Introduction
Metastasis is the tendency of cancer cells to spread
to distant organs in the body, which is considered responsible for
more than 90% of cancer-associated deaths (1–4). It
involves a multistep process including migration from the primary
tumors, invasion to surrounding tissues, and proliferation leading
to the colonization at distant sites (1,4).
Accordingly, 25–30% of patients with renal cell carcinoma (RCC)
have metastatic spread by the time they are diagnosed (5–7) and
in these cases, the 5-year survival rate of patients is <10%
(8,9). Moreover, 20–25% of suffers remain
unresponsive to all treatments and the disease progresses rapidly
(10,11). Honokiol, a small-molecule
biphenolic compound isolated from Magnolia spp. Bark, has
been shown to exhibit anticancer effects in different cancer types
(12–17). The most widely investigated
mechanism of its anticancer activities is apoptosis, which is
induced in vitro and in vivo through multiple facets
of signal transduction (12,14,16–25).
Recently, several studies demonstrated that honokiol could also
inhibit metastasis of breast, brain, gastric, lung and prostate
cancer cells (13,21,26–32).
However, only one study shows the metastasis suppression of RCC
cells A-498 by honokiol through reversing epithelial-mesenchymal
transition and blocking cancer stem cell properties (33). Definitely, there are other
important targets involved in the process of RCC metastasis
suppression by honokiol.
In this study, we found that honokiol inhibits the
invasion and colony formation of highly metastatic RCC cells 786-0
(34) in a dose-dependent manner.
DNA-microarray data showed significant upregulation of
metastasis-suppressor gene KISS1 and its receptor,
KISS1R. Both of the upregulation were confirmed by qRT-PCR
analysis. Overexpression levels of KISS1 and KISS1R were detected
by western blotting at the translation level as well. Of note,
inhibition of invasion and colony formation were reversed by
KISS1 knockdown. Taken together, our results indicate that
honokiol suppresses the multistep process of metastasis, including
invasion and colony formation, in RCC cells 786-0 via stimulation
of KISS1/KISS1R signaling pathway.
Materials and methods
Cell culture and reagents
Human RCC cells 786-0 were obtained from ATCC
(Manassas, VA, USA). Cancer cells were maintained according to the
ATCC procedures. Honokiol (98%) (HonoPure®) was provided
by Econugenics Inc. (Santa Rosa, CA, USA) and dissolved in DMSO at
a concentration of 80 mM then stored at −20°C. DMSO was purchased
from Sigma (St. Louis, MO, USA). Anti-KISS1, anti-KISS1R and
anti-β-actin antibodies were obtained from Santa Cruz Biotechnology
(Santa Cruz, CA, USA).
Cell invasion assay
Cell invasion of 786-0 cells treated with honokiol
(0–20 μM) was performed as previously described (35). Data points represent the mean ± SD
of three individual filters within one representative experiment
repeated at least twice.
Colony formation assay
Colony formation of the 786-0 cells incubated in the
presence of honokiol (0–40 μM) was evaluated as previously
described (36). Data points
represent the mean ± SD in one representative experiment repeated
at least twice.
DNA-microarray and quantitative RT-PCR
analysis
The 786-0 cells were treated with honokiol (0, 40
μM) for 24 h and TaqMan® Array Human Tumor metastasis
was performed as previously described (37). In qRT-PCR analysis, the 786-0 cells
were treated with honokiol (0–40 μM) for 24 h. Isolation,
quantification, reverse transcription of RNA and PCR were performed
as previously described (37).
Relative quantity (RQ) of gene expression was normalized to β-actin
and performed using the 2−ΔΔCt method (38).
Western blot analysis
The 786-0 cells were treated with honokiol (0–40 μM)
for 24 h. Whole protein extracts isolated from cells were prepared
and western blot analysis with KISS1 and KISS1R antibodies were
performed as previously described (39). Western blots were quantified with
HP-Scanjet 550c and analyzed by UN-SCAN-IT software (Silk
Scientific, Orem, UT, USA).
siRNA transfection
The 786-0 cells were transfected with human
KISS1 siRNA or control siRNA-A as previously described
(37). After 48 h of transfection,
the cells were harvested and KISS1 knockdown was verified by
western blot analysis.
Statistical analysis
All the statistical analysis was performed using
SigmaPlot 11.2.0 (Systat Software Inc., San Jose, CA, USA). Data
are presented as mean ± SD. Statistical comparisons were carried
out using ANOVA with the significance level adjusted using the
repeated t-tests with Bonferroni correction. P-value <0.05 was
considered to be significant.
Results
Honokiol inhibits invasion and colony
formation of highly metastatic RCC cells
To evaluate whether honokiol (Fig. 1) suppresses invasive behavior of
highly metastatic RCC cells, the 786-0 cells were treated with
honokiol (0–20 μM) for 24 h and cell invasion was determined as
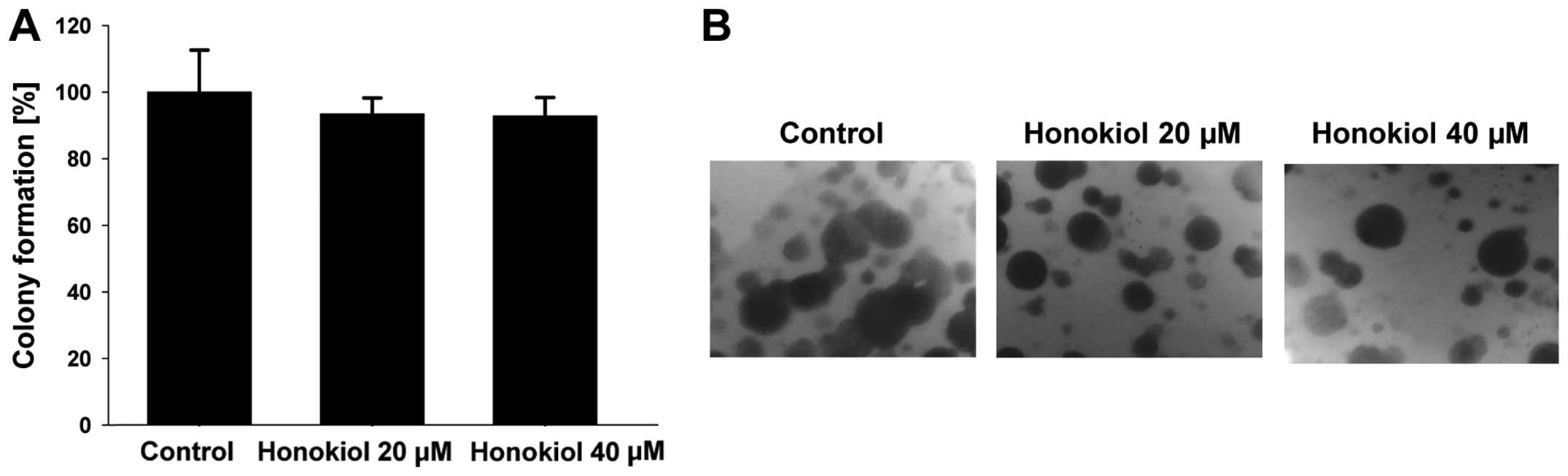
described in Materials and methods. As shown in Fig. 2A, honokiol inhibits cell invasion
through Matrigel in a dose-dependent manner. Moreover, honokiol
significantly decreases the number of anchorage-independent
colonies formed, which is a key step in cancer metastasis (Fig. 2B and C). In summary, honokiol
significantly inhibits invasion as well as colony formation of
highly meta-static RCC 786-0 cells in a dose-dependent manner.
Effect of honokiol on the expression of
genes related to human tumor metastasis
In order to gain further mechanistic insight into
the molecular events underlying metastasis inhibition of the 786-0
cells treated with honokiol, DNA-microarray analysis of 92 tumor
metastasis-associated genes and 4 candidate endogenous control
genes was performed. Table I
summarizes the genes with large recurring expression differences
compared with control. For example, significant upregulation was
observed including the expression of metastasis suppressor gene
(KISS-1, 28.56±11.17), genes encoding TIMP metalloproteinase
inhibitor 4 (TIMP4, 14.25±4.04) and KISS-1 receptor
(KISS-1R, 13.33±5.11). In addition, honokiol markedly
suppresses expression of genes encoding chemokine (C-X-C motif)
ligand 12 (CXCL12, 0.13±0.05), chemokine (C-C motif) ligand 7
(CCL7, 0.14±0.04), interleukin-18 (IL18, 0.23±0.05) and matrix
metallo proteinase 7 (MMP7, 0.26±0.09).
 | Table IEffect of honokiol on the expression
of human tumor metastasis genes. |
Table I
Effect of honokiol on the expression
of human tumor metastasis genes.
| Gene | Description | RQ |
|---|
| KISS1 | KISS-1 metastasis
suppressor | 28.56±11.17a |
| TIMP4 | TIMP
metalloproteinase inhibitor 4 | 14.25±4.04a |
| KISS1R | KISS1 receptor | 13.33±5.11a |
| TP53 | P53 tumor
suppressor | 2.24±0.16 |
| CXCL12 | Chemokine (C-X-C
motif) ligand 12 | 0.13±0.05 |
| CCL7 | Chemokine (C-C
motif) ligand 7 | 0.14±0.04 |
| IL18 | Interleukin-18 | 0.23±0.05 |
| MMP7 | Matrix
metalloproteinase 7 | 0.26±0.09 |
| VEGFC | Vascular
endothelial growth factor C | 0.42±0.04 |
| FGFR4 | Fibroblast growth
factor receptor 4 | 0.53±0.04 |
Honokiol activates KISS1/KISS1R signaling
in highly meta-static RCC cells
Since recent studies showed that activation of
KISS1/KISS1R signaling by kisspeptin treatment decreases the
motility and invasive capacity of conventional RCC, and
overexpression of KISS1 inhibits invasion of RCC cells Caki-1
(40,41), we confirmed the significant
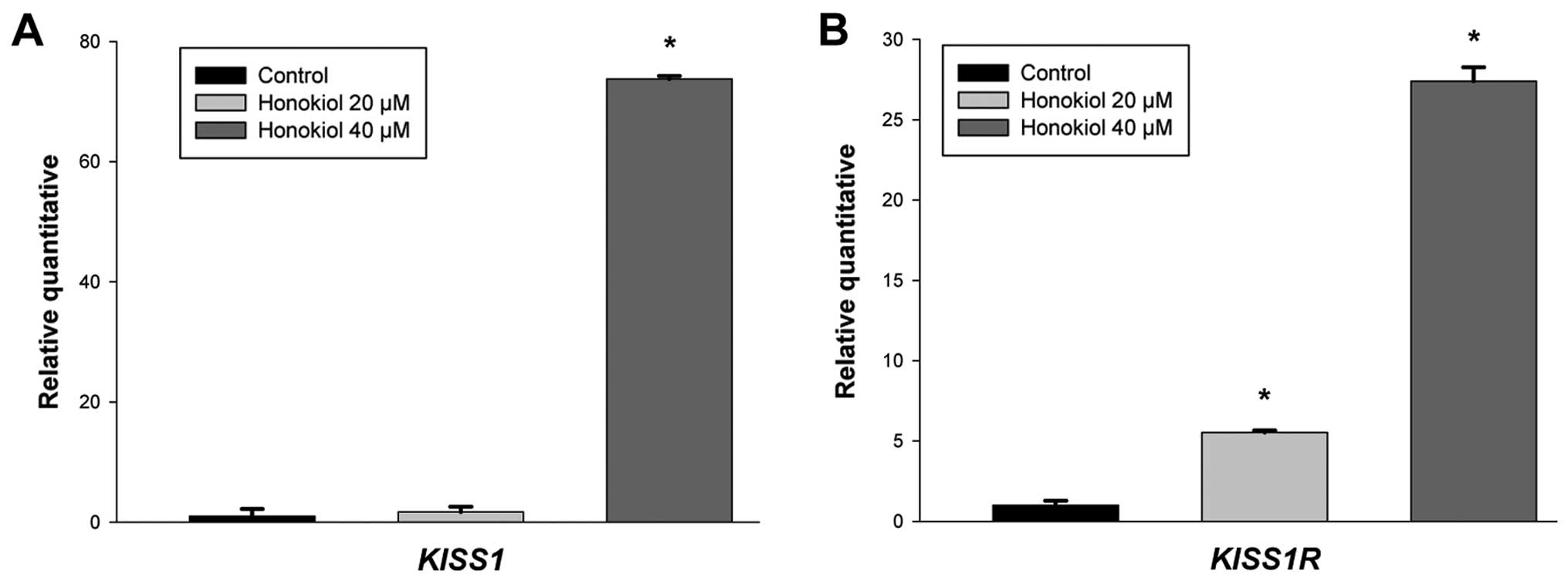
upregulation of KISS1 and KISS1R in the 786-0 cells
treated with honokiol by qRT-PCR (Fig.
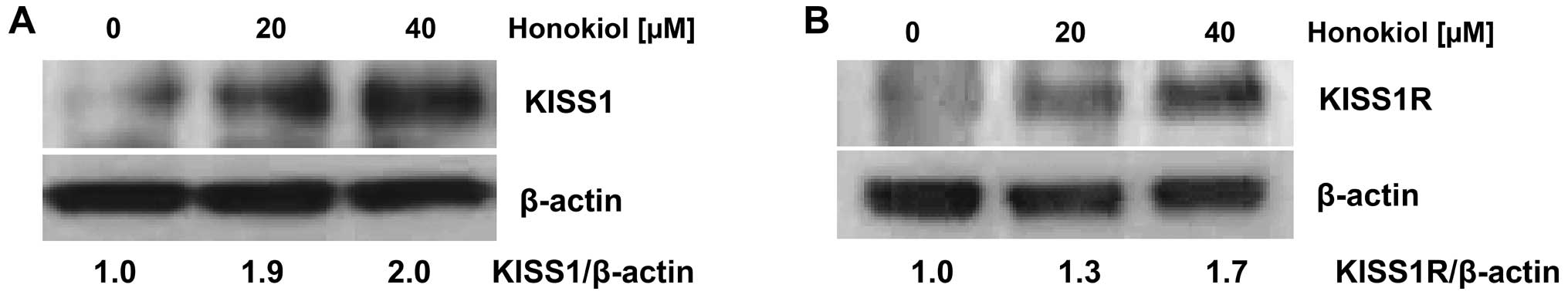
3). In accordance with the change in mRNA, western blot
analysis showed that honokiol stimulates expression of KISS1 and
KISS1R in the 786-0 cells dose-dependently at the protein level
(Fig. 4).
Silencing KISS1 reverses suppression of
invasion and colony formation
To determine whether the suppression of invasion and
colony formation by honokiol are associated with the activation of
KISS1/KISS1R signaling in the 786-0 cells, we silenced KISS1
with siRNA as described in Materials and methods. As shown in
Fig. 5, knockdown of KISS1
partially rescues the effect of honokiol on cell invasion by more
than 40%. Moreover, the effect of honokiol on colony formation of
the 786-0 cells is markedly reversed by KISS1 silencing
(Fig. 6). These results further
indicate that KISS1/KISS1R signaling is a major target of honokiol
in suppressing metastasis of RCC cells.
Discussion
In the present study, we investigated the role of
honokiol in the metastasis of RCC cells. Our results showed that
honokiol significantly inhibited the invasion and colony formation
of highly metastatic RCC cells 786-0 in a dose-dependent manner.
Moreover, honokiol markedly upregulated metastasis-suppressor gene
KISS1 and its receptor, KISS1R, at both transcription
and translation levels. Interestingly, knockdown of KISS1
partially rescued the effect of honokiol on cell invasion and its
effect on colony formation of the 786-0 cells is reversed as well,
indicating that KISS1/KISS1R signaling is a major target of
honokiol in suppressing metastasis of RCC cells.
Metastasis suppressors are defined as molecules
whose expression results in the suppression of metastasis processes
and since 1986, more than 13 metastasis suppressors have been
identified (42). The KISS1
gene, initially discovered as a novel human malignant melanoma
metastasis-suppressor gene (43),
has been validated as an anti-metastatic gene by preclinical and
clinical evidence in various types of cancer (44). The encoded KISS1 protein can be
processed to a C-terminally amidated peptide termed metastin
binding and activating the G-protein coupled receptor GPR54
(KISS1R) (45). Shoji et al
found that metastin inhibited migration and invasion of RCC with
overexpression of KISS1R (46). In
addition, a recent study demonstrated that an absence of KISS1R
expression was associated with rapid progression of conventional
RCC in patients (40), suggesting
KISS1/KISS1R signaling as a promising target in RCC.
Honokiol targets multiple signaling pathways such as
nuclear factor κB (NF-κB), signal transducers and activator of
transcription 3 (STAT3), mammalian target of rapamycin (mTOR) and
epidermal growth factor receptor (EGFR), which have great relevance
during cancer initiation and progression (47). Moreover, pharmacokinetic studies
revealed that honokiol crossed the blood-brain barrier (BBB), the
blood-cerebrospinal fluid barrier (BCSFB) and had a desirable
bioavailability after intravenous administration in animal models
(48) thus making it a suitable
agent for clinical trials.
In summary, our results indicate that activation of
KISS1/KISS1R signaling by honokiol decreases the invasiveness and
colonized capacity of highly metastatic RCC cells. Furthermore, we
confirmed that honokiol stimulated the expression of TIMP4
dose-dependently (data not shown). It is in accordance with the
finding that metastin suppresses the motility and invasive ability
of RCC cells which possess KISS1R through the downregulation of
MMP-2 (49). As emerging studies
show that KISS1R activates a series of signaling molecules such as
protein kinase C (PKC), extra-cellular signal-regulated kinases 1
and 2 (ERK1/2), p38, and phosphatidylinositol-3-kinase (PI3K)
(50), further studies are in
progress to investigate the specific mechanism of honokiol, which
may have the potential for use as a natural agent against RCC
metastasis.
Acknowledgments
We thank Dr Zizheng Dong, Indiana University School
of Medicine, for his technical assistance with the colony formation
assay. This study was supported by EcoNugenics, Inc., Santa Rosa,
CA, USA. One of the authors, I. Eliaz, acknowledges his interest as
the formulator and owner of EcoNugenics, Inc.
References
|
1
|
Mehlen P and Puisieux A: Metastasis: A
question of life or death. Nat Rev Cancer. 6:449–458. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nguyen DX and Massagué J: Genetic
determinants of cancer metastasis. Nat Rev Genet. 8:341–352. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Monteiro J and Fodde R: Cancer stemness
and metastasis: Therapeutic consequences and perspectives. Eur J
Cancer. 46:1198–1203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deep G and Agarwal R: Antimetastatic
efficacy of silibinin: Molecular mechanisms and therapeutic
potential against cancer. Cancer Metastasis Rev. 29:447–463. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gupta K, Miller JD, Li JZ, Russell MW and
Charbonneau C: Epidemiologic and socioeconomic burden of metastatic
renal cell carcinoma (mRCC): A literature review. Cancer Treat Rev.
34:193–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sadler GJ, Anderson MR, Moss MS and Wilson
PG: Metastases from renal cell carcinoma presenting as
gastrointestinal bleeding: Two case reports and a review of the
literature. BMC Gastroenterol. 7:42007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Czarnecka AM, Kornakiewicz A, Kukwa W and
Szczylik C: Frontiers in clinical and molecular diagnostics and
staging of metastatic clear cell renal cell carcinoma. Future
Oncol. 10:1095–1111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cairns P: Renal cell carcinoma. Cancer
Biomark. 9:461–473. 2010.
|
|
9
|
Patil S, Manola J, Elson P, Negrier S,
Escudier B, Eisen T, Atkins M, Bukowski R and Motzer RJ:
Improvement in overall survival of patients with advanced renal
cell carcinoma: Prognostic factor trend analysis from an
international data set of clinical trials. J Urol. 188:2095–2100.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buti S, Bersanelli M, Sikokis A, Maines F,
Facchinetti F, Bria E, Ardizzoni A, Tortora G and Massari F:
Chemotherapy in metastatic renal cell carcinoma today? A systematic
review. Anticancer Drugs. 24:535–554. 2013.PubMed/NCBI
|
|
11
|
Lin J, Deng Z, Tanikawa C, Shuin T, Miki
T, Matsuda K and Nakamura Y: Downregulation of the tumor suppressor
HSPB7, involved in the p53 pathway, in renal cell carcinoma by
hypermethylation. Int J Oncol. 44:1490–1498. 2014.PubMed/NCBI
|
|
12
|
Kim DW, Ko SM, Jeon YJ, Noh YW, Choi NJ,
Cho SD, Moon HS, Cho YS, Shin JC, Park SM, et al:
Anti-proliferative effect of honokiol in oral squamous cancer
through the regulation of specificity protein 1. Int J Oncol.
43:1103–1110. 2013.PubMed/NCBI
|
|
13
|
Joo YN, Eun SY, Park SW, Lee JH, Chang KC
and Kim HJ: Honokiol inhibits U87MG human glioblastoma cell
invasion through endothelial cells by regulating membrane
permeability and the epithelial-mesenchymal transition. Int J
Oncol. 44:187–194. 2014.
|
|
14
|
Tian W, Deng Y, Li L, He H, Sun J and Xu
D: Honokiol synergizes chemotherapy drugs in multidrug resistant
breast cancer cells via enhanced apoptosis and additional
programmed necrotic death. Int J Oncol. 42:721–732. 2013.
|
|
15
|
Hahm ER, Sakao K and Singh SV: Honokiol
activates reactive oxygen species-mediated cytoprotective autophagy
in human prostate cancer cells. Prostate. 74:1209–1221. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lai YJ, Lin CI, Wang CL and Chao JI:
Expression of survivin and p53 modulates honokiol-induced apoptosis
in colorectal cancer cells. J Cell Biochem. 115:1888–1899.
2014.PubMed/NCBI
|
|
17
|
Chilampalli C, Zhang X, Kaushik RS, Young
A, Zeman D, Hildreth MB, Fahmy H and Dwivedi C: Chemopreventive
effects of combination of honokiol and magnolol with α-santalol on
skin cancer developments. Drug Discov Ther. 7:109–115.
2013.PubMed/NCBI
|
|
18
|
Liang WZ, Chou CT, Chang HT, Cheng JS, Kuo
DH, Ko KC, Chiang NN, Wu RF, Shieh P and Jan CR: The mechanism of
honokiol-induced intracellular Ca(2+) rises and apoptosis in human
glioblastoma cells. Chem Biol Interact. 221:13–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Beitler JJ, Wang H, Lee MJ, Huang
W, Koenig L, Nannapaneni S, Amin AR, Bonner M, Shin HJ, et al:
Honokiol enhances paclitaxel efficacy in multi-drug resistant human
cancer model through the induction of apoptosis. PLoS One.
9:e863692014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang KH, Yan MD, Yao CJ, Lin PC and Lai
GM: Honokiol-induced apoptosis and autophagy in glioblastoma
multiforme cells. Oncol Lett. 6:1435–1438. 2013.PubMed/NCBI
|
|
21
|
Pan HC, Lai DW, Lan KH, Shen CC, Wu SM,
Chiu CS, Wang KB and Sheu ML: Honokiol thwarts gastric tumor growth
and peritoneal dissemination by inhibiting Tpl2 in an orthotopic
model. Carcinogenesis. 34:2568–2579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martin S, Lamb HK, Brady C, Lefkove B,
Bonner MY, Thompson P, Lovat PE, Arbiser JL, Hawkins AR and Redfern
CP: Inducing apoptosis of cancer cells using small-molecule plant
compounds that bind to GRP78. Br J Cancer. 109:433–443. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yao CJ, Lai GM, Yeh CT, Lai MT, Shih PH,
Chao WJ, Whang-Peng J, Chuang SE and Lai TY: Honokiol eliminates
human oral cancer stem-like cells accompanied with suppression of
Wnt/β-catenin signaling and apoptosis induction. Evid Based
Complement Alternat Med. 2013:1461362013. View Article : Google Scholar
|
|
24
|
Chae JI, Jeon YJ and Shim JH:
Downregulation of Sp1 is involved in honokiol-induced cell cycle
arrest and apoptosis in human malignant pleural mesothelioma cells.
Oncol Rep. 29:2318–2324. 2013.PubMed/NCBI
|
|
25
|
Wang Y, Zhu X, Yang Z and Zhao X: Honokiol
induces caspase-independent paraptosis via reactive oxygen species
production that is accompanied by apoptosis in leukemia cells.
Biochem Biophys Res Commun. 430:876–882. 2013. View Article : Google Scholar
|
|
26
|
Avtanski DB, Nagalingam A, Bonner MY,
Arbiser JL, Saxena NK and Sharma D: Honokiol inhibits
epithelial-mesenchymal transition in breast cancer cells by
targeting signal transducer and activator of transcription
3/Zeb1/E-cadherin axis. Mol Oncol. 8:565–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh T and Katiyar SK: Honokiol inhibits
non-small cell lung cancer cell migration by targeting
PGE2-mediated activation of β-catenin signaling. PLoS
One. 8:e607492013. View Article : Google Scholar
|
|
28
|
Liu SH, Wang KB, Lan KH, Lee WJ, Pan HC,
Wu SM, Peng YC, Chen YC, Shen CC, Cheng HC, et al: Calpain/SHP-1
interaction by honokiol dampening peritoneal dissemination of
gastric cancer in nu/nu mice. PLoS One. 7:e437112012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeong JJ, Lee JH, Chang KC and Kim HJ:
Honokiol exerts an anticancer effect in T98G human glioblastoma
cells through the induction of apoptosis and the regulation of
adhesion molecules. Int J Oncol. 41:1358–1364. 2012.PubMed/NCBI
|
|
30
|
Singh T and Katiyar SK: Honokiol, a
phytochemical from Magnolia spp., inhibits breast cancer cell
migration by targeting nitric oxide and cyclooxygenase-2. Int J
Oncol. 38:769–776. 2011.PubMed/NCBI
|
|
31
|
Wen J, Fu AF, Chen LJ, Xie XJ, Yang GL,
Chen XC, Wang YS, Li J, Chen P, Tang MH, et al: Liposomal honokiol
inhibits VEGF-D-induced lymphangiogenesis and metastasis in
xenograft tumor model. Int J Cancer. 124:2709–2718. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shigemura K, Arbiser JL, Sun SY, Zayzafoon
M, Johnstone PA, Fujisawa M, Gotoh A, Weksler B, Zhau HE and Chung
LW: Honokiol, a natural plant product, inhibits the bone metastatic
growth of human prostate cancer cells. Cancer. 109:1279–1289. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li W, Wang Q, Su Q, Ma D, An C, Ma L and
Liang H: Honokiol suppresses renal cancer cells’ metastasis via
dual-blocking epithelial-mesenchymal transition and cancer stem
cell properties through modulating miR-141/ZEB2 signaling. Mol
Cells. 37:383–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Modulation of human renal cell carcinoma
786-0 MMP-2 and MMP-9 activity by inhibitors and inducers in vitro.
Med Oncol. 23:245–250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lloyd FP Jr, Slivova V, Valachovicova T
and Sliva D: Aspirin inhibits highly invasive prostate cancer
cells. Int J Oncol. 23:1277–1283. 2003.PubMed/NCBI
|
|
36
|
Slivova V, Valachovicova T, Jiang J, et
al: Ganoderma lucidum inhibits invasiveness of breast cancer cells.
J Cancer Integr Med. 2:25–30. 2004.
|
|
37
|
Cheng S, Eliaz I, Lin J, Thyagarajan-Sahu
A and Sliva D: Triterpenes from Poria cocos suppress growth and
invasiveness of pancreatic cancer cells through the downregulation
of MMP-7. Int J Oncol. 42:1869–1874. 2013.PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
39
|
Jiang J, Slivova V, Harvey K,
Valachovicova T and Sliva D: Ganoderma lucidum suppresses growth of
breast cancer cells through the inhibition of Akt/NF-kappaB
signaling. Nutr Cancer. 49:209–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Y, Yusenko MV and Kovacs G: Lack of
KISS1R expression is associated with rapid progression of
conventional renal cell carcinomas. J Pathol. 223:46–53. 2011.
View Article : Google Scholar
|
|
41
|
Zhang H, Guo Y, Shang C, Song Y and Wu B:
miR-21 down-regulated TCF21 to inhibit KISS1 in renal cancer.
Urology. 80:1298–302.e1. 2012. View Article : Google Scholar
|
|
42
|
Hurst DR and Welch DR: Metastasis
suppressor genes at the interface between the environment and tumor
cell growth. Int Rev Cell Mol Biol. 286:107–180. 2011.PubMed/NCBI
|
|
43
|
Lee JH, Miele ME, Hicks DJ, Phillips KK,
Trent JM, Weissman BE and Welch DR: KiSS-1, a novel human malignant
melanoma metastasis-suppressor gene. J Natl Cancer Inst.
88:1731–1737. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Beck BH and Welch DR: The KISS1 metastasis
suppressor: A good night kiss for disseminated cancer cells. Eur J
Cancer. 46:1283–1289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ohtaki T, Shintani Y, Honda S, Matsumoto
H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, et
al: Metastasis suppressor gene KiSS-1 encodes peptide ligand of a
G-protein-coupled receptor. Nature. 411:613–617. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shoji S, Tang XY, Umemura S, Itoh J,
Takekoshi S, Shima M, Usui Y, Nagata Y, Uchida T, Osamura RY, et
al: Metastin inhibits migration and invasion of renal cell
carcinoma with overexpression of metastin receptor. Eur Urol.
55:441–449. 2009. View Article : Google Scholar
|
|
47
|
Arora S, Singh S, Piazza GA, Contreras CM,
Panyam J and Singh AP: Honokiol: A novel natural agent for cancer
prevention and therapy. Curr Mol Med. 12:1244–1252. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang X, Duan X, Yang G, Zhang X, Deng L,
Zheng H, Deng C, Wen J, Wang N, Peng C, et al: Honokiol crosses BBB
and BCSFB, and inhibits brain tumor growth in rat 9L intracerebral
gliosarcoma model and human U251 xenograft glioma model. PLoS One.
6:e184902011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yoshioka K, Ohno Y, Horiguchi Y, Ozu C,
Namiki K and Tachibana M: Effects of a KiSS-1 peptide, a metastasis
suppressor gene, on the invasive ability of renal cell carcinoma
cells through a modulation of a matrix metalloproteinase 2
expression. Life Sci. 83:332–338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cvetković D, Babwah AV and Bhattacharya M:
Kisspeptin/KISS1R System in breast cancer. J Cancer. 4:653–661.
2013. View
Article : Google Scholar
|