Introduction
Diabetes mellitus is a severe metabolic disease, and
numerous complications are associated with the characteristic
hyperglycemia of this disease (1–3). Of
these, diabetic retinopathy is one of the major microvascular
complications amongst diabetic patients, and is the primary cause
of visual loss through neovascularization (1).
Vascular endothelial growth factor (VEGF) is a
potent angiogenic and vascular hyperpermeability factor, and has a
key role in the pathogenesis underlying diabetic retinopathy
(2). VEGF is produced by multiple
types of cell, including retinal pigment epithelium, ganglion
cells, Müller cells, pericytes and the smooth muscle cells of the
human retina and choroid, and is mainly modulated by tissue oxygen
content (3). The transcriptional
regulation of VEGF is mediated by hypoxia-inducible factor (HIF)-1
(4).
HIF-1 is the primary hypoxic signaling protein in
cells for regulating angiogenesis, and is a transcription factor
that is regarded as a ‘master switch’, responsible for the
regulation of all oxygen-dependent retinal diseases (5). Downregulation of HIF-1 inhibits
neovascularization in proliferative diabetic retinopathy (4). HIF-1 is a heterodimeric
transcriptional factor composed of a labile α subunit (120 kDa) and
a stable β subunit (92 kDa) (5).
HIF-1α activity is regulated by posttranslational
modification-associated processes, whereas HIF-1β is constitutively
expressed (6). HIF-1 induces the
transcription of genes whose protein products function to enhance
O2 delivery, for example erythropoietin and VEGF, which
stimulate erythropoiesis and angiogenesis, respectively; or to
induce metabolic adaptations to facilitate function under reduced
O2 conditions, including glucose transporters and
glycolytic enzymes (5). HIF-1α
promotes neovascularization (7,8), by
increasing VEGF expression (9).
Akt is a member of a class of serine or threonine
protein kinases, and is a major effector in the phosphoinositide
3-kinase (PI3K) signaling pathway (10,11).
Akt has a significant role in multiple cellular processes,
including cell survival, metabolism, growth, proliferation and
mobility (10), and additionally
regulates vascular homeostasis and angiogenesis (11). Growth factors, cytokines and other
signaling molecules stimulate HIF-1α protein synthesis via
activation of the PI3K/Akt signaling pathways (10,11).
Phosphorylated (p)Akt denotes activated Akt, and the PI3K/Akt
pathway is involved in modulating the expression of HIF1-α and VEGF
(9).
Betaine is an alkaloid, which is occasionally
classified as an amino acid, that is found in capsicum, silybum
(the source of liver-protective flavonoid, silymarin) and Beta
vulgaris (12,13). Betaine is found amongst various
animals, plants and microorganisms, and dietary sources rich in
betaine include seafood, in particular marine invertebrates, wheat
germ or wheat bran and spinach. The primary physiological role of
betaine is as an osmolyte and methyl donor for transmethylation. In
its capacity as an osmolyte, betaine protects cells, proteins and
enzymes against environmental stressors. In addition, betaine is a
crucial nutrient required for the prevention of chronic diseases
(12). Betaine is a component of
Fructus lycii, and is known to enhance visual acuity
(13). However, the effect of
betaine on the neovascularization of the retinas of patients with
diabetes, which is the underlying cause of diabetic retinopathy,
has remained to be elucidated.
In the present study, the effect of betaine on the
expression of VEGF and HIF-1α in association with Akt activation in
the retinas of streptozotocin (STZ)-induced diabetic rats was
investigated using western blot analysis and
immunohistochemistry.
Materials and methods
Animals and treatments
Forty male Sprague-Dawley rats (Dae Han Bio Link
Co., Ltd., Seoul, Korea) weighing 120±10 g (aged five weeks) were
used in the present study. The rats were housed under controlled
temperature (20±2°C) and lighting conditions (07:00 to 19:00 h),
with ad libitum access to food and water throughout the
experimental period. The experimental procedures were performed in
accordance with the animal care guidelines of the National
Institutes of Health and the Korean Academy of Medical Sciences
(Seoul, Korea), and the study was approved by the Kyung Hee
Insitutional Animal Care and Use Committee (Seoul, Korea). The
animals were randomly divided into four groups (n=10 per group):
The control group, the STZ-induced diabetes group, STZ-induced
diabetes and 250 mg/kg betaine-treated group and the STZ-induced
diabetes and 500 mg/kg betaine-treated group. The control group
received the same volume of water for the same duration. Betaine
was purchased from Sigma-Aldrich (St. Louis, MO, USA). Four weeks
following STZ administration, betaine was orally administrated to
the rats once a day for 14 consecutive days at the respective doses
for each group.
Induction of diabetes
Diabetes was induced in the experimental animals
with a single intraperitioneal (i.p.) injection of STZ (60 mg/kg,
dissolved in 10 mM citrate buffer; pH 4.5; Sigma-Aldrich)
administered to each animal. Blood glucose levels were determined
two days after STZ injection using a blood glucose tester (Arkray,
Kyoto, Japan). Only those rats with blood glucose levels of ≥300
mg/dl were confirmed to have diabetes and used in the diabetes
groups. Subsequently, blood glucose levels were measured at 0, 2, 4
and 6 weeks following commencement of the experiment.
Tissue preparation
The animals were anesthetized using Zoletil
50® (10 mg/kg, i.p.; Vibac Laboratories, Carros,
France), transcardially perfused with 50 mM phosphate-buffered
saline and fixed with a freshly prepared solution of 4%
paraformaldehyde (Sigma-Aldrich) in 100 mM phosphate buffer (pH
7.4; Sigma-Aldrich). The retinas were dissected and postfixed
overnight in 4% paraformaldehyde with 100 mM phosphate buffer, and
then transferred into a 30% sucrose solution (Sigma-Aldrich) for
cryoprotection. A freezing microtome (CM 1510-3; Leica Microsystems
GmbH, Nussloch, Germany). was used to slice 20-µm coronal
sections of the retinas.
Western blot analysis of VEGF, HIF1-α and
pAkt expression
Western blot analyses were conducted according to a
previously described method (14,15).
Retinal tissues were lysed in ice-cold whole cell lysate buffer,
which comprised 50 mM HEPES (pH 7.5), 150 mM NaCl, 10% glycerol, 1%
Triton X-100, 1.5 mM magnesium chloride hexahydrate, 1 mM
ethyleneglycol-bis-(β-aminoethyl ether)-N,N′-tetraacetic
acid, 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 µg/ml
leupeptin, 1 µg/ml pepstatin, 1 mM sodium orthovanadate and
100 mM sodium fluoride. Additionally, rat retina tissues were lysed
in a lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl,
0.5% deoxycholic acid, 1% Nonidet P40, 0.1% SDS, 1 mM PMSF and 100
mg/ml leupeptin. The mixture was incubated at 4°C for 30 min. Cell
debris was removed by microcentrifugation at 19,000 x g for 20 min
at 4°C, followed by snap freezing of the supernatant. The protein
concentration was measured using a Bio-Rad colorimetric protein
assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Protein
(30 µg) was separated on 12% SDS-PAGE and transferred onto a
nitrocellulose membrane (Schleicher & Schuell GmbH, Dassel,
Germany). The membranes were incubated with the following primary
antibodies: Mouse monoclonal anti-actin antibody (cat. no.
sc-8432), rabbit polyclonal anti-HIF-1α antibody (cat. no.
sc-10790) and mouse monoclonal anti-VEGF antibody (cat. no.
sc-7269) (1:1,000; Santa Cruz Biotechnology Inc., Dallas, TX, USA);
rabbit polyclonal anti-Akt antibody (cat. no. 9272) and rabbit
monoclonal anti-pAkt antibody (cat. no. 4060) (1:1,000; Cell
Signaling Technology Inc., Beverly, MA, USA) at 4°C overnight. The
membranes were then incubated with anti-mouse (1:2,000; cat. no.
RPN4201; Amersham Pharmacia Biotechnology GmbH, Freiburg, Germany)
and anti-rabbit (1:2,000; cat. no. sc-2054; Santa Cruz
Biotechnology, Inc.) antibodies at room temperature for 1 h.
Protein bands were detected using an enhanced chemiluminescence
detection system (Santa Cruz Biotechnology, Inc.).
Immunohistochemical analysis of VEGF,
HIF1-α and pAkt expression
Immunohistochemical analyses were conducted as
previously described (16,17). The frozen retinal sections were
incubated overnight at 4°C with mouse monoclonal anti-VEGF antibody
(1:200; cat. no. sc-7269; Santa Cruz Biotechnology, Inc.), rabbit
polyclonal anti-HIF1-α antibody (1:500; cat. no. sc-10790; Santa
Cruz Biotechnology, Inc.) and rabbit monoclonal anti-pAkt antibody
(1:200; cat. no. 4060; Cell Signaling Technology, Inc.).
Subsequently, the sections were incubated for a further 1 h at room
temperature with biotinylated anti-mouse secondary antibody (1:200;
cat. no. BA2000; Vector Laboratories, Inc., Burlingame, CA, USA)
and biotinylated anti-rabbit secondary antibody (1:200; cat. no.
BA1000; Vector Laboratories, Inc.). The bound secondary antibody
was then amplified using a Vector Elite ABC kit® (1:100;
Vector Laboratories, Inc.). The antibody-biotin-avidin-peroxidase
complexes were visualized using 0.03% 3,3′diaminobenzidine
(Sigma-Aldrich) and the sections were mounted onto gelatin-coated
slides (Marienfeld-Superior, Lauda-Königshofen, Germany). The
slides were air dried at room temperature overnight, and
cover-slips were subsequently mounted with Permount®
(Thermo Fisher Scientific, Waltham, MA, USA).
Statistical analysis
Following staining, the number of immunoreactive
cells per 1,000-µm length of retinal section were counted
for each rat. Results were analyzed using one-way analysis of
variance followed by Duncan’s post-hoc test, and values are
presented as the mean ± standard error of the mean. Statistical
analyses were conducted using SPSS version 21.0 (IBM, Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference between values.
Results
Effect of betaine on blood glucose
levels
At 0, 2, 4 and 6 weeks of the experiment, blood
glucose levels were 134.08±0.84, 134.57±4.67, 137.29±12.14 and
137.79±9.45 mg/dl, respectively, in the control group; 134.08±0.84,
414.14±9.75, 458.00±40.01 and 457.14±19.16 mg/dl, respectively, in
the STZ-induced diabetes group; 134.08±0.84, 419.13±12.53,
460.75±35.84 and 405.75±19.88 mg/dl, respectively, in the
STZ-induced diabetes and 250 mg/kg betaine-treated group and
134.08±0.84, 419.00±13.22, 453.37±35.36 and 391.75±22.75 mg/dl,
respectively, in the STZ-induced diabetes and 500 mg/kg
betaine-treated group. Blood glucose levels were significantly
increased following STZ injection (P<0.05). Two weeks of betaine
treatment decreased blood glucose level in the diabetic rats,
however this decrease was not statistically significant
(P>0.05).
Betaine attenuates the STZ-induced
increase in VEGF expres- sion in the retina
The expression of VEGF in the retinas of the control
group was set as 1.0. The expression of VEGF was 5.64±0.12 in the
STZ-induced diabetes group, 2.11±0.37 in the STZ-induced diabetes
and 250 mg/kg betaine-treated group and 1.69±0.35 in the
STZ-induced diabetes and 500 mg/kg betaine-treated group.
STZ-induced diabetic rats demonstrated enhanced VEGF expression in
the retina (P<0.05) compared with that of the control group. By
contrast, betaine treatment suppressed VEGF expression in the
retinas of the diabetic rats (P<0.05), compared with that of the
STZ-induced diabetes group. A higher dose of betaine exerted a more
potent suppressive effect on VEGF expression levels (Fig. 1, upper panel).
The number of VEGF-positive cells in the retinas was
7.70±0.62/section in the control group, 21.48±1.56/section in the
STZ-induced diabetes group, 11.09±0.99/section in the STZ-induced
diabetes and 250 mg/kg betaine-treated group and 12.75±1.79/section
in the STZ-induced diabetes and 500 mg/kg betaine-treated group. An
increased number of VEGF-positive cells were detected in the
retinas of STZ-induced diabetes rats compared with those of the
control group (P<0.05). By contrast, betaine treatment inhibited
this increase in the number of VEGF-positive cells in the retinas
of the diabetic rats (P<0.05). A lower dose of betaine exerted a
more potent inhibitory effect on the number VEGF-positive cells
(Fig. 1, lower panel).
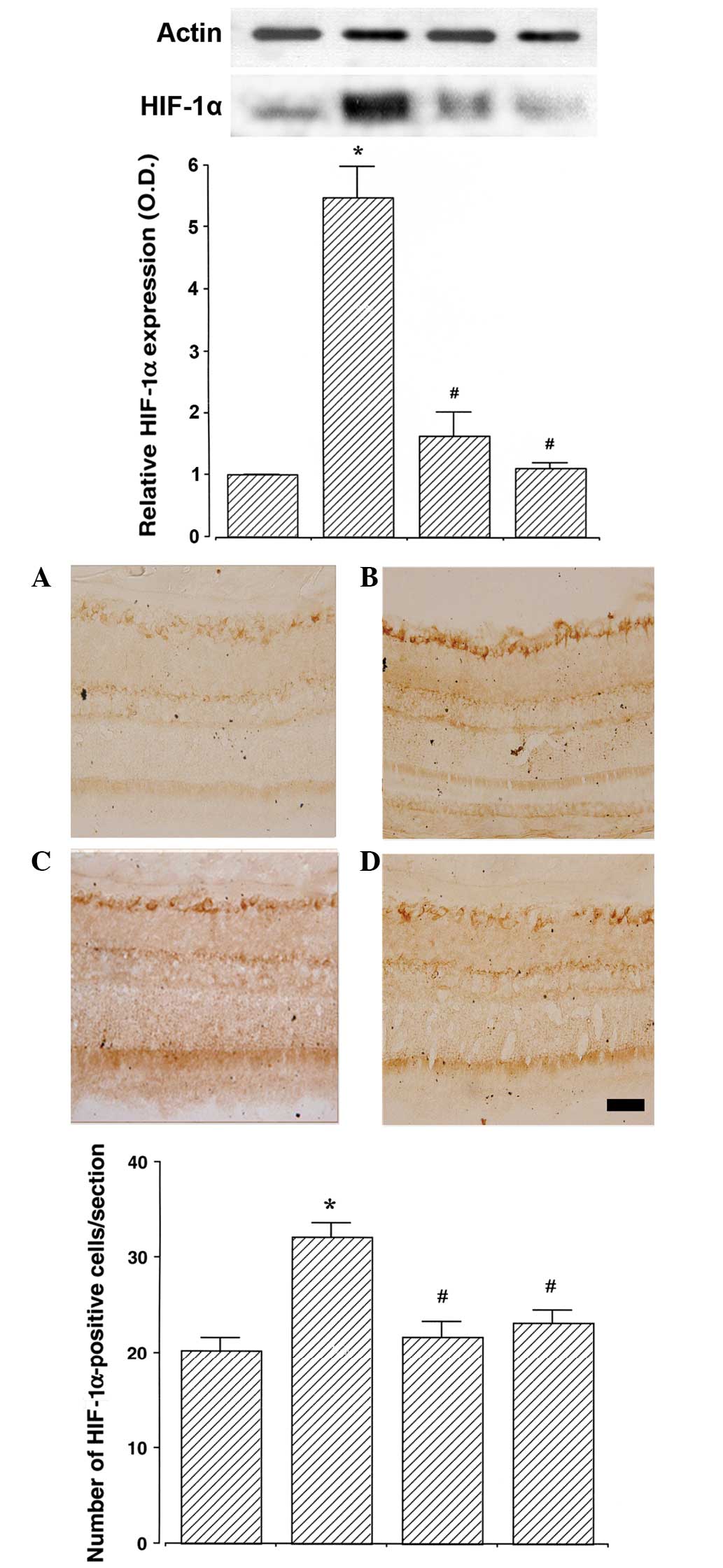
Betaine attenuates the STZ-induced
increase in HIF-1α expression in the retina
The expression of HIF-1α in the retinas of the
control group was set as 1.0. The expression of HIF-1α was
5.48±0.51 in the STZ-induced diabetes group, 1.64±0.38 in the
STZ-induced diabetes and 250 mg/kg betaine-treated group and
1.11±0.10 in the STZ-induced diabetes and 500 mg/kg betaine-treated
group. STZ-induced diabetic rats demonstrated enhanced levels of
HIF-1α expression in the retina compared with those of the control
group (P<0.05). Conversely, betaine treatment suppressed HIF-1α
expression in the retinas of the STZ-induced diabetes rats
(P<0.05). Higher doses of betaine exerted a more potent
suppressive effect on the HIF-1α expression, although this effect
was not significant (Fig. 2,
upper).
The number of HIF-1α-positive cells in the retinas
was 20.26±1.29/section in the control group, 32.16±1.49/section in
the STZ-induced diabetes group, 21.73±1.56/section in the
STZ-induced diabetes and 250 mg/kg betaine-treated group and
23.19±1.31/section in the STZ-induced diabetes and 500 mg/kg
betaine-treated group. STZ-induced diabetes rats exhibited an
increased number of HIF-1α-positive cells in the retinas, compared
with those of the control group (P<0.05). By contrast, betaine
treatment inhibited this increase in the number of HIF-1α-positive
cells in the retinas of the diabetic rats (P<0.05; Fig. 2, lower panel).
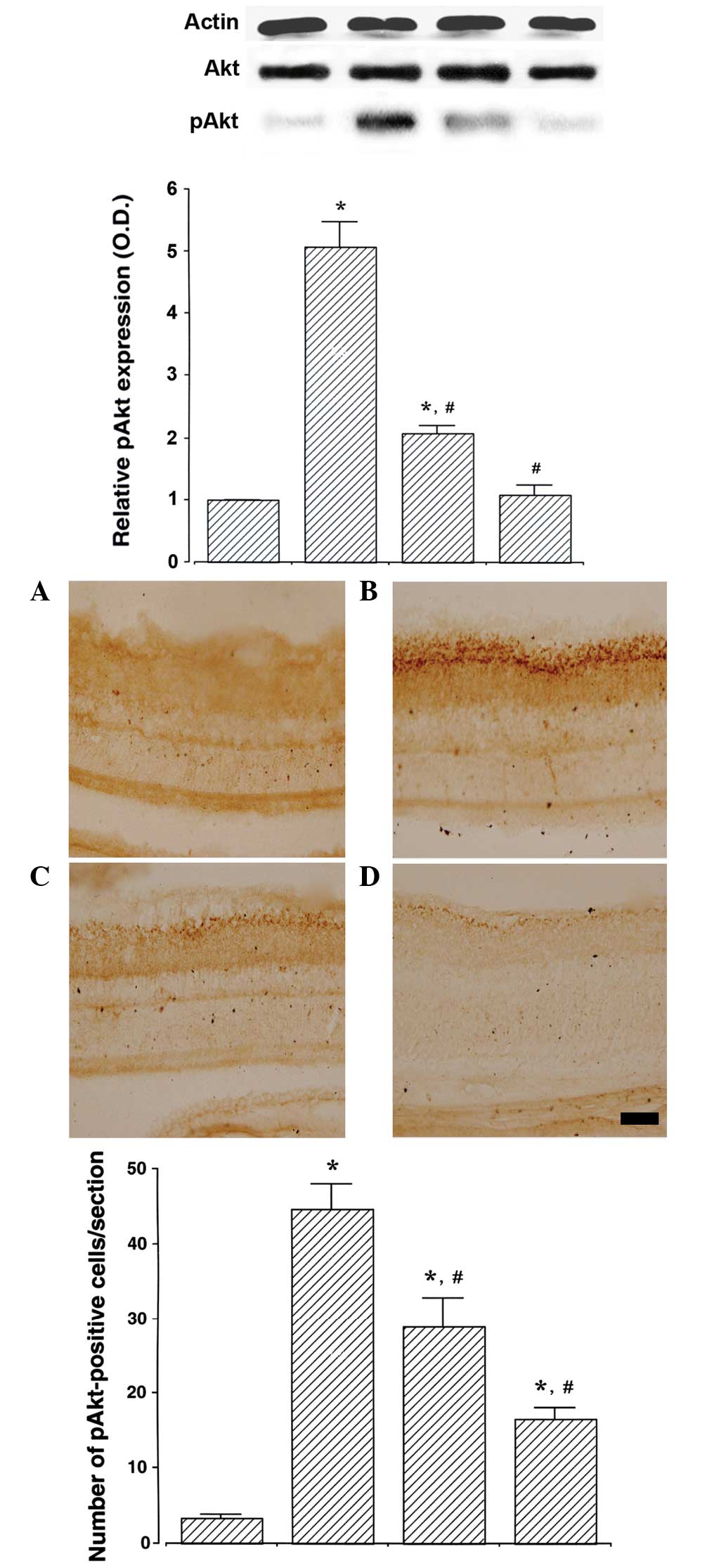
Betaine ameliorates the STZ-induced
increase in pAkt expression in the retina
The expression of pAkt in the retinas of the control
group was set as 1.0. The expression of pAkt was 5.07±0.40 in the
STZ-induced diabetes group, 2.08±0.13 in the STZ-induced diabetes
and 250 mg/kg betaine-treated group and 1.09±0.15 in the
STZ-induced diabetes and 500 mg/kg betaine-treated group.
STZ-induced diabetes rats revealed enhanced pAkt expression in the
retinas compared with those of the control rats (P<0.05).
However, betaine treatment was able to suppress this increase in
pAkt expression in the retinas of the diabetic rats (P<0.05). A
higher dose of betaine exerted a more potent suppressive effect on
pAkt expression (Fig. 3, upper
panel).
The number of pAkt-positive cells in the retina was
3.41±0.36/section in the control group, 44.71±3.25/section in the
STZ-induced diabetes group, 28.87±3.82/section in the STZ-induced
diabetes and 250 mg/kg betaine-treated group and 16.63 ±
1.42/section in STZ-induced diabetes and 500 mg/kg betaine-treated
group. STZ-induced diabetes resulted in an increase in the number
of pAkt-positive cells in the rat retinas compared with those in
the control group (P<0.05). By contrast, betaine treatment
inhibited this increase in the number of pAkt-positive cells in the
retinas of the diabetic rats (P<0.05). A higher dose of betaine
exerted a more potent inhibitory effect on the number of
pAkt-positive cells (Fig. 3, lower
panel). These results suggest that betaine is able to suppress Akt
activation.
Discussion
Suppression of angiogenesis is a key therapeutic
strategy in the prevention of the progression of diabetic
retinopathy (18,19). Steroid dexamethasone, laser
photocoagulation and vitrectomy have clinically been used for the
prevention of neovascularization amongst patients with diabetes
(1,18).
In the present study, the anti-angiogenic effect of
betaine was evaluated using an STZ-induced hyperglycemic rat model.
Betaine has previously been demonstrated to protect internal
organs, reduce vascular risk factors and enhance athletic
performance (12). Betaine reduces
homocysteine level in homocystinuria, decreases serum homocysteine
level and increases brain methionone and S-adenosylmethionine,
functions which may delay the progression of Alzheimer’s disease
(20).
The STZ-induced diabetic rat model is the most
widely used animal model for diabetes (16,17),
and is also extensively used in the study of diabetic neuropathy in
particular (21,22). Vascular changes, including
microaneurysms, decreased pericyte number, increased vascular
permeability, breakdown of blood-retinal barrier and early changes
in growth factor expression, were observed in the STZ-induced
diabetic rats (23,24).
In the present study STZ-induced diabetic rats
demonstrated enhanced VEGF expression in the retina, an effect
which was attenuated following betaine treatment. VEGF is an
endothelial angiogenic and vasopermeability factor, which induces
functional changes in the retinal pigment epithelium (25). VEGF expression is upregulated by
hypoxia and ischemia (26), and
VEGF directly stimulates retinal neovascularization (27,28).
Enhanced expression of VEGF occurs as a result of the upregulation
of survivin, which is activated by the PI3K/Akt signaling pathway
(29). Suppression of VEGF
expression in the retina has been demonstrated to enhance vision in
multiple neovascular eye diseases, including diabetic retinopathy
and age-associated macular degeneration (30,31).
In the present study, STZ-induced diabetic rats
additionally demonstrated enhanced HIF-1 expression in the retina.
Betaine treatment suppressed this increase in HIF-1 expression in
the STZ-induced diabetic rats. Previously, increased levels of
HIF-1α were observed in the ischemic retinas of the retinopathy
mouse model, and HIF-1α expression was correlated with VEGF
expression (27). HIF-1α regulates
the expression of numerous genes required for normal cellular
function and survival under various stressful conditions (32). The retina is sensitive to oxygen
tension, and oxygen has a key role in the stabilization of HIF-1α
function. When oxygen tension is normal, HIF-1α is rapidly oxidized
by hydroxylase enzymes; however, when cells enter a hypoxic state,
HIF-1α degradation is inhibited, and HIF-1α activates VEGF and
erythropoietin (4–6). HIF-1α is closely associated with
oxygen-dependent retinal diseases, including von Hippel-Lindau,
proliferative diabetic retinopathy, retinopathy of prematurity and
glaucoma (4). HIF-1 mediates the
role of Akt by promoting VEGF expression; therefore, promotion of
the Akt-HIF-1α-VEGF signaling pathway contributes to the induction
of angiogenesis (33).
The results of the present study revealed an
increase in pAkt expression in the retinas of STZ-induced diabetic
rats, an effect that was ameliorated by betaine treatment.
VEGF-induced endothelial cell migration requires Akt activation
(34). Activation of Akt signaling
in the endothelial cells stimulates endothelial cell bioactivity
and angiogenesis (35), and pAkt
is predominantly expressed in the inner nuclear layer of the
diabetic retina (36).
Furthermore, the hypoxia-induced expression of HIF-1α and VEGF
requires activation of the PI3K/Akt pathway (9).
The results of the present study supported the
hypothesis that Akt activation is an upper signaling pathway, which
triggers a proliferative response in the endothelial cells by
enhancing VEGF expression, leading to the induction of
neovascularization. The results also suggested that the inhibition
of Akt activation may ameliorate neovascularization via suppression
of VEGF expression. In addition, betaine treatment alleviated
diabetes-induced vascularization by suppressing VEGF and HIF-1α
expression via downregulation of pAkt expression in the retina of
the STZ-induced diabetic rats. In conclusion, the results of the
present study suggested a potential role for betaine in the
prevention and/or delay of complications of diabetic retinopathy by
inhibiting retinal neovascularization in patients with
diabetes.
References
|
1
|
Laaksonen DE, Niskanen L, Punnonen K,
Nyyssönen K, Tuomainen TP, Valkonen VP, Salonen R and Salonen JT:
Testosterone and sex hormone-binding globulin predict the metabolic
syndrome and diabetes in middle-aged men. Diabetes Care.
27:1036–1041. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qaum T, Xu Q, Joussen AM, Clemens MW, Qin
W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD
and Adamis AP: VEGF-initiated blood-retinal barrier breakdown in
early diabetes. Invest Ophthalmol Vis Sci. 42:2408–2413.
2001.PubMed/NCBI
|
|
3
|
Ishida S, Usui T, Yamashiro K, Kaji Y,
Ahmed E, Carrasquillo KG, Amano S, Hida T, Oguchi Y and Adamis AP:
VEGF164 is proinflammatory in the diabetic retina. Invest
Ophthalmol Vis Sci. 44:2155–2162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arjamaa O and Nikinmaa M: Oxygen-dependent
diseases in the retina: role of hypoxia-inducible factors. Exp Eye
Res. 83:473–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Semenza GL: Expression of
hypoxia-inducible factor 1: mechanisms and consequences. Biochem
Pharmacol. 59:47–53. 2000. View Article : Google Scholar
|
|
6
|
Hewitson KS and Schofield CJ: The HIF
pathway as a therapeutic target. Drug Discov Today. 9:704–711.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Makino Y, Kanopka A, Wilson WJ, Tanaka H
and Poellinger L: Inhibitory PAS domain protein (IPAS) is a
hypoxia-inducible splicing variant of the hypoxia-inducible
factor-3alpha locus. J Biol Chem. 277:32405–32408. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuda T, Abe T, Wu JL, Fujiki M and
Kobayashi H: Hypoxia-inducible factor-1alpha DNA induced
angiogenesis in a rat cerebral ischemia model. Neurol Res.
27:503–508. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang XM, Wang YS, Zhang J, Li Y, Xu JF,
Zhu J, Zhao W, Chu DK and Wiedemann P: Role of PI3K/Akt and MEK/ERK
in mediating hypoxia-induced expression of HIF-1alpha and VEGF in
laser-induced rat choroidal neovascularization. Invest Ophthalmol
Vis Sci. 50:1873–1879. 2009. View Article : Google Scholar
|
|
10
|
Brazil DP and Hemmings BA: Ten years of
protein kinase B signalling: a hard Akt to follow. Trends Biochem
Sci. 26:657–664. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shiojima I and Walsh K: Role of Akt
signaling in vascular homeostasis and angiogenesis. Circ Res.
90:1243–1250. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Craig SA: Betaine in human nutrition. Am J
Clin Nutr. 80:539–549. 2004.PubMed/NCBI
|
|
13
|
Chang RC and So KF: Use of anti-aging
herbal medicine, Lycium barbarum, against aging-associated
diseases. What do we know so far? Cell Mol Neurobiol. 28:643–652.
2008. View Article : Google Scholar
|
|
14
|
Chang HK, Shin MS, Yang HY, Lee JW, Kim
YS, Lee MH, Kim J, Kim KH and Kim CJ: Amygdalin induces apoptosis
through regulation of Bax and Bcl-2 expressions in human DU145 and
LNCaP prostate cancer cells. Biol Pharm Bull. 29:1597–1602. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim DY, Jung SY, Kim CJ, Sung YH and Kim
JD: Treadmill exercise ameliorates apoptotic cell death in the
retinas of diabetic rats. Mol Med Rep. 7:1745–1750. 2013.PubMed/NCBI
|
|
16
|
Jee YS, Ko IG, Sung YH, Lee JW, Kim YS,
Kim SE, Kim BK, Seo JH, Shin MS, Lee HH, et al: Effects of
treadmill exercise on memory and c-Fos expression in the
hippocampus of the rats with intracerebroventricular injection of
streptozotocin. Neurosci Lett. 443:188–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shin MS, Kim SK, Kim YS, Kim SE, Ko IG,
Kim YS, Kim CJ, Kim YM, Kim BK and Kim TS: Aqueous extract of
Anemarrhena rhizome increases cell proliferation and neuro-peptide
Y expression in the hippocampal dentate gyrus on
streptozotocin-induced diabetic rats. Fitoterapia. 79:323–327.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J and Smith LE: Retinopathy of
prematurity. Angiogenesis. 10:133–140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JH, Lee BJ, Kim JH, Yu YS, Kim MY and
Kim KW: Rosmarinic acid suppresses retinal neovascularization via
cell cycle arrest with increase of p21(WAF1) expression. Eur J
Pharmacol. 615:150–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knopman D and Patterson M: An open-label,
24-week pilot study of the methyl donor betaine in Alzheimer
disease patients. Alzheimer Dis Assoc Disord. 15:162–165. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coggeshall RE, Tate S and Carlton SM:
Differential expression of tetrodotoxin-resistant sodium channels
Nav1.8 and Nav1.9 in normal and inflamed rats. Neurosci Lett.
355:45–48. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coppey LJ, Davidson EP, Dunlap JA, Lund DD
and Yorek MA: Slowing of motor nerve conduction velocity in
streptozotocin-induced diabetic rats is preceded by impaired
vasodilation in arterioles that overlie the sciatic nerve. Int J
Exp Diabetes Res. 1:131–143. 2000. View Article : Google Scholar
|
|
23
|
Cherdshewasart W and Sutjit W: Correlation
of antioxidant activity and major isoflavonoid contents of the
phytoestrogen-rich Pueraria mirifica and Pueraria lobata tubers.
Phytomedicine. 15:38–43. 2008. View Article : Google Scholar
|
|
24
|
Xiong FL, Sun XH, Gan L, Yang XL and Xu
HB: Puerarin protects rat pancreatic islets from damage by hydrogen
peroxide. Eur J Pharmacol. 529:1–7. 2006. View Article : Google Scholar
|
|
25
|
Hartnett ME, Lappas A, Darland D, McColm
JR, Lovejoy S and D’Amore PA: Retinal pigment epithelium and
endothelial cell interaction causes retinal pigment epithelial
barrier dysfunction via a soluble VEGF-dependent mechanism. Exp Eye
Res. 77:593–599. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ozaki H, Seo MS, Ozaki K, Yamada H, Yamada
E, Okamoto N, Hofmann F, Wood JM and Campochiaro PA: Blockade of
vascular endothelial cell growth factor receptor signaling is
sufficient to completely prevent retinal neovascularization. Am J
Pathol. 156:697–707. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wenger RH: Cellular adaptation to hypoxia:
O2-sensing protein hydroxylases, hypoxia-inducible
transcription factors, and O2-regulated gene expression.
FASEB J. 16:1151–1162. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang Y, Hua K, Zhou X, Jin H, Chen X, Lu
X, Yu Y, Zha X and Feng Y: Activation of the PI3K/AKT pathway
mediates FSH-stimulated VEGF expression in ovarian serous
cystadeno-carcinoma. Cell Res. 18:780–791. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dugel PU: Ranibizumab treatment of
patients with ocular diseases. Int Ophthalmol Clin. 46:131–140.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nguyen QD, Tatlipinar S, Shah SM, Haller
JA, Quinlan E, Sung J, Zimmer-Galler I, Do DV and Campochiaro PA:
Vascular endothelial growth factor is a critical stimulus for
diabetic macular edema. Am J Ophthalmol. 142:961–969. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vengellur A, Woods BG, Ryan HE, Johnson RS
and LaPres JJ: Gene expression profiling of the hypoxia signaling
pathway in hypoxia-inducible factor 1alpha null mouse embryonic
fibroblasts. Gene Expr. 11:181–197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee BL, Kim WH, Jung J, Cho SJ, Park JW,
Kim J, Chung HY, Chang MS and Nam SY: A hypoxia-independent
up-regulation of hypoxia-inducible factor-1 by AKT contributes to
angio-genesis in human gastric cancer. Carcinogenesis. 29:44–51.
2008. View Article : Google Scholar
|
|
34
|
Dimmeler S, Dernbach E and Zeiher AM:
Phosphorylation of the endothelial nitric oxide synthase at
ser-1177 is required for VEGF-induced endothelial cell migration.
FEBS Lett. 477:258–262. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kureishi Y, Luo Z, Shiojima I, Bialik A,
Fulton D, Lefer DJ, Sessa WC and Walsh K: The HMG-CoA reductase
inhibitor simvastatin activates the protein kinase Akt and promotes
angiogenesis in normocholesterolemic animals. Nat Med. 6:1004–1010.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Q, Pfister F, Dorn-Beineke A, vom
Hagen F, Lin J, Feng Y and Hammes HP: Low-dose erythropoietin
inhibits oxidative stress and early vascular changes in the
experimental diabetic retina. Diabetologia. 53:1227–1238. 2010.
View Article : Google Scholar : PubMed/NCBI
|