properties of FK-3000 revealed a compound isolated
from S. cepharantha that inhibits nuclear factor κB activity
(2) and exhibits antiviral effects
against herpes simplex virus type-1 (3), by inhibiting DNA synthesis (4), and against human immunodeficiency
virus type-1 (5).
Mammalian cell division is controlled by cyclins and
cyclin-dependent kinases (CDKs), which form various cyclin-CDK
heterodimeric complexes (protein kinase holoenzymes) that regulate
different phases of the cell cycle. Positive regulators of CDK
function are upregulated in most cancer cells, whereas the
expression of negative regulators are downregulated. Accordingly,
cyclin D1, CDK4, cyclin E, cyclin A, and Wee1 are upregulated in
the Long-Evans Cinnamon rat model of hepatocellular carcinoma
(6); CDK4 plays a pivotal role in
the progression from preneoplastic to neoplastic status in
diethylnitrosamine-induced hepatocellular carcinoma in rats
(7); increased expression of cell
cycle regulatory proteins and kinase activities of cyclin D1, CDK4,
cyclin E, cyclin A, and Wee1 was revealed by epidemiological
studies of patients with liver disease (8); and inhibitors of cell division cycle
25 (CDC25) phosphatases have shown promise as anticancer agents
(9). Targeting CDKs or cell cycle
protein kinases is an important strategy in the discovery of novel
anticancer drugs, and several preclinical and clinical trials are
assessing these proteins as targets (10).
In humans, there are three homologues of CDC25:
CDC25A, CDC25B, and CDC265C. In an earlier CDC25 regulation model,
CDC25A controls the G1/S cell cycle transition, and
CDC25B and CDC25C control mitosis (11) but in recent studies it was found
that all three homologues have function to control both
G1/S and G2/M phase transitions and mitosis
(12). CDC25B facilitates
dephosphorylation of the key cell cycle regulator CDC2 (also called
CDK1) at Tyr15 or Thr14, thereby initiating the G2/M
transition (13). Moreover, CDC25B
is overexpressed in most tumor types, including head and neck,
ovary, colon, and breast cancers, suggesting its potential as a
target for novel anticancer drugs (14). Examples of CDC25 inhibitors include
the compound BN82002, which strongly inhibits CDC25 activation and
delays cell cycle progression at the G1/S transition, in
S phase, and at the G2/M transition (15); silymarin and silibinin, which
arrest human prostate cancer PC3 cells at the G1 and
G2/M phases and specifically decrease levels of cyclin
B1, cyclin A, phospho-CDC2 (Tyr15), and CDC2 (16); naphthofurandione
3-benzoyl-naphtho[1,2-b] furan-4,5-dione, which inhibits
recombinant CDC25B in vitro, exhibits 96-h half maximal
inhibitory concentration (IC50) of 6.5 μM against MCF-7
cells and 1.2 μM against MDA-MB-231 cells, and causes
G1/S and G2/M phase arrest (17); BN82685, which inhibits recombinant
CDC25A, -B, and -C, and inhibits growth of the human pancreatic
tumor Mia PaCa-2 xenografted in athymic mice (18); and IRC-083864, which inhibits cell
proliferation by p21 induction and apoptosis (19).
Activation of p38 mitogen-activated protein kinase
(p38 MAPK) arrests cells in the G2/M phase by inhibiting
CDC25B phosphorylation (19) and
blocking participation of the CDC2/cyclin B complex in the
G2/M phase transition (20,21).
In the present study, we investigated the
antiproliferative mechanisms of FK-3000 by examining its effect on
cell cycle regulatory proteins. Our results show that FK-3000
decreased levels of phosphorylated CDC25B (phospho-CDC25B) but
neither CDC25A nor CDC25C, and induced G2/M phase arrest
in human breast carcinoma cell lines MDA-MB231 and MCF-7.
Materials and methods
Isolation of 6,7,-di-O-acetylsinococuline
(FK-3000)
The methanol extract (1 g) of S. delavayi
Diels. was separated by chromatography on a Sephadex LH-20 column
(GE Healthcare, Uppsala, Sweden, 40i.d.x860 mm, 25–100 μm, eluted
with methanol). Fraction 3 (700 mg) was further purified by C18
high performance liquid chromatography [YMC-Pack Pro, YMC GmbH,
Leicestershire, UK, S-5 μm, 20i.d.x250 mm) with 10–30% aqueous
acetonitrile (0.05% trifluoroacetic acid, Sigma-Aldrich Co., St.
Louis, MO, USA) for 90 min at 7 ml/min, yielding FK-3000 (76 mg;
retention time, 82.14 min) as pale brown needles. The
1H, 13C, and two-dimensional nuclear magnetic
resonance (2D NMR) spectra of the isolate were in good agreement
with those of FK-3000 isolated from S. cepharantha (22).
Cell culture and cell viability
assay
The human breast carcinoma cell lines MDA-MB-231 and
MCF-7 were obtained from the Korean Cell Line Bank (Seoul, Korea).
Cells were cultivated in RPMI-1640 (Gibco/BRL, Grand Island, NY,
USA) containing 10% fetal bovine serum (Gibco/BRL), 2 mg/ml sodium
bicarbonate (Gibco/BRL), 100 U/ml penicillin (Gibco/BRL), and 100
μg/ml streptomycin (Gibco/BRL).
Cells were seeded in 96-well plates
(1.5×104 cells/well) and incubated at 37°C in a 5%
CO2 atmosphere. To determine the IC50 of
FK-3000, MDA-MB-231 and MCF-7 cells were treated with 0.1% dimethyl
sulfoxide (DMSO; vehicle only control) (Sigma-Aldrich Co.) or
FK-3000 (0–5 μg/ml) 24 h after seeding. Cell proliferation was
analyzed after 24 and 48 h using the cell counting kit-8 (Dojindo
Molecular Technologies, Rockville, MD, USA) according to the
manufacturer’s instructions.
To evaluate cell viability, cells were treated with
0.1% DMSO, the 48 h IC50 of FK-3000 (0.52 μg/ml for
MDA-MB-231 cells; 0.77 μg/ml for MCF-7 cells), 5.0 μM
trans-1-(4-hydroxy-cyclohexyl)-4-(4-fluorophenyl)-5-(2-methoxypyridimidin-4-yl)-imidazole
(p38 MAPK inhibitor SB 239063, Sigma-Aldrich Co.), or cotreated
with the 48 h IC50 of FK-3000 and 5.0 μM SB 239063. Cell
viability was evaluated after 48 h using the cell counting kit-8
(Dojindo Molecular Technologies). All experiments were performed in
quadruplicate on different days.
Cell cycle distribution assay
Cells were seeded in 100-mm culture dishes
(1.0×106 cells/well). After attachment, cells were
synchronized by fetal bovine serum withdrawal for 6 h and then
treated in quadruplicate with DMSO only, FK-3000 (MDA-MB-231 cells,
0.5 μg/ml; MCF-7 cells, 0.7 μg/ml), SB 239063 (both cell lines, 5.0
μM), or combination treatment (MDA-MB-231 cells, 0.5 μg/ml FK-3000
+ 5.0 μM SB 239063; MCF-7 cells, 0.7 μg/ml FK-3000 + 5.0 μM SB
239063). Cells were harvested after 24 or 48 h of treatment and
fixed with ice-cold 70% ethanol at 4°C. After 24 h, the fixed cells
were centrifuged at 1,200 rpm using a Gyro 416 G (Gyrozen, Daejeon,
Korea) for 6 min and the supernatant was discarded. The cell
pellets were resuspended in binding buffer consisting of 0.01 M
HEPES/NaOH (pH 7.4) (Sigma-Aldrich Co.) containing 0.14 M NaCl
(Sigma-Aldrich Co.), 2.5 mM CaCl2 (Sigma-Aldrich Co.), 5
μl propidium iodide (Sigma-Aldrich Co.), and 80 μl/ml ribonuclease
A (Sigma-Aldrich Co.). After 20–30 min of incubation at room
temperature in the dark, the DNA content of the cells was examined
using a BD Model FACScan flow cytometer (Becton-Dickinson, San
Jose, CA, USA).
Protein extraction and western blot
analysis
Cells were seeded in 100-mm culture dishes
(1.0×106 cells/well), incubated for 24 h, and then
treated in quadruplicate with DMSO, FK-3000 (MDA-MB-231 cells, 0.5,
2.5, or 5.0 μg/ml; MCF-7 cells, 0.7, 3.5 or 7.0 μg/ml), 5.0 μM SB
239063 (both cell lines), or combination treatment (MDA-MB-231
cells, 0.5 μg/ml FK-3000 + 5.0 μM SB 239063; MCF-7 cells, 0.7 μg/ml
FK-3000 + 5.0 μM SB 239063). After incubation for 45 min to 48 h,
cells were harvested by trypsinization and washed twice with cold
phosphate-buffered saline (PBS, Sigma-Aldrich Co.). Total protein
was prepared with Pro-Prep™ (iNtRON Biotechnology, Seongnam,
Korea), and the protein content of each sample was determined using
the Bio-Rad DC protein assay kit (Bio-Rad, Hercules, CA, USA).
Equal amounts of protein were separated by 10% SDS-polyacrylamide
gel electrophoresis and transferred to a nitrocellulose membrane in
Trans-Blot® Transfer Medium (Bio-Rad). Membranes were
incubated with anti-phospho-p38 MAPK monoclonal antibody (Cell
Signaling Technology, Danvers, MA, USA; cat no. 9215),
anti-phospho-CDC25C antibody (Cell Signaling Technology, cat no.
9527), anti-phospho-CDC25B antibody (Abgent, San Diego, CA, USA;
AP3053a), anti-cyclin B antibody (Santa Cruz Biotechnology, Santa
Cruz, CA, USA; SC-245), anti-phospho-CDC-2 antibody (Cell Signaling
Technology, cat no. 9112), anti-cyclin A antibody (Santa Cruz
Biotechnology, SC-751), anti-phospho-retinoblastoma (RB) antibody
(Cell Signaling Technology, cat no. 9308), and anti-β-actin
monoclonal antibody (Sigma-Aldrich Co., cat no. A-5316).
Horseradish peroxidase-conjugated goat anti-rabbit IgG (Cayman, Ann
Arbor, MI, USA; cat no. 10004301) was used as the secondary
antibody. Stained bands were analyzed using the ECL detection kit
(Amersham Biosciences, Buckinghamshire, UK).
p38 MAPK phosphorylation assay
Attached cells were treated with DMSO alone, FK-3000
(MDA-MB-231 cells, 0.5 μg/ml; MCF-7 cells, 0.7 μg/ml), 5.0 μM SB
239063 (both cell lines), or combination treatment (MDA-MB-231
cells, 0.5 μg/ml FK-3000 + 5.0 μM SB 239063; MCF-7 cells, 0.7 μg/
ml FK-3000 + 5.0 μM SB 239063), and incubated for 2 h in a confocal
dish (SPL Life Science, Pochoen, Korea). Cells were washed three
times in cold PBS, fixed with 4% paraformaldehyde (Sigma-Aldrich
Co.) at room temperature, treated with 0.5% Triton X-100, blocked
with Animal-Free Blocker™ (Vector, Burlingame, CA, USA) for 1 h and
incubated overnight at 4°C with anti-phospho-p38 MAPK monoclonal
antibody. Cells were then incubated for 1 h with fluorescein
isothio-cyanate (FITC)-conjugated goat anti-rabbit IgG (Cayman)
followed by 7 μg/ml bisbenzimide H 33342 trihydrochloride
(Sigma-Aldrich Co.) for nuclear staining, and photographed using an
LSM510 Meta Fluorescent Microscope with Plan-Apochromat 100x/1.4
Oil DIC (Carl-Zeiss, Jena, Germany).
Analysis of apoptosis
Attached cells were treated for 48 h with DMSO
alone, FK-3000 (MDA-MB-231 cells, 0.5 μg/ml; MCF-7 cells, 0.3
μg/ml), 5.0 μM SB 239063 (both cell lines), or combination
treatment (MDA-MB-231 cells, 0.5 μg/ml FK-3000 + 5.0 μM SB 239063;
MCF-7 cells, 0.3 μg/ml FK-3000 + 5.0 μM SB 239063). Cells were
harvested by trypsinization, washed in cold PBS, and resuspended in
binding buffer consisting of 0.01 M HEPES/NaOH (pH 7.4) containing
0.14 M NaCl and 2.5 mM CaCl2. FITC-conjugated Annexin V
(BioVision, Milpitas, CA, USA) and propidium iodide (5 μl each)
(Becton-Dickinson) were added to the cells, which were gently mixed
and incubated for 15 min at room temperature in the dark. Binding
buffer was then added, and the cells were analyzed with BD Model
FACScan (Becton-Dickinson).
Statistical analysis
Results are expressed as mean ± standard deviation
(SD). Groups were compared using Tukey’s studentized range (HSD)
test with SPSS Statics (IBM, Armonk, NY, USA); p<0.01 was
considered statistically significant.
Results
FK-3000 isolated from S. delavayi Diels
inhibits proliferation of human carcinoma cell-lines MDA-MB-231 and
MCF-7
We screened 509 natural products for anticancer
activity and identified 14 candidates. The compound
6,7-di-O-acetylsinococuline (FK-3000) was isolated from S.
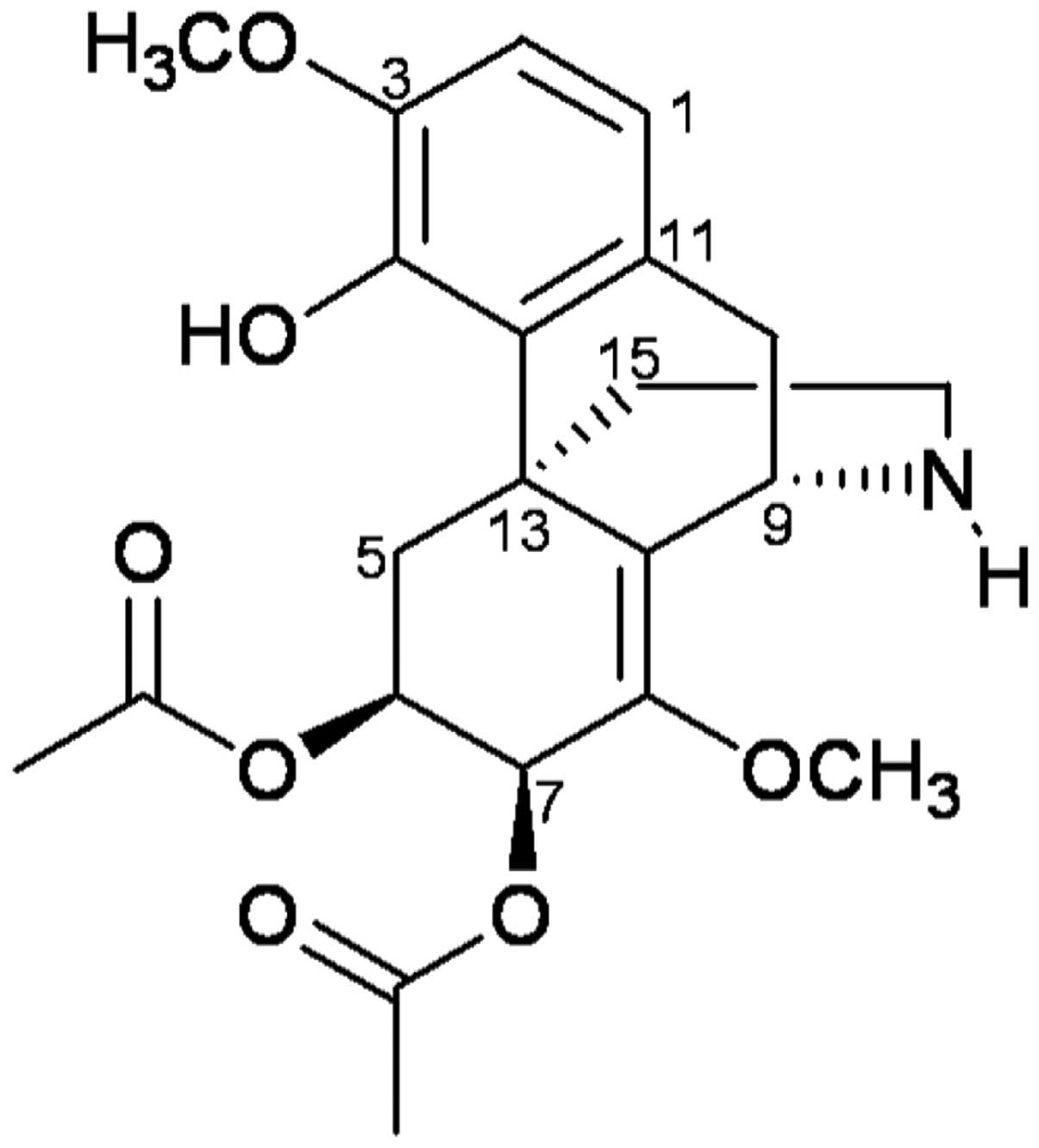
delavayi Diels. (Fig. 1;
molecular weight, 417.45), and its chemical structure was confirmed
by 1H, 13C, and 2D NMR. The
chemical structure of FK-3000 isolated from S. delavayi
Diels. was in good agreement with the compound previously isolated
from S. cepharantha (22).
Antiproliferative effects of FK-3000 against cancer
cells have not previously been reported, we found that FK-3000
inhibited cell proliferation in a dose-and time-dependent manner in
two human breast cancer cell lines. The antiproliferative effect of
FK-3000 against MDA-MB-231 cells (24 h IC50, 0.89 μg/ml;
48 h IC50, 0.52 μg/ml) was greater than its effect
against MCF-7 cells (24 h IC50, 2.53 μg/ml; 48 h
IC50, 0.77 μg/ml).
FK-3000 arrests MDA-MB-231 and MCF-7
cells at G2/M phase
Carcinogenesis is caused by cell cycle deregulation,
typically an increase in positive regulators such as CDKs and/or
decrease in negative regulators such as cyclin D1, CDK4, cyclin E,
cyclin A, and Wee1. Because the cell cycle is no longer controlled,
cell proliferation is excessive (6). We therefore measured the effect of
FK-3000 on cell cycle regulation in MDA-MB-231 and MCF-7 cells.
Doses were based on the 48 h IC50 of FK-3000 for each
cell line, corresponding to 1× IC50 to 10×
IC50 for each cell line (MDA-MB-231, 0.5–5.0 μg/ml;
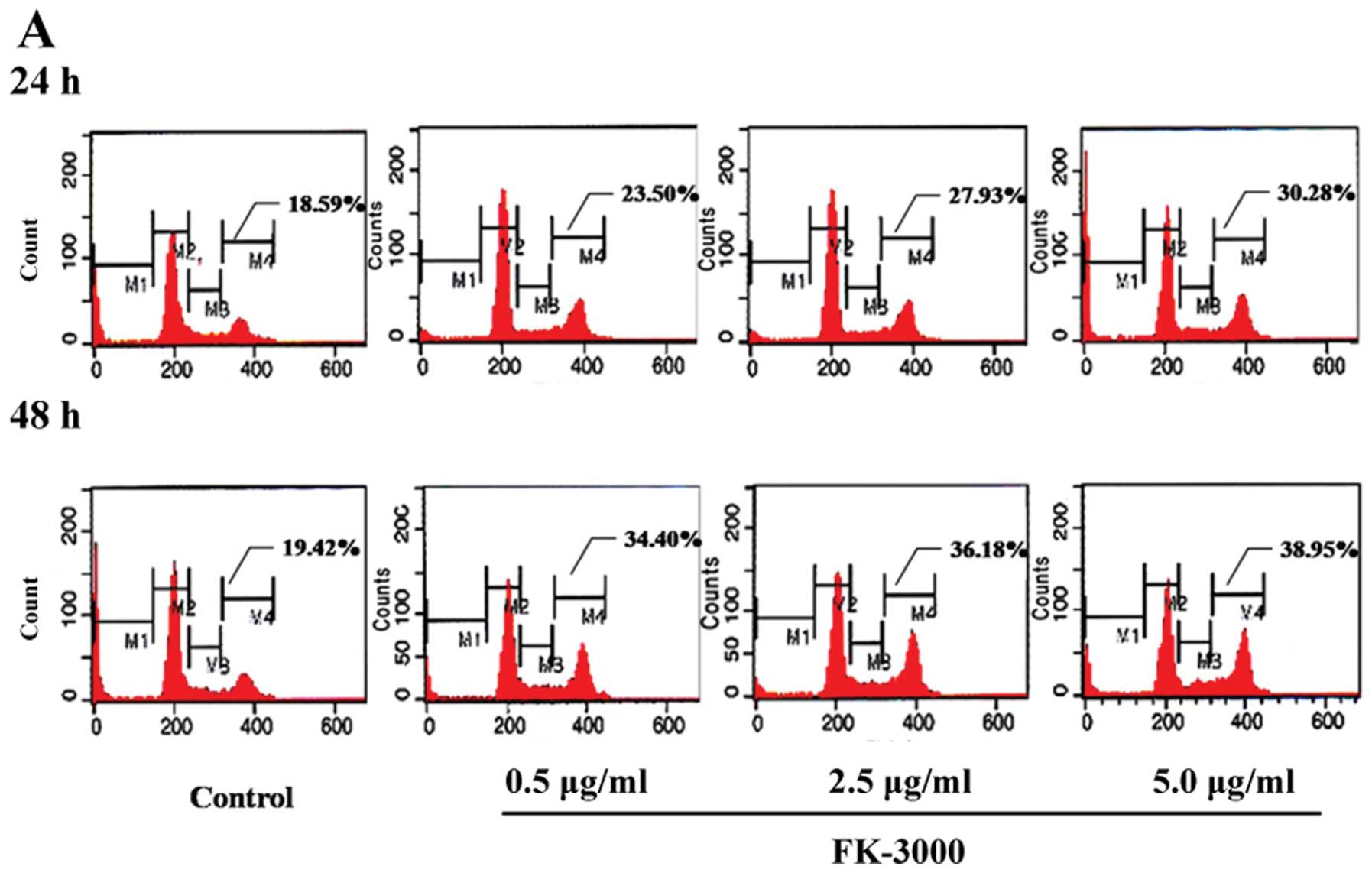
MCF-7, 0.7–7.0 μg/ml). As shown in Fig. 2A and B, FK-3000 treatment resulted
in G2/M phase arrest in a time- and dose-dependent
manner. In MDA-MB-231 cells treated with 1× IC50 FK-3000
for 24 h, the percentage of G2/M phase arrested cells
was 23.50%, increasing to 38.95% after 48-h treatment with 10×
IC50 FK-3000. In MCF-7 cells treated with 1×
IC50 FK-3000 for 24 h, the percentage of G2/M
phase arrested cells was 28.93%, increasing to 40.13% after 48-h
treatment with 10× IC50 FK-3000.
FK-3000 induces dephosphorylation of
CDC25 through p38 MAPK signaling
In cancer cells, levels of phosphorylated p38 MAPK
proteins are low whereas phosphorylated CDC25B protein levels are
high. CDC25B plays a key role in G2/M phase transition
and CDC2 activation (23);
phosphorylation of CDC25B is an important step leading to
proliferation and metastasis of neoplastic cells. P38 MAPK induces
G2/M arrest by inhibiting CDC25B phosphorylation and
blocking participation of the CDC2/cyclin B complex in
G2/M transition (20,21).
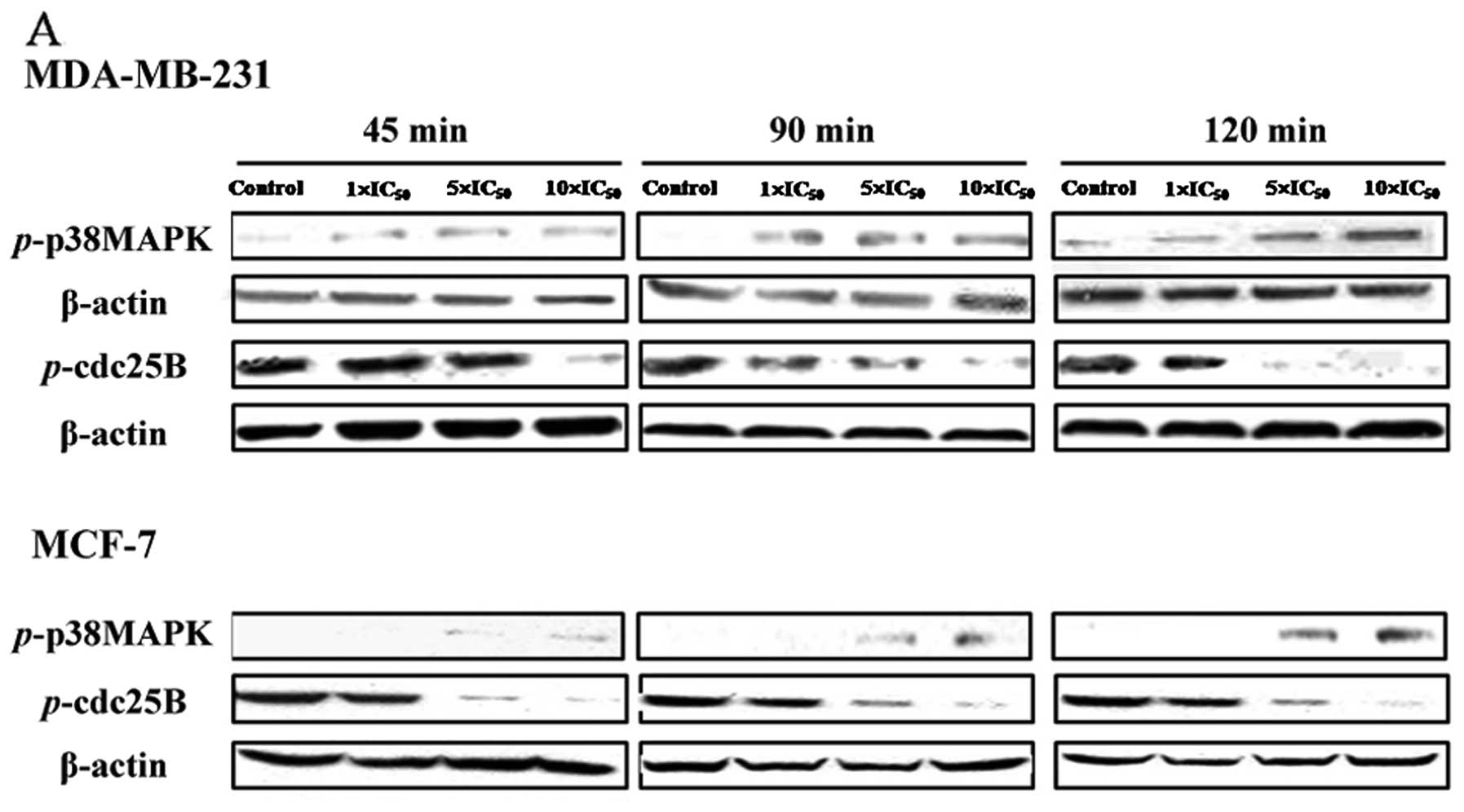
As shown in Fig.
3A, FK-3000 increased phosphorylation of p38 MAPK and decreased
phosphorylation of CDC25B in both MDA-MB-231 and MCF-7 cell lines.
Levels of phosphorylated p38 MAPK increased in a dose- and
time-dependent manner in MCF-7 cells, whereas the level of
phosphorylated p38 MAPK at 90 min differed from that of other time
points in MDA-MB-231 cells. In MDA-MB-231 cells, phosphorylation of
CDC25B in cells treated with 1× IC50 FK-3000 was similar
to that of the 5× IC50 group at 45 min, but was almost
completely abolished by 90-min treatment with 10× IC50
FK-3000 and 120-min treatment with 5× IC50 FK-3000. In
MCF-7 cells, FK-3000 significantly reduced phospho-CDC25B in a
dose-and time-dependent manner, and 10× IC50 FK-3000
almost completely suppressed phosphorylation of CDC25B at all time
points. These results suggest that FK-3000 inhibits CDC25B through
p38 MAPK activation.
We next determined the effect of FK-3000 on the
G2/M phase regulatory factors and related proteins
CDC-2, cyclin A, cyclin B, and RB. With the exception of cyclin B
and phospho-CDC-2, we did not observe changes in these proteins
(data not shown). Cyclin B levels were not altered by 24 h FK-3000
treatment in either MDA-MB-231 or MCF-7 cells, except in cells
treated with 10× IC50 FK-3000; however, this increase
was attenuated at 48 h, and cyclin B was barely detectable after
48-h treatment with 10× IC50 FK-3000 in both cell lines
(Fig. 3B). FK-3000 decreased
phosphorylation of CDC2 in a dose- and time-dependent manner, and
48-h treatment with 10× IC50 FK-3000 in MDA-MB-231 cells
and 5× IC50 or 10× IC50 FK-3000 in MCF-7 cell
completely abolished phosphorylation of CDC2 (Fig. 3B).
p38 MAPK inhibition attenuated the
antiproliferative action of FK-3000 but did not completely block
FK-3000-induced apoptosis
CDC25B phosphorylation is regulated by p38 MAPK,
which also blocks participation of the CDC2/cyclin B complex in
G2/M transition (20,21).
Phosphorylation of p38 MAPK plays a role in cell death, cell
differentiation, and cell cycle progression. Following DNA damage,
phospho-p38 MAPK translocates from the cytoplasm into the nucleus
(24), where accumulation of
phospho-p38 MAPK triggers G2/M phase arrest and DNA
repair.
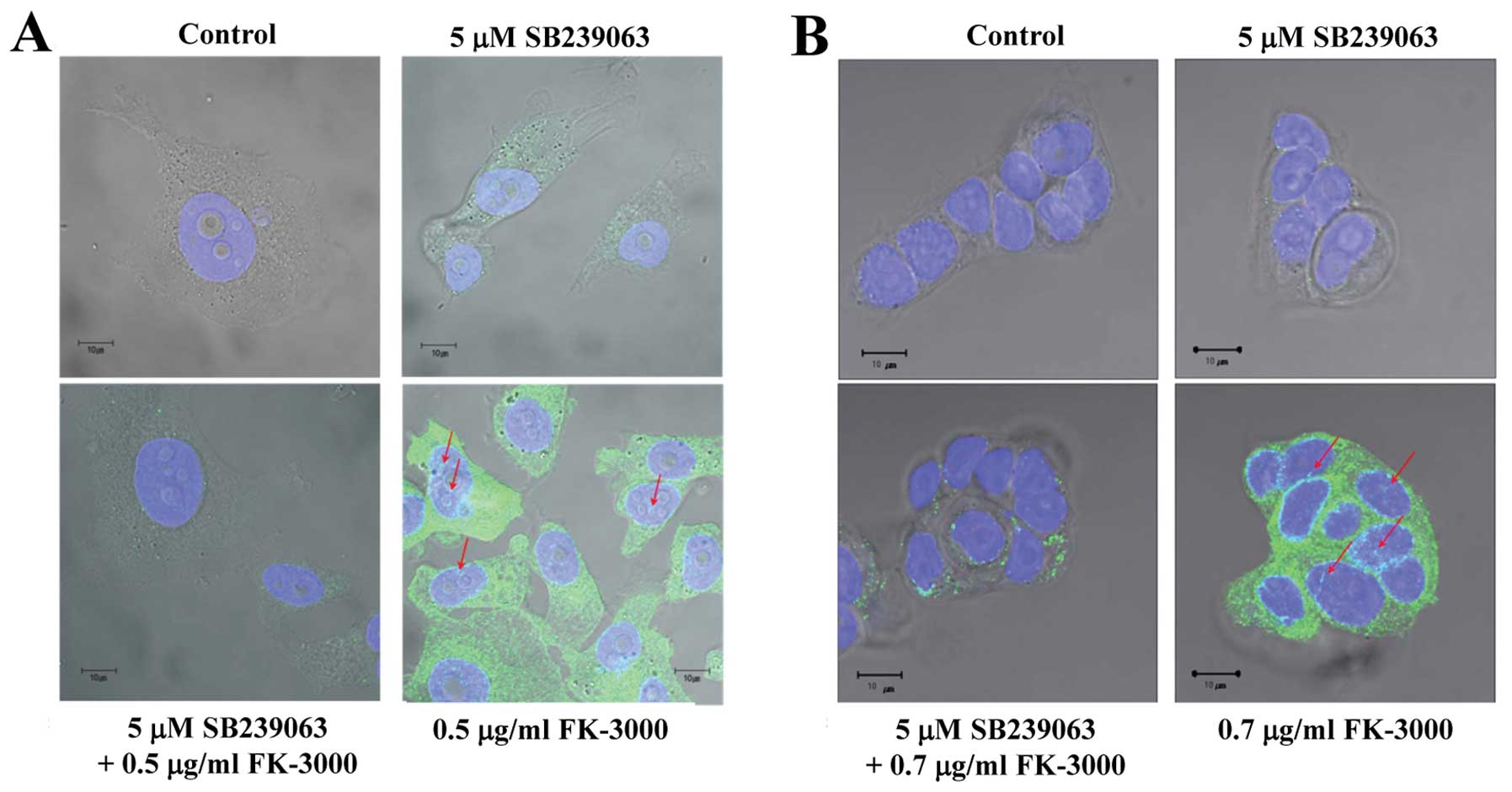
We assumed that FK-3000 induced p38 MAPK
phosphorylation and then suppressed CDC25B phosphorylation. Our
results showed that a 90-min FK-3000 treatment stimulated p38 MAPK
phosphorylation and nuclear translocation in MDA-MB-231 and MCF-7
cells (Fig. 4A and B), and this
effect was suppressed by SB 239063, a potent and selective
inhibitor of p38 MAPK (25). We
compared phospho-p38 MAPK and phospho-CDC25B levels in FK-3000
treated cells with that of untreated cells at 90 min (Fig. 4C). Phosphorylation of CDC25B was
abolished in cells treated with FK-3000 in the presence or absence
of SB 239063. Together, these findings indicate that FK-3000
inhibits CDC25B phosphorylation directly as well as indirectly
through p38 MAPK phosphorylation.
To evaluate the mechanism of cell cycle arrest by
FK-3000, we analyzed the cell cycle distribution of treated cells.
Although the distribution of cells treated with SB 239063 was
similar to that of the vehicle control, SB 239063 could not
completely reverse FK-3000-induced G2/M phase arrest
(Fig. 4D).
To confirm that FK-3000 inhibited cell proliferation
through p38 MAPK activation, we evaluated whether the p38 MAPK
inhibitor SB 239063 could rescue the antiproliferative effect of
FK-3000. Our results showed that SB 239063 attenuated but could not
completely block the antiproliferative action of FK-3000. SB 239063
increased viability from 52.93 to 62.52% in FK-3000-treated
MDA-MB-231 cells and increased viability from 50.59 to 60.63% in
FK-3000-treated MCF-7 cells (Fig.
4E). As shown in Fig. 4E, the
viability of cells treated with both SB 239063 and FK-3000 (77.69%
in MDA-MB-231, 60.63% in MCF-7) did not fully recover to the level
of control cells, suggesting that FK-3000 inhibits cell
proliferation by an additional mechanism besides G2/M
phase arrest through p38 MAPK phosphorylation and CDC25B
dephosphorylation. We therefore analyzed the effect of SB 239063 on
the rate of apoptosis in cells treated with FK-3000 (Fig. 4F). Apoptosis in cells treated with
FK-3000 (SB 239063 + FK-3000 cotreatment or FK-3000 only) was
significantly higher than that of cells treated with the vehicle
control or SB 239063 only. Thus, FK-3000 appears to induce
apoptosis by a pathway independent of the p38 MAPK-CDC25B
pathway.
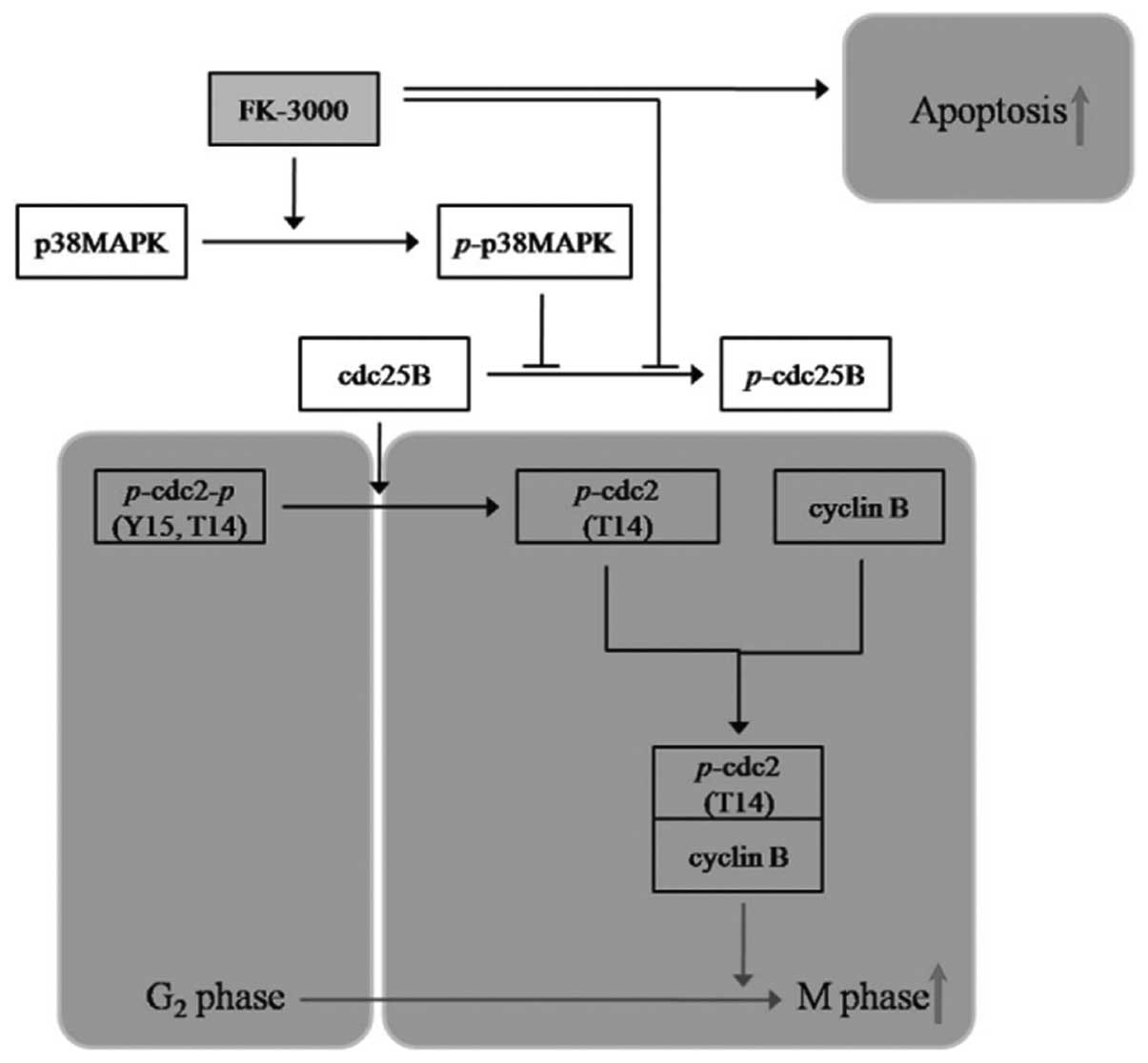
Taken together, these findings indicate that FK-3000
is a promising anticancer drug candidate that exerts its
antiproliferative activity through two pathways: induction of
G2/M phase arrest by p38 MAPK-CDC25B-CDC2-cyclin B
modulation and stimulation of apoptosis independent of the p38
MAPK-CDC25B pathway.
Discussion
Cell cycle regulatory factors and related proteins
(e.g., cyclin A, cyclin B, CDC2, CDC25A, CDC25B, CDC25C and p38
MAPK) are associated with G2/M transition; in
particular, the CDC2-cyclin B heterodimeric complex regulates entry
into mitosis (26). We found that
FK-3000 induced G2/M phase arrest in the human breast
carcinoma cell lines MDA-MB-231 and MCF-7 in a dose- and
time-dependent manner. Further, phospho-CDC2 levels were
significantly decreased after 24 h and cyclin B levels were
decreased after 48 h, and phospho-p38 MAPK was upregulated, whereas
phospho-CDC25B was downregulated in a dose- and time-dependent
manner. Taken together, our findings suggest that FK-3000 induces
G2/M arrest by inhibiting CDC2 activation via p38 MAPK
phosphorylation and CDC25B dephosphorylation. To confirm these
results, we evaluated the ability of the selective p38 MAPK
inhibitor SB 239063 to block the antiproliferative action of
FK-3000. SB 239063 increased viability from 52.93 to 62.52% in
FK-3000-treated MDA-MB-231 cells and from 50.59 to 60.63% in
FK-3000-treated MCF-7 cells. Moreover, SB 239063 inhibited
FK-3000-induced p38 MAPK phosphorylation and nuclear accumulation
(Fig. 4A–C).
However, SB 239063 did not completely rescue the
effects of FK-3000, suggesting the involvement of another pathway
in the antiproliferative action of FK-3000. Although SB 239063
suppressed FK-3000-induced p38 MAPK phosphorylation, it did not
inhibit apoptosis (Fig. 4F). We
therefore propose that FK-3000 exerts its cytostatic effect through
p38 MAPK activation and its cytotoxic effect through apoptosis.
CDC25B has been proposed as a target for the development of
anticancer agents (14,23). EK-6136 is a synthetic CDC25B
inhibitor that inhibits cell proliferation in MCF-7 (48 h
IC50, 7.2±1.0 μM), HT-29 (48 h IC50, 8.4±1.0
μM), and A549 cells (48 h IC50, 7.7±1.0 μM) (27). BN82002 is a synthetic pan-CDC25
inhibitor that reduces proliferation of the carcinoma cell lines
Mia PaCa-2, DU-145, U-87 MG, LNCaP, HT-29, and U2OS, with 96-h
IC50 values in the range 7.2–32.6 μM (15). Another synthetic pan-CDC25
inhibitor, naphthofurandione
3-benzoyl-naphtho[1,2-b]furan-4,5-dione, inhibits cell
proliferation in PC-3 cells (96 h IC50, 6.5 μM) and
MDA-MB-435 cells (96 h IC50, 1.2 μM) (17). FK-3000 suppresses activation of
CDC25B but not CDC25C. Compared with the previously described CDC25
inhibitors, FK-3000 is a more potent inhibitor of proliferation in
various cell lines and appears to be safe as assessed by animal
studies at doses <10 mg/kg of body weight, administered
intraperitoneally once a day for 5 days (data not shown).
Cell cycle regulators may be positive (e.g., CDKs,
cyclins) or negative [e.g., INK4 family (p16ink4a,
p15ink4b, p18ink4c, and p19ink4d),
p21waf1, p27Kip1, and p57Kip2]
(28–30). Carcinogenesis is the result of an
imbalance between these positive and negative regulatory factors;
therefore, modulating these proteins is a common therapeutic
strategy against neoplasms. Recent studies have evaluated CDK
modulators as anticancer agents. For example, the staurosporine
analogue, 7-hydroxystaurosporine (UCN-01), is in phase I/II
clinical trials for leukemia, lymphoma, ovarian epithelial, primary
peritoneal or fallopian tube cancer, and unspecified solid tumors
(31), and the flavonoid
flavopiridol is in phase I/II clinical trials for non-Hodgkin
lymphoma, renal, prostate, colon, and gastric cancers (32,33).
UCN-01 induces CDC2 dephosphorylation at Tyr-15, promoting early
entry into mitosis and ultimately inducing arrest at the
G2/M phase (32). The
24-h IC50 of UCN-01 in MDA-MB-231 cells is ~1 μM
(34). Like UCN-01, FK-3000
dephosphorylates CDC2 at Tyr-15, activates CDC/cyclin B, and
facilitates initiation of mitosis. Although flow cytometric
analysis in the present study showed that FK-3000 induced
G2/M phase arrest in MDA-MB-231 and MCF-7 cells, most of
these cells may be in mitosis.
We demonstrated that FK-3000 exerts an
antiproliferative effect through two pathways: i) G2/M
phase arrest via downregulation of cyclin B and phospho-CDC2 by
dephosphorylation of CDC25B and phosphorylation of p38 MAPK; and
ii) p38 MAPK-independent induction of apoptosis (Fig. 5). Although further studies are
needed to evaluate FK-3000 in other cancer cell types and elucidate
the antiproliferative mechanisms, therapeutic index, and margin of
safety, our findings indicate that FK-3000 is a promising
anticancer agent.
Acknowledgements
This study was supported by National Research
Foundation of Korea Grant funded by the Korean Government
(2009-0073116) and by Fishery Commercialization Technology
Development Program (112098-03-2-SB010).
References
|
1
|
Park DH, Xu HD, Shim J, Li YC, Lee JH, Cho
SC, Han SS, Lee YL, Lee MJ and Kwon SW: Stephania delavayi Diels.
Inhibits breast carcinoma proliferation through the p38MAPK/
NF-κB/COX-2 pathway. Oncol Rep. 26:833–841. 2011.PubMed/NCBI
|
|
2
|
Baba M and Ono M: NF-κB activity
inhibitor. US Patent 6123943. Filed March 10, 1998; issued
September 26, 2000.
|
|
3
|
Nawawi A, Nakamura N, Meselhy MR, Hattori
M, Kurokawa M, Shiraki K, Kashiwaba N and Ono M: In vivo antiviral
activity of Stephania cepharantha against herpes simplex virus
type-1. Phytother Res. 15:497–500. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohsaki M, Kurokawa M, Nawawi A, Nakamura
N, Hattori M and Shiraki K: Characterization of anti-herpes simplex
virus type 1 activity of an alkaloid FK-3000 from Stephania
cepharantha. J Trad Med. 19:129–136. 2002.
|
|
5
|
Ma C-M, Nakamura N, Miyashiro H, Hattori
M, Komatsu K, Kawahata T and Otake T: Screening of Chinese and
Mongolian herbal drugs for anti-human immunodeficiency virus type 1
(HIV-1) activity. Phytother Res. 16:186–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masaki T, Shiratori Y, Rengifo W, Igarashi
K, Matsumoto K, Nishioka M, Hatanaka Y and Omata M: Hepatocellular
carcinoma cell cycle: study of Long-Evans Cinnamon rats.
Hepatology. 32:711–720. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park DH, Shin JW, Park SK, Seo JN, Li L,
Jang JJ and Lee MJ: Diethylnitrosamine (DEN) induces irreversible
hepatocellular carcinogenesis through overexpression of G1/S-phase
regulatory proteins in rat. Toxicol Lett. 191:321–326. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Masaki T, Shiratori Y, Rengifo W,
Igarashhi K, Yamagata M, Kurokohchi K, Uchida N, Miyauchi Y,
Yoshiji H, Watanabe S, Omata M and Kuriyama S: Cyclins and
cyclin-dependent kinases: comparative study of hepatocellular
carcinoma versus cirrhosis. Hepatology. 37:534–543. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bana E, Sibille E, Valente S, Cerella C,
Chaimbault P, Kirsch G, Dicato M, Diederich M and Bagrel D: A novel
coumarin-quinone derivative SV37 inhibits CDC25 phosphatases,
impairs proliferation, and induces cell death. Mol Carcinog. Oct
24–2013.(Epub ahead of print). View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Falco MD and Luca AD: Cell cycle as a
target of antineoplastic drugs. Curr Pharm Des. 16:1417–1426. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nilsson I and Hoffmann I: Cell cycle
regulation by the cdc25 phosphatase family. Prog Cell Cycle Res.
4:107–114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boutros R, Dozier C and Ducommun B: The
When and where of CDC25 phosphatase. Curr Opin Cell Biol.
18:185–191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Norbury C, Blow and Nurse P: Regulatory
phosphorylation of the p34cdc2 protein kinase in
vertebrates. EMBO J. 10:3321–3329. 1991.PubMed/NCBI
|
|
14
|
Lavecchia A, Di Giovanni C and Novellino
E: Inhibitors of Cdc25 phosphatases as anticancer agents: a patent
review. Expert Opin Ther Pat. 20:405–425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brezak MC, Quaranta M, Mondésert O,
Galacera MO, Lavergne O, Alby F, Cazales M, Baldin V, Thurieau C,
Harnett J, Lanco C, Kasprzyk PG, Prevost GP and Ducommun B: A novel
synthetic inhibitor of CDC25 phosphastases: BN82002. Cancer Res.
64:3320–3325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deep G, Singh RP, Agarwal C, Kroll DJ and
Agarwal R: Silymarin and silibinin cause G1 and G2-M cell cycle
arrest via distinct circuitries in human prostate cancer PC3 cells:
a comparison of flavanone silibinin with flavanolignan mixture
silymarin. Oncogene. 25:1053–1069. 2006. View Article : Google Scholar
|
|
17
|
Brisson M, Nguyen T, Vogt A, Yalowich J,
Giorgianni A, Tobi D, Bahar I, Stephenson CRJ, Wipf P and Lazo JS:
Discovery and Characterization of novel small molecule inhibitors
of human cdc25B dual specificity phosphatase. Mol Pharmacol.
66:824–833. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brezak MC, Quaranta M, Contour-Galcera MO,
Lavergne O, Mondesert O, Auvray P, Kasprzyk PG, Prevost GP and
Ducommun B: Inhibition of human tumor cell growth in vivo by an
orally bioavailable inhibitor of CDC25 phosphatases. Mol Cancer
Ther. 4:1378–1387. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brezak MC, Valette A, Quaranta M,
Contour-Galcera MO, Jullien D, Lavergne O, Frongia C, Bigg D,
Kasprzyk PG, Prevost GP and Ducommun B: IRC-083864, a novel bis
quinine inhibitor of CDC25 phosphatases active against human cancer
cells. Int J Cancer. 124:1449–1456. 2009. View Article : Google Scholar
|
|
20
|
Mikhailov A, Shinohara M and Rieder C: The
p38-mediated stress-activated checkpoint. Cell Cycle. 4:57–62.
2005. View Article : Google Scholar
|
|
21
|
Hirose Y, Katayama M, Mirzoeva OK, Berger
MS and Pieper RO: Akt activation suppresses chk2-mediated,
methylating agent-induced G2 arrest and protects from
Temozolomide-induced mitotic catastrophe and cellular senescence.
Cancer Res. 65:4861–4869. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kashiwaba N, Morooka S, Kimura M, Ono M,
Toda J, Suzuki H and Sano T: New morphinane and hasubanane
alkaloids form Stephania cepharantha. J Nat Prod. 59:476–480. 1996.
View Article : Google Scholar
|
|
23
|
Boutros R, Lobjois V and Ducommun B: CDC25
phosphastases in cancer cells: key players? Good targets? Nature.
7:495–507. 2007.
|
|
24
|
Wood CD, Thornton TM, Sabio G, Davis RA
and Rincon M: Nuclear localization of p38MAPK in response to DNA
damage. Int J Biol Sci. 5:428–437. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Underwood DC, Osborn RR, Koitzer CJ, Adams
JL, Lee JC, Webb EF, Carpenter DC, Bochnowicz S, Thomas HC, Hay DW
and Griswold DE: SB 239063, a potent p38 MAP kinase inhibitor,
reduces inflammatory cytokine production, airways eosinophil
infiltration, and persistence. J Pharmacol Exp Ther. 293:281–288.
2000.PubMed/NCBI
|
|
26
|
O’Farrell PH: Triggering the
all-or-nothing switch into mitosis. Trends Cell Biol. 11:512–519.
2001. View Article : Google Scholar
|
|
27
|
Kim KR, Kwon JL, Kim JS, No Z, Kim HR and
Cheon HG: EK-6136
(3-methyl-4-(O-methyl-oximino)-1-phenylpyrazolin-5-one): a novel
Cdc25B inhibitor with antiproliferative activity. Eur J Pharmacol.
528:37–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sherr CJ and Roberts JM: CDK inhibitors:
positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1997. View Article : Google Scholar
|
|
29
|
LaBaer J, Garrett MD, Stevenson LF,
Slingerland JM, Sandhu C, Chou HS, Fattaey A and Harlow E: New
functional activities for the p21 family of CDK inhibitors. Genes
Dev. 11:847–862. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng M, Olivier P, Diehl JA, Fero M,
Roussel MF, Foberts JM and Sherr CJ: The p21(CIP1) and p27(Kip1)
CDK ‘inhibitors’ are essential activators of cyclin-D-dependent
kinases in murine fibroblasts. EMBO J. 18:1571–1583. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
|
|
32
|
Senderowicz AM and Sausville EA:
Preclinical and clinical development of cyclin-dependent kinase
modulators. J Natl Cancer Inst. 92:376–387. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
|
|
34
|
Jones CB, Clements MK, Wasi S and Daoud
SS: Enhancement of camptothecin-induced cytotoxicity with UCN-01 in
breast cancer cells: abrogation of S/G2 arrest. Cancer Chemother
Pharmacol. 45:252–258. 2000. View Article : Google Scholar
|