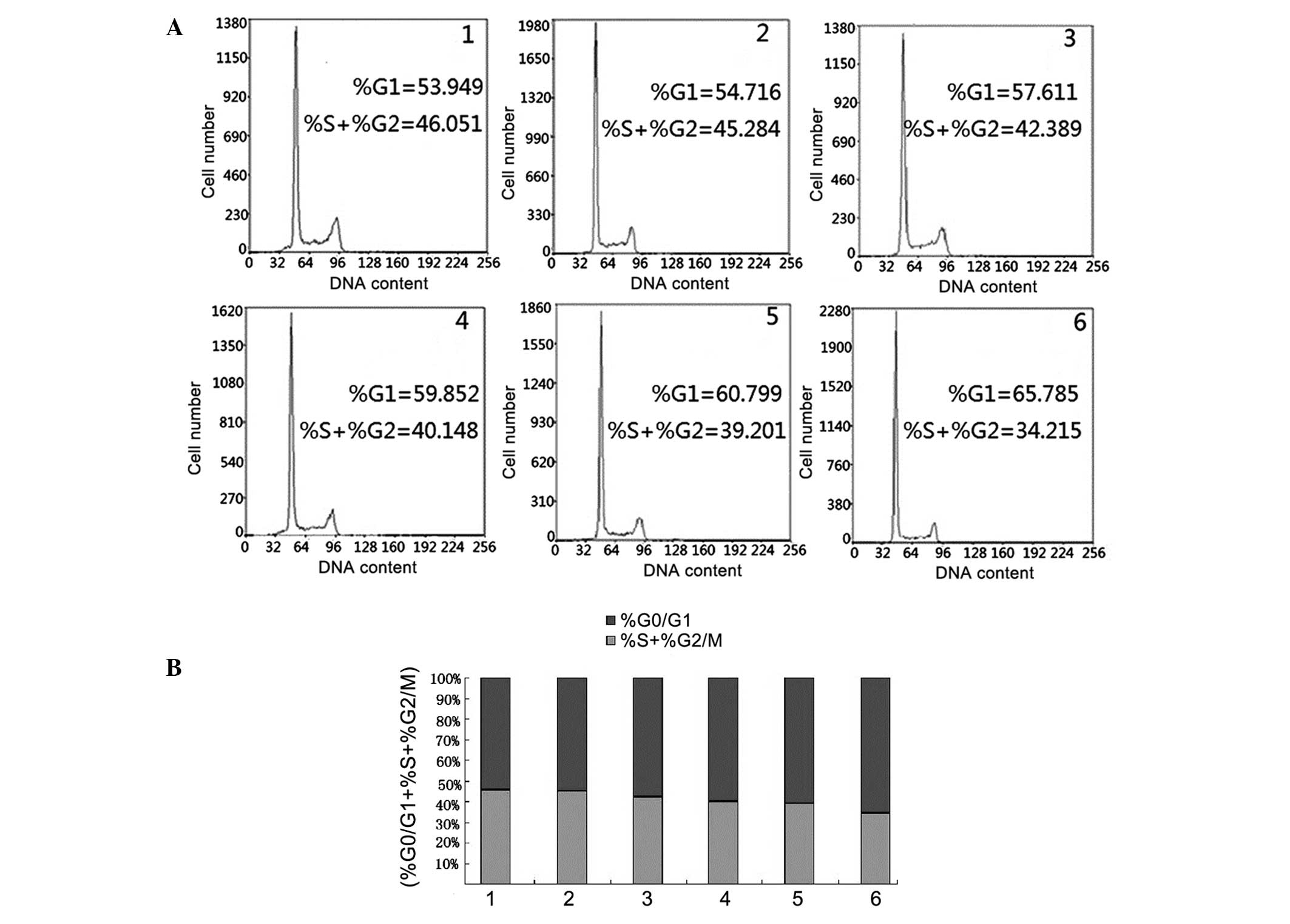
|
1
|
Kato T, Suzuki M, Murata T and Park EY:
The effects of N-glycosylation sites and the N-terminal region on
the biological function of beta1,3-N-acetylglucosaminyltransferase
2 and its secretion. Biochem Biophys Res Commun. 329:699–705. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Narimatsu H: Human glycogene cloning:
focus on beta 3-glycosyltransferase and beta 4-glycosyltransferase
families. Curr Opin Struct Biol. 16:567–575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hakomori S: Glycosylation defining cancer
malignancy: new wine in an old bottle. Proc Natl Acad Sci USA.
99:10231–10233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saitoh O, Wang WC, Lotan R and Fukuda M:
Differential glycosylation and cell surface expression of lysosomal
membrane glycoproteins in sublines of a human colon cancer
exhibiting distinct metastatic potentials. J Biol Chem.
267:5700–5711. 1992.PubMed/NCBI
|
|
5
|
Kinoshita M, Mitsui Y, Kakoi N, Yamada K,
Hayakawa T and Kakehi K: Common glycoproteins expressing
polylactosamine-type glycans on matched patient primary and
metastatic melanoma cells show different glycan profiles. J
Proteome Res. 2013.PubMed/NCBI
|
|
6
|
Mitsui Y, Yamada K, Hara S, Kinoshita M,
Hayakawa T and Kakehi K: Comparative studies on glycoproteins
expressing polylactosamine-type N-glycans in cancer cells. J Pharm
Biomed Anal. 70:718–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Togayachi A, Kozono Y, Ishida H, et al:
Polylactosamine on glycoproteins influences basal levels of
lymphocyte and macrophage activation. Proc Natl Acad Sci USA.
104:15829–15834. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang W, Chang SB and Hemler ME: Links
between CD147 function, glycosylation, and caveolin-1. Mol Biol
Cell. 15:4043–4050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang W, Luo WJ, Zhu P, et al: Modulation
of CD147-induced matrix metalloproteinase activity: role of CD147
N-glycosylation. Biochem J. 449:437–448. 2013. View Article : Google Scholar
|
|
10
|
Gabison EE, Hoang-Xuan T, Mauviel A and
Menashi S: EMMPRIN/CD147, an MMP modulator in cancer, development
and tissue repair. Biochimie. 87:361–368. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agrawal SM and Yong VW: The many faces of
EMMPRIN - roles in neuroinflammation. Biochim Biophys Acta.
1812:213–219. 2011. View Article : Google Scholar
|
|
12
|
Hall ET, Yan JP, Melançon P and Kuchta RD:
3′-Azido-3′-deoxythymidine potently inhibits protein glycosylation.
A novel mechanism for AZT cytotoxicity. J Biol Chem.
269:14355–14358. 1994.PubMed/NCBI
|
|
13
|
Steet RA, Melancon P and Kuchta RD:
3′-Azidothymidine potently inhibits the biosynthesis of highly
branched N-linked oligosaccharides and poly-N-acetyllactosamine
chains in cells. J Biol Chem. 275:26812–26820. 2000.PubMed/NCBI
|
|
14
|
Liu Z, Shen L, Xu L, Sun X, Zhou J and Wu
S: Down-regulation of β-1,3-N-acetylglucosaminyltransferase-8 by
siRNA inhibits the growth of human gastric cancer. Mol Med Rep.
4:497–503. 2011.PubMed/NCBI
|
|
15
|
van den Eijnden DH, Koenderman AH and
Schiphorst WE: Biosynthesis of blood group i-active
polylactosaminoglycans. Partial purification and properties of an
UDP-GlcNAc:N-acetyllactosaminide beta 1 - 3-N-acetylglucos
aminyltransferase from Novikoff tumor cell ascites fluid. J Biol
Chem. 263:12461–12471. 1988.PubMed/NCBI
|
|
16
|
Ishida H, Togayachi A, Sakai T, et al: A
novel beta1,3-N-acetylglucosaminyltransferase (beta3Gn-T8), which
synthesizes poly-N-acetyllactosamine, is dramatically upregulated
in colon cancer. FEBS Lett. 579:71–78. 2005. View Article : Google Scholar
|
|
17
|
Do KY and Cummings RD: 2,6-branched
mannose and the regulation of poly-N-acetyllactosamine biosynthesis
in N-linked oligosaccharides of Chinese hamster ovary cells. J Biol
Chem. 268:22028–22035. 1993.PubMed/NCBI
|
|
18
|
Hakomori S: Tumor malignancy defined by
aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer
Res. 56:5309–5318. 1996.PubMed/NCBI
|
|
19
|
Pierce M, Buckhaults P, Chen L and Fregien
N: Regulation of N-acetylglucosaminyltransferase V and Asn-linked
oligosaccharide beta(1,6) branching by a growth factor signaling
pathway and effects on cell adhesion and metastatic potential.
Glycoconj J. 14:623–630. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Datti A, Orlacchio A, Siminovitch KA and
Dennis JW: A coupled assay for UDP-GlcNAc:Gal beta 1-3GalNAc-R beta
1,6-N-acetylglucosaminyltransferase (GlcNAc to GalNAc). Anal
Biochem. 206:262–266. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sawada R, Lowe JB and Fukuda M:
E-selectin-dependent adhesion efficiency of colonic carcinoma cells
is increased by genetic manipulation of their cell surface
lysosomal membrane glycoprotein-1 expression levels. J Biol Chem.
268:12675–12681. 1993.PubMed/NCBI
|
|
22
|
Krishnan V, Bane SM, Kawle PD, Naresh KN
and Kalraiya RD: Altered melanoma cell surface glycosylation
mediates organ specific adhesion and metastasis via lectin
receptors on the lung vascular endothelium. Clin Exp Metastasis.
22:11–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishida H, Togayachi A, Sakai T, et al: A
novel beta1,3-N-acetyl-glucosaminyltransferase (beta3Gn-T8), which
synthesizes poly-N-acetyllactosamine, is dramatically upregulated
in colon cancer. FEBS Lett. 579:71–78. 2005. View Article : Google Scholar
|
|
24
|
Ni J, Jiang Z, Shen L, et al: β3GnT8
regulates the metastatic potential of colorectal carcinoma cells by
altering the glycosylation of CD147. Oncol Rep. 31:1795–1801.
2014.PubMed/NCBI
|
|
25
|
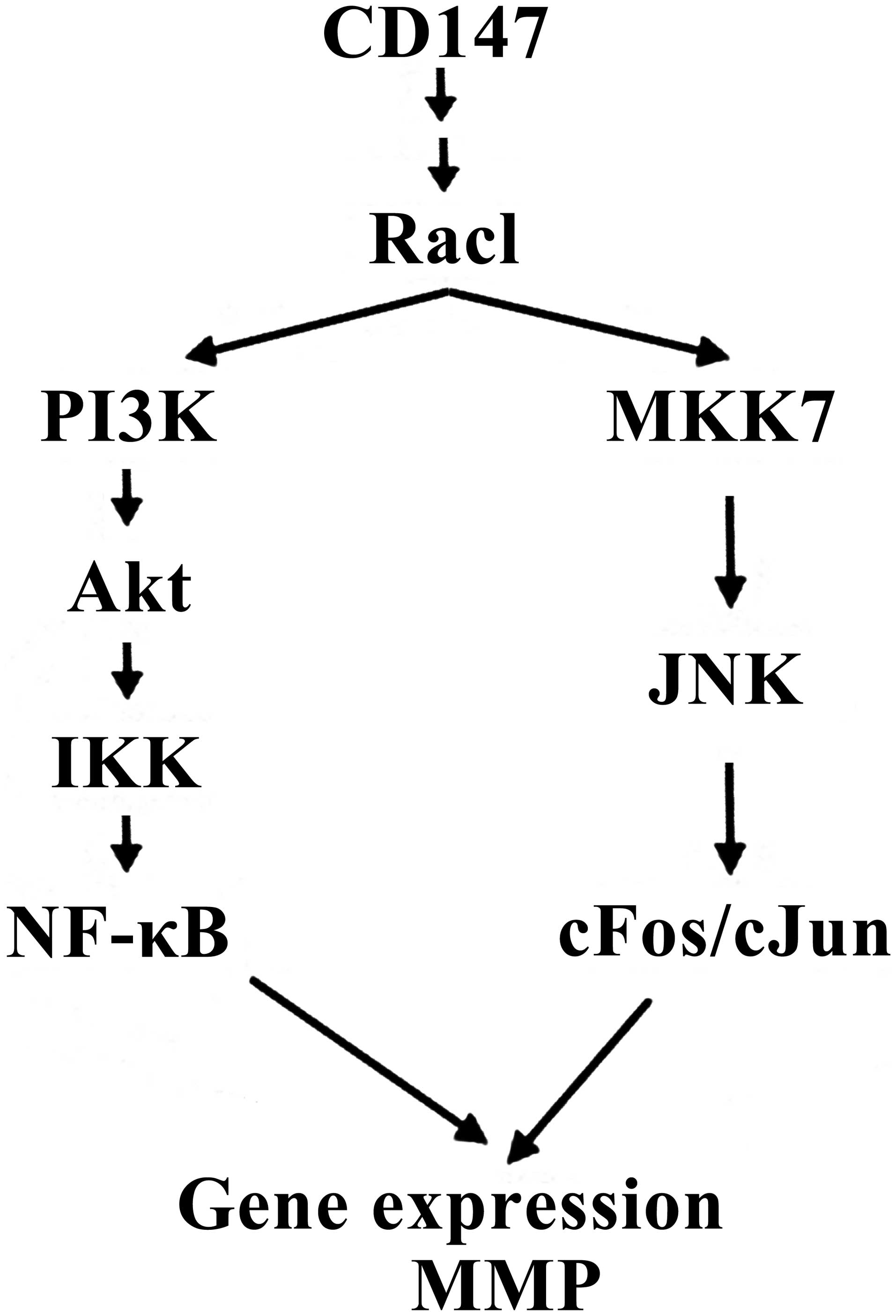
Venkatesan B, Valente AJ, Prabhu SD,
Shanmugam P, Delafontaine P and Chandrasekar B: EMMPRIN activates
multiple transcription factors in cardiomyocytes, and induces
interleukin-18 expression via Rac1-dependent PI3K/Akt/IKK/NF-kappaB
and MKK7/JNK/AP-1 signaling. J Mol Cell Cardiol. 49:655–663. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li G, Zhang Y, Qian Y, Zhang H, et al:
Interleukin-17A promotes rheumatoid arthritis synoviocytes
migration and invasion under hypoxia by increasing MMP2 and MMP9
expression through NF-κB/HIF-1α pathway. Mol Immunol. 53:227–236.
2013. View Article : Google Scholar
|
|
27
|
Sun P, Mu Y and Zhang S: A novel
NF-κB/MMP-3 signal pathway involves in the aggressivity of glioma
promoted by Bmi-1. Tumour Biol. 35:12721–12727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han YP, Tuan TL, Wu H, Hughes and Garner
WL: TNF-alpha stimulates activation of pro-MMP2 in human skin
through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci.
114:131–139. 2001.
|