Introduction
Venous thromboembolism (VTE), which includes deep
vein thrombosis (DVT) and pulmonary embolism (PE), is a common
disorder with a high annual incidence of ~131.5 cases per 100,000
people in the general population (1). DVT is characterized by the formation
of an occlusive blood clot in the venous vascular system, which can
potentially result in PE, due to detachment and embolization of
thrombi into the lung. Therefore, DVT and PE are regarded as two
different stages of the same disease, and are associated with
common risk factors caused by genetic, environmental and behavioral
interactions (2,3). DVT is a major cause of
cardiovascular-associated mortality and its treatment is limited to
palliation, including mechanical leg compression and
anticoagulation therapy (4).
The underlying cellular mechanisms of DVT have
remained elusive. Slow blood flow, abnormal blood components and
vein endothelial injury are considered to be the three classical
factors that participate in the pathogenesis of DVT (5). Reduced blood flow leads to adherence
of circulating neutrophils and monocytes to the activated
endothelium within hours, thus activating platelets, circulating
tissue factor and factor XII, resulting in the initiation and
propagation of DVT (6). Platelets
perform surveillance on vascular injury and are capable of vascular
repair and hemostasis by quick adhesion and aggregation at the site
of injury. However, disruption of hemostasis may lead to abnormally
enhanced platelet adhesion and aggregation, which can cause
thrombotic disorders such as DVT (7). Out of all of these processes,
platelet-endothelial adhesion has a key role in the initiation and
promotion of DVT. von Willebrand factor (VWF) was previously shown
to be required for thrombus formation in a murine model of DVT
through promoting platelet adhesion and the subsequent adherence of
platelets to endothelial cells (8). Furthermore, platelets can communicate
with monocytes and neutrophils to initiate and propagate venous
thrombosis in mice with DVT by promoting leukocyte recruitment and
neutrophil-dependent coagulation (9). The mechanisms underlying
platelet-endothelial adhesion and DVT remain to be fully
elucidated; however, the process is known to be mediated by various
cell adhesion molecules, including platelet endothelial cell
adhesion molecule-1 (PECAM-1) (10).
PECAM-1 is a 130-kDa cell surface glycoprotein that
belongs to the immunoglobulin gene superfamily of cell adhesion
molecules. PECAM-1 is expressed on the surface of circulating
platelets, monocytes, neutrophils and endothelial cell
intercellular junctions (11).
PECAM-1 is involved in inhibition of platelet function (12) and maintenance of endothelial
barrier integrity (13), both of
which are major determinants of venous thrombosis. Furthermore,
PECAM-1-deficient mice with DVT exhibited larger thrombi over
longer periods of time. In addition, higher plasma levels of
sPECAM-1 were detected in patients with delayed resolution of
thrombi, as compared with patients whose thrombi resolved normally
(14).
The PECAM-1 gene is located on human chromosome
17q23 and consists of 16 exons. A number of single nucleotide
polymorphisms (SNPs) have been identified in PECAM-1 (15). Out of the 11 SNPs in human PECAM-1,
only three have been shown to be associated with disease: Leu125Val
(C373 G), Asn563Ser (T1688C) and Gly670Arg (C2008T) (16). The Leu125Val polymorphism is
located in exon 3 and consists of a leucine to valine alteration,
Asn563Ser is located in exon 8 and consists of an asparagine to
serine alteration, and Gly670Arg is located in exon 12 and consists
of a glycine to arginine alteration. PECAM-1 gene polymorphisms
have previously been shown to be associated with atherosclerosis
and myocardial infarction (17,18).
However, no studies have yet examined the association between
PECAM-1 polymorphisms and DVT. Furthermore, plasma sPECAM-1 levels
were previously shown to be increased in DVT patients with delayed
thrombus resolution; however, the association between plasma
sPECAM-1 levels and PECAM-1 polymorphisms in DVT remains
elusive.
The present study investigated the frequency of
PECAM-1 Leu125Val, Asn563Ser, and Gly670Arg polymorphisms in
patients with DVT as compared with those in healthy controls.
Furthermore, the association between these polymorphisms and plasma
sPECAM-1 levels, vascular PECAM-1 expression, and thrombus burden
was evaluated in the patients with DVT.
Materials and methods
Subjects
The present study consisted of 115 patients with DVT
(67 males and 48 females, between 30 and 83 years of age). All of
the patients were diagnosed with lower extremity DVT by duplex
ultrasonography between September 2011 and March 2013, at Shandong
Provincial Hospital affiliated to Shandong University (Jinan,
China) and were recruited into the DVT group. The exclusion
criteria were as follows: Hematological diseases, liver or kidney
dysfunction, tumors, infections, autoimmune diseases, or coexisting
symptomatic pulmonary embolism. The DVT group consisted of 63
patients with left lateral DVT (54.8%), 35 patients with right
lateral DVT (30.4%) and 17 patients with bilateral DVT (14.8%). A
total of 104 healthy unrelated subjects, both age- and
gender-matched, were recruited into the control group (60 males and
44 females, between 29 and 79 years of age). The control subjects
underwent a routine medical check-up and none of them were
diagnosed with DVT or other associated diseases. Written informed
consent, in accordance with the Declaration of Helsinki, was
obtained from all of the study subjects, and the study was approved
by the Shandong University Research Ethics Committee (Jinan,
China).
Determination of PECAM-1 genotype
Peripheral venous blood was obtained from all of the
patients with DVT and the healthy controls, and was promptly
centrifuged at 1,000 × g for 10 min. Genomic DNA was extracted from
the leucocytes using a DNA Extraction kit (Qiagen, Manchester, UK),
according to the manufacturer’s instructions. The extracted DNA was
then stored at −70°C until further use. PECAM-1 Leu125Val (C373G),
Asn563Ser (T1688C) and Gly670Arg (C2008T) genotype frequencies were
determined by polymerase chain reaction-restriction fragment length
polymorphism (PCR-RFLP) analysis. The PCR primers were designed and
synthesized based on the GenBank reference sequence (accession no.
NC_000017), as previously reported (19), with the following sequences:
Leu125Val sense, 5′-GCTCCATCTGCTTGCCTGT-3′ and anti-sense,
5′-TGTCAGCACCACCTCTCACG-3′; Asn563Ser sense,
5′-TGGAGACCCTGACTCACCTC-3′ and anti-sense,
5′-TGCAATGTGCTGTGAATGAA-3′; and Gly670Arg sense,
5′-TGGGAAATTATCCACAGTCCTTCA-3′ and antisense,
5′-CAACTAGGTCACAATGACGATGCC-3′ by Sangon Biotech Co., Ltd.
(Shanghai, China). The PCR amplification was performed in a total
volume of 25 µl using the PCR Amplification kit (Takara
Biotechnology Co., Ltd., Dalian, China) on PCR amplification
reaction apparatus (Tgradient; Biometra, Göttingen, Germany). The
cycling conditions for PCR were set as follows: Initiation at 94°C
for 5 min, followed by 30 cycles of denaturation at 94°C for 40
sec, annealing at 60°C for 4 sec and extension at 72°C for 10 sec,
with a final extension step at 72°C for 7 min. The amplified PCR
products were then incubated with restriction endonuclease
PvuII (Leu125Val), NheI (Asn563Ser) or MspI
(Gly670Arg) (Takara Biotechnology Co., Ltd.) overnight for
digestion. All of the PCR products were subsequently separated by
8% polyacrylamide gel electrophoresis. The molecular weight marker
(GM303) was obtained from BBI Life Sciences Corporation (Shanghai,
China).
Determination of sPECAM-1 levels
Plasma was collected from the venous blood samples
of the patients with DVT within 24 h of diagnosis by centrifugation
at 1,000 × g for 20 min. The plasma samples were then stored in
aliquots at −70°C until further use. Measurements of sPECAM-1
levels were performed using the Human sPECAM-1 ELISA kit (Bender
MedSystems GmbH, Vienna, Austria), according to the manufacturer’s
instructions. The color density of the samples was measured at a
wavelength of 450 nm using an ELISA plate reader (Ricso RK201;
Shenzhen Ricso Technology Co., Ltd, Shenzhen, China). A standard
curve was constructed using the standards supplied in the ELISA kit
(Range, 0–1000 ng/ml), in order to determine the concentrations of
sPECAM-1.
Western blot analysis
Human samples of venous vessel walls were obtained
from patients with DVT during variceal surgeries (n=6). Venous
vessel walls were harvested and were immediately snap frozen in
liquid nitrogen. Prior to the experiment, the venous vessel walls
were lysed by RIPA buffer [50 mM Tris-HCl (pH 7.5), 1% Nonidet
P-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA and complete
protease inhibitor cocktail (Roche, Indianapolis, IN, USA)].
Following centrifugation at 20,000 × g and 4°C for 15 min, the
supernatant was collected. The protein concentrations were
determined using a Bicinchoninic Acid Protein Concentration Assay
kit (Beijing Biosea Biotechnology Co. Ltd., Beijing, China). Equal
amounts of protein (50 µg) were separated by 10% SDS-PAGE
and electrophoretically transferred to a polyvinylidene fluoride
(PVDF) membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The membrane was then blocked with 3% bovine serum albumin
(Sigma-Aldrich, St. Louis, MO, USA) in Tris-buffered saline
containing 0.05% Tween® 20 (Sigma-Aldrich) for 1 h,
followed by incubation with a primary mouse monoclonal antibody
targeting human PECAM-1 (1:1,000 dilution; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 37°C overnight. The PVDF
membrane was then incubated with horseradish peroxidase-conjugated
rabbit anti-mouse polyclonal secondary antibody (1:1,000 dilution;
Santa Cruz Biotechnology, Inc.) for 2 h at room temperature. An
Enhanced Chemiluminescence substrate (Pierce ECL Plus Western
Blotting substrate; Pierce Biotechnology, Inc., Rockford, IL, USA)
was used to visualize the density of the targeted bands. β-actin
was used as an internal control.
Determination of thrombus burden
The thrombus score reflects the thrombus burden in
the leg vein and was calculated in the present study by manual
complete compression ultrasonography using ProSound Alpha 7 Doppler
ultrasonography (Hitachi-Aloka Medical Ltd., Tokyo, Japan) at 75
MHz (20). The score for each
affected venous segment was calculated from thrombus diameter under
full compressed conditions, relative to the diameter of the
corresponding artery. When the compressed thrombus diameter was
>1.5 times the arterial diameter, the segment score was
increased 1.5-fold (14). When the
compressed thrombus diameter was <0.5 times the arterial
diameter, the segment score was reduced 0.5-fold. The thrombus
score was calculated as the sum of each segment: External iliac
vein, 8; common femoral vein, 4; proximal superficial femoral vein,
4; distal superficial femoral vein, 3; deep femoral vein, 2,
popliteal vein, 2; peroneal veins, 2; and posterior tibial veins,
2. A baseline score >4 was an inclusion criterion for the DVT
group in the present study. Follow-up ultrasonography was performed
at 28 days after diagnosis in each of the patients with DVT, and a
duplex scan was performed to evaluate residual vein thrombus as
well as to calculate the change in the thrombus score (Δ thrombus
score=baseline thrombus score − thrombus score on day 28). A Δ
thrombus score <4 was defined as delayed thrombus resolution.
All of the patients with DVT received compression stockings and
oral anticoagulation (warfarin) therapy (Coumadin®;
Bristol-Myers Squibb, New York City, NY, USA).
Statistical analysis
All quantitative data are expressed as the mean ±
standard deviation. The commercially available software SPSS
version 14.0 (SPSS Inc., Chicago, IL, USA) was used for all
statistical analyses. An independent t-test was used to compare
results between two groups. A χ2 analysis was used to
determine the differences in genotype and allele frequencies.
Unconditional logistic regression models were used to determine
odds ratios (ORs) and 95% confidence intervals [95% confidence
intervals (CIs)]. The Hardy-Weinberg equilibrium test was performed
on the control and DVT groups to determine whether the samples were
a typical representative groups of the whole population. P<0.05
was considered to indicate a statistically significant
difference.
Results
Val/Val genotype of PECAM-1 is associated
with an increased risk of DVT
No statistically significant differences were
observed between the age and gender of the control and DVT groups,
thus indicating that the two groups were matched for age as well as
gender. The plasma sPECAM-1 levels were significantly higher in the
DVT group (83.4±23.5 ng/ml), as compared with those in the control
group (60.4±19.4 ng/ml) (P<0.05; Table I). PECAM-1 genotype and allele
frequencies of the Leu125Val, Asn563Ser and Gly670Arg polymorphisms
were evaluated in the patients with DVT and the healthy controls.
There were significant differences in the genotype and allele
frequencies of the PECAM-1 Leu125Val polymorphism between the
control and DVT groups (P<0.01). The frequencies of Leu/Leu,
Leu/Val and Val/Val genotypes of the Leu125Val polymorphism were
24.0, 53.9 and 22.1% in controls, and 12.2, 47.8 and 40.0% in the
patients with DVT, respectively (Table II). However, there were no
significant differences in the genotype and allele frequencies of
the Asn563Ser and Gly670Arg polymorphisms between the control and
DVT groups. The genotype distributions of the three polymorphisms
were in Hardy-Weinberg equilibrium among the control and DVT groups
(Table III). A PCR-RFLP assay
was used to analyze the Leu125Val polymorphisms of PECAM-1, and the
following fragments were detected: One 245 bp DNA fragment in CC
homozygous subjects, three DNA fragments of 52, 193 and 245 bp in
CG heterozygous subjects and two DNA fragments of 52 and 193 bp in
GG homozygous subjects (Fig. 1).
The 52-bp fragment was not visible in the electrophoresis gel due
to its small size. The Leu/Val and Val/Val genotypes were
associated with a significantly increased risk of DVT, as compared
with the Leu/Leu genotype (P<0.01) (Table IV).
 | Figure 1PECAM-1 Leu125Val (C373G) polymorphism
was analyzed by enzyme-cut electrophoresis with restriction
endonuclease PvuII. M, DNA molecular weight marker; lanes 1,
4 and 7, 373 CC (125 Leu/Leu) homozygous genotype, 245 bp; lanes 2,
3 and 6, 373 CG (125Leu/Val) heterozygous genotype, 245 bp and 193
bp; lane 5, 373 GG (125Val/Val) homozygous genotype, 193 bp.
PECAM-1, platelet endothelial cell adhesion molecule-1. |
 | Table ICharacteristics of the patients with
DVT and the healthy controls. |
Table I
Characteristics of the patients with
DVT and the healthy controls.
| Characteristic | Controls
(n=104)
n | DVT (n=115)
n | P-value |
|---|
| Age (years) | 51.6±9.2 | 53.8±9.5 | 0.078 |
| Gender
(male/female) | 60/44 | 67/48 | 0.11 |
| sPECAM-1 (ng/ml) | 60.4±19.4 | 83.4±23.5 | <0.01 |
 | Table IIGenotype and allele frequencies of
PECAM-1 polymorphisms in patients with DVT and healthy
controls. |
Table II
Genotype and allele frequencies of
PECAM-1 polymorphisms in patients with DVT and healthy
controls.
| Polymorphism | Controls
(n=104)
n (%) | DVT (n=115)
n (%) | χ2 | P-value |
|---|
| Leu125Val
genotypes | | | 10.25 | <0.01 |
| Leu/Leu | 25 (24.0) | 14 (12.2) | | |
| Leu/Val | 56 (53.9) | 55 (47.8) | | |
| Val/Val | 23 (22.1) | 46 (40.0) | | |
| Alleles | | | 9.85 | <0.01 |
| Leu | 106 (51.0) | 83 (36.1) | | |
| Val | 102 (49.0) | 147 (63.9) | | |
| Asn563Ser
genotypes | | | 3.94 | >0.05 |
| Asn/Asn | 27 (26.0) | 24 (20.9) | | |
| Asn/Ser | 55 (52.9) | 53 (46.1) | | |
| Ser/Ser | 22 (21.1) | 38 (33.0) | | |
| Alleles | | | 3.16 | >0.05 |
| Asn | 109 (52.4) | 101 (43.9) | | |
| Ser | 99 (47.6) | 129 (56.1) | | |
| Gly670Arg
genotypes | | | 2.47 | >0.05 |
| Gly/Gly | 26 (25.0) | 22 (19.1) | | |
| Gly/Arg | 53 (51.0) | 58 (50.4) | | |
| Arg/Arg | 25 (24.0) | 35 (30.5) | | |
| Alleles | | | 1.65 | >0.05 |
| Gly | 105 (50.5) | 102 (44.3) | | |
| Arg | 103 (49.5) | 128 (55.7) | | |
 | Table IIIPECAM-1 genotype distribution of
Leu125Val, Asn563Ser and Gly670Arg in Hardy-Weinberg
equilibrium. |
Table III
PECAM-1 genotype distribution of
Leu125Val, Asn563Ser and Gly670Arg in Hardy-Weinberg
equilibrium.
| Group | Genotype | Observed
value
n (%) | Predicted
value
n (%) | χ2 | P-value |
|---|
| Leu125Val
controls | | | | 0.31 | >0.05 |
| Leu/Leu | 25 (24.0) | 27 (26.0) | | |
| Leu/Val | 56 (53.9) | 52 (50.0) | | |
| Val/Val | 23 (22.1) | 25 (24.0) | | |
| DVT | | | | 0.08 | >0.05 |
| Leu/Leu | 14 (12.2) | 15 (13.0) | | |
| Leu/Val | 55 (47.8) | 53 (46.1) | | |
| Val/Val | 46 (40.0) | 47 (40.9) | | |
| Asn563Ser
controls | | | | 0.19 | >0.05 |
| Asn/Asn | 27 (26.0) | 28 (26.9) | | |
| Asn/Ser | 55 (52.9) | 52 (50.0) | | |
| Ser/Ser | 22 (21.1) | 24 (23.1) | | |
| DVT | | | | 0.29 | >0.05 |
| Asn/Asn | 24 (20.9) | 22 (19.1) | | |
| Asn/Ser | 53 (46.1) | 57 (49.6) | | |
| Ser/Ser | 38 (33.0) | 36 (31.3) | | |
| Gly670Arg
controls | | | | 0.03 | >0.05 |
| Gly/Gly | 26 (25.0) | 27 (26.0) | | |
| Gly/Arg | 53 (51.0) | 52 (50.0) | | |
| Arg/Arg | 25 (24.0) | 25 (24.0) | | |
| DVT | | | | 0.03 | >0.05 |
| Gly/Gly | 22 (19.1) | 23 (20.0) | | |
| Gly/Arg | 58 (50.4) | 57 (49.6) | | |
| Arg/Arg | 35 (30.5) | 35 (30.4) | | |
 | Table IVPECAM-1 polymorphisms and risk of
DVT. |
Table IV
PECAM-1 polymorphisms and risk of
DVT.
| Genotype | Controls
(n=104)
n (%) | DVT
(n=115)
n (%) | OR (95% CI) | P-value |
|---|
| Leu/Leu | 25 (24.0) | 14 (12.2) | 1 | |
| Leu/Val | 56 (53.9) | 55 (47.8) | 1.75
(1.22–2.53) | <0.01 |
| Val/Val | 23 (22.1) | 46 (40.0) | 3.57
(2.47–5.16) | <0.01 |
|
Leu/Val+Val/Val | 79 (76.0) | 101 (87.8) | 2.28
(1.58–3.29) | <0.01 |
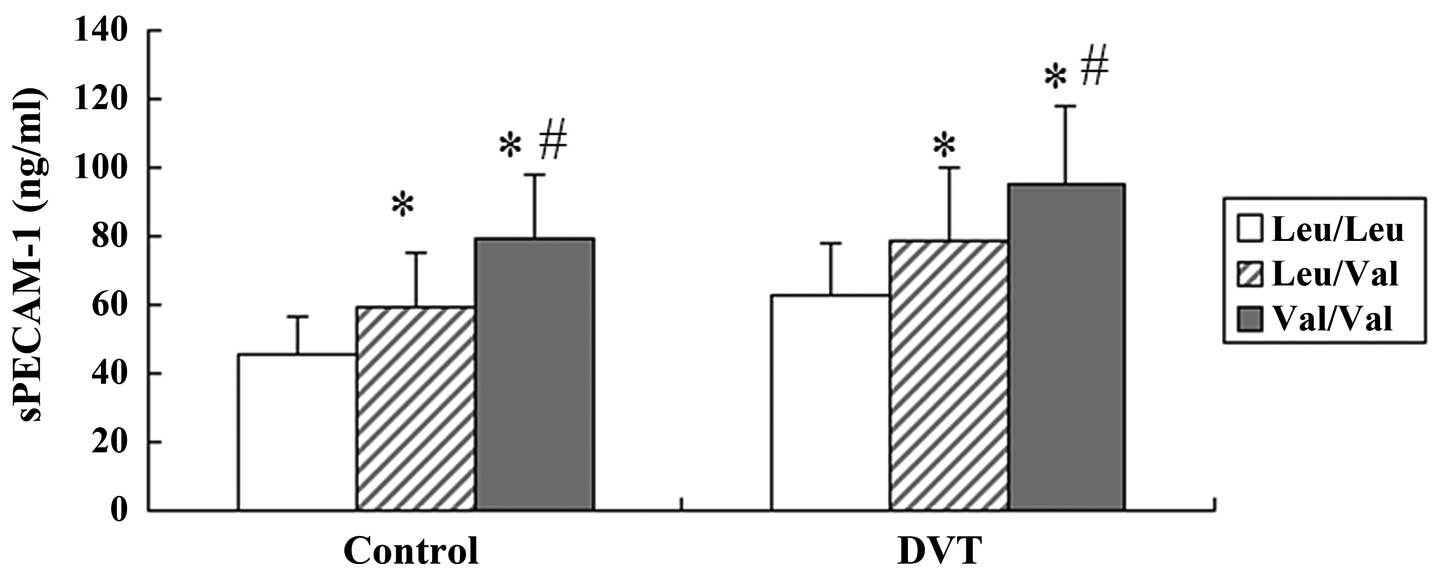
Association between sPECAM-1 levels and
PECAM-1 Leu125Val polymorphism
An ELISA was conducted to detect the plasma sPECAM-1
levels in the control and DVT groups. Significantly higher sPECAM-1
levels were observed in the patients with DVT, as compared with
those in the healthy controls (Table
I). The sPECAM-1 levels were further analyzed in both groups
with regards to the three Leu125Val genotypes of PECAM-1. sPECAM-1
levels were significantly associated with the Leu125Val
polymorphism in the control as well as in the DVT group. The plasma
sPECAM-1 levels were significantly higher in DVT patients with the
homozygous Val/Val genotype (95.2±22.4 ng/ml, n=46) or the
heterozygous Leu/Val genotype (78.7±20.9 ng/ml, n=55), as compared
with those in the patients with the homozygous Leu/Leu genotype
(62.8±15.9 ng/ml, n=14, P<0.01, respectively). Furthermore,
significantly higher plasma sPECAM-1 levels were detected in the
subjects with the Val/Val genotype as compared with those in the
Leu/Val genotype (Fig. 2).
Protein expression levels of PECAM-1 in
the three Leu125Val genotypes of PECAM-1
Western blot analysis was performed to detect the
PECAM-1 protein expression levels in the venous vessels of the
patients with DVT. PECAM-1 protein expression levels were
significantly lower in the patients with the Val/Val genotype, as
compared with those with the Leu/Leu and Leu/Val genotypes
(P<0.01, respectively). Furthermore, significantly lower PECAM-1
protein expression levels were detected in the patients with the
Leu/Val genotype, as compared with those with the Leu/Leu genotype
(P<0.05; Fig. 3A and B).
Patients with Leu/Val and Val/Val
genotypes have a higher thrombus burden and delayed thrombus
resolution
Complete compression ultrasonography was performed
to evaluate the thrombus burden in each patient with DVT at the
time of diagnosis and 28 days thereafter. The baseline thrombus
score was significantly higher in patients with the Val/Val
genotype as compared with those in patients with the Leu/Leu and
Leu/Val genotypes. At 28 days post-diagnosis, the Δ thrombus scores
for the patients with the Leu/Val and Val/Val genotypes were
significantly lower as compared with those for patients with the
Leu/Leu genotype (Table V). These
results indicated that the presence of the Val allele may promote
delayed thrombus resolution in patients with DVT.
 | Table VPECAM-1 polymorphisms and thrombus
resolution in DVT. |
Table V
PECAM-1 polymorphisms and thrombus
resolution in DVT.
| Genotype | Leu/Leu | Leu/Val | Val/Val |
|---|
| Mean thrombus score
baseline (±SD) | 14.1 (±2.5) | 15.5 (±2.8) | 16.9 (±2.9)a,b |
| Mean thrombus score
d 28 (±SD) | 9.3 (±2.2) | 11.9 (±2.5)a | 13.7 (±2.3)a,b |
| Mean Δ thrombus
score (±SD) | 4.8 (±1.3) | 3.6 (±1.5)a | 3.3 (±1.7)a |
| Normal thrombus
resolution | 11 | 27 | 17 |
| Delayed thrombus
resolution | 5 | 30 | 25 |
Discussion
The present study identified a significant
association between DVT and the Leu125Val polymorphism of PECAM-1
and plasma sPECAM-1 levels. In patients with DVT, those with the
Leu/Val and Val/Val genotypes exhibited increased levels of
sPECAM-1 and decreased PECAM-1 protein expression levels in venous
vessels. Furthermore, the Leu/Val and Val/Val genotypes were
associated with an increased thrombus burden and delayed thrombus
resolution in patients with DVT. However, associations were not
observed between DVT and PECAM-1 Asn563Ser or Gly670Arg genotypes.
These data suggested that PECAM-1 may have an important role in the
development and thrombus resolution of DVT, and the Leu125Val
polymorphism of PECAM-1 may serve as a novel genetic marker of
susceptibility to DVT.
Virchow’s triad, which includes abnormal blood
composition and vessel wall components, and decreased blood flow,
has been proposed to explain the pathophysiological mechanisms
underlying the development of venous thrombosis (21). Out of the three components of the
triad, blood composition, including circulating blood cells and
plasma proteins, has been well studied, whereas the underlying
mechanisms associated with the vessel wall and blood flow remain
elusive. The early phase of venous thrombosis involves the
recruitment of circulating leukocytes and platelets by adhesion
molecules to sites of vascular damage. Platelet adhesion and
enhanced procoagulant activity on endothelial cells is then
modulated by shear stress (21).
Therefore, adhesion molecules are considered to have a critical
role in the process of venous thrombosis, which is supported by an
increased risk of venous thrombosis associated with SNPs in the
cell adhesion molecule 1 gene (22). PECAM-1 is another cell adhesion
molecule, which is constitutively expressed in leucocytes,
platelets and endothelial cells, and is involved in endothelial
integrity and platelet function. A previous study suggested that
PECAM-1 may be important in the inhibition of the adhesion cascade
that leads to platelet activation and aggregation during the venous
thrombosis process (23).
The present study identified an association between
Leu125Val polymorphisms in PECAM-1 and DVT, with a significant
increase in Leu/Val and Val/Val genotype frequencies and the Val
allele in patients with DVT, as compared with those in control
subjects. These results indicated a significantly increased risk of
DVT in subjects with Leu/Val and Val/Val genotypes as compared with
those possessing the Leu/Leu genotype. To the best of our
knowledge, the present study was the first to examine PECAM-1
polymorphisms in patients with DVT. PECAM-1 SNPs have been widely
reported to be associated with atherosclerosis and myocardial
infarction (17,18), and previous studies have mainly
focused on Leu125Val, Asn563Ser and Gly670Arg polymorphisms.
However, in the present study, no significant differences were
observed in the genotype and allele frequencies of Asn563Ser and
Gly670Arg polymorphisms between the control and DVT groups. This
may be due to the small number of study subjects and disparities
between DVT and arterial diseases, including atherosclerosis and
myocardial infarction.
Significantly higher plasma sPECAM-1 levels were
detected in patients with DVT, as compared with the control
subjects. In patients with DVT, sPECAM-1 levels were significantly
higher in those with the Val/Val and Leu/Val genotypes, as compared
with levels in those with the Leu/Leu genotype. Plasma sPECAM-1
levels were previously shown to be elevated in patients with
coronary artery disease (CAD), and patients with the Val/Val
genotype of the Leu125Val polymorphism had higher sPECAM-1 levels
(24). sPECAM-1 is generated
either by alternative splicing upon cell activation, or PECAM-1
proteolytic cleavage at the cell surface (25,26).
Leu125Val of PECAM-1 is located at exon 3, which encodes the first
extracellular immunoglobulin (Ig)-like domain that mediates the
homophilic binding of PECAM-1. These results indicated that
Leu125Val is a functional SNP site and 125Val may enhance the
production of sPECAM-1 in DVT.
The present study demonstrated that subjects with
Leu/Val and Val/Val genotypes had an increased risk of DVT and
higher sPECAM-1 levels, which contradict the previously observed
inhibitory effects of PECAM-1 on thrombosis (27). Whether sPECAM-1 acts as an
anti-thrombotic protein or only as a marker for DVT requires
further study. The present study aimed to detect the protein
expression levels of PECAM-1 in the venous vessels of patients with
DVT, and PECAM-1 protein expression levels were shown to be
significantly decreased in patients with Leu/Val and Val/Val
genotypes, as compared with levels in those with the Leu/Leu
genotype. Decreased PECAM-1 protein expression levels were
previously detected in the venous vessels of patients with DVT with
delayed thrombus resolution as compared with those in patients with
normal thrombus resolution (14).
The results of the present study further demonstrated that the
Leu/Val and Val/Val genotypes of the Leu125Val polymorphism may
decrease PECAM-1 protein expression in the venous vessels of
patients with DVT. Previous studies have shown that PECAM-1 protein
can be cleaved at the cell surface (28) and may exert competitive inhibition
on membrane-bound PECAM-1 (29).
Therefore, elevated sPECAM-1 plasma levels are most likely due to
cleavage of PECAM-1 at the cell surface, which may lead to
decreased PECAM-1 protein expression in the venous vessels of
subjects with Leu/Val and Val/Val genotypes. These results
indicated that plasma sPECAM-1 levels may be a marker for decreased
PECAM-1 protein expression in venous vessels, and be associated
with a high risk of thrombosis. This hypothesis was supported by a
previous study that detected increased levels of plasma sPECAM-1 in
polycystic ovary syndrome (30),
which is a predisposing condition for VTE (31).
PECAM-1 has previously been shown to inhibit
thrombus formation and PECAM-1-deficient mice exhibited larger
thrombi as compared with those of control mice (32). To investigate whether decreased
venous PECAM-1 protein expression due to the presence of the 125Val
allele is associated with thrombosis in DVT patients, the thrombus
burden of the patients was evaluated by complete compression
ultrasonography. Patients with the 125Val allele had a
significantly higher baseline thrombus score and lower Δ thrombus
score, as compared with the 125Leu allele, thus indicating that the
125Val allele may promote thrombosis and delay thrombus resolution
in patients with DVT. These results were concordant with the
findings of a previous study that identified an association between
delayed thrombus resolution and increased plasma sPECAM-1 levels,
and decreased venous PECAM-1 protein expression in patients with
DVT (14). The 125Leu allele of
PECAM-1 gene was also shown to promote the thrombotic process and
was associated with larger thrombi. The Leu125Val allele of PECAM-1
is located in the first extracellular (Ig)-like domain that
mediates the homophilic binding of PECAM-1, and has an important
role in maintaining the integrity of endothelial cell junctions
(33). Further investigation is
required to determine whether decreased thrombosis by 125Val is
cause by decreased PECAM-1 expression, or defects in homophilic
binding of the PECAM-1 protein. Furthermore, delayed thrombus
resolution has a higher risk in developing into post-thrombotic
syndrome (PTS) (34). In the
present study, patients possessing the 125Val allele had a lower Δ
thrombus score and delayed thrombus resolution. If these findings
can be replicated by larger population studies, Leu125Val
genotyping at DVT diagnosis may be used as a predictive marker to
determine which patients are prone to PTS and require more
aggressive treatment.
In conclusion, the present study demonstrated that
subjects with the 125Val allele of PECAM-1 had an increased risk of
DVT, increased plasma sPECAM-1 levels, decreased venous PECAM-1
protein expression, increased thrombus burden and delayed thrombus
resolution. In DVT patients with the 125Val allele, higher plasma
sPECAM-1 levels may be due to enhanced cleavage of PECAM-1 protein
at the cell surface. The present study provides evidence suggesting
that the Leu125Val polymorphism of PECAM-1 may be a potential
genetic marker to predict susceptibility to DVT and PTS. Additional
studies are required with larger sample sizes to confirm these
findings. Further studies should focus on determining the pathways
through which Leu125Val polymorphisms affect the anti-thrombotic
effects of PECAM-1 in DVT.
References
|
1
|
Martinez C, Cohen AT, Bamber L and
Rietbrock S: Epidemiology of first and recurrent venous
thromboembolism: A population-based cohort study in patients
without active cancer. Thromb Haemost. 112:255–263. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Monreal M, Barba R, Tolosa C, et al: Deep
vein thrombosis and pulmonary embolism: the same disease?
Pathophysiol Haemost Thromb. 35:133–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nielsen JD: The incidence of pulmonary
embolism during deep vein thrombosis. Phlebology. 28:29–33. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blättler W and Partsch H: Leg compression
and ambulation is better than bed rest for the treatment of acute
deep venous thrombosis. Int J Angiol. 22:393–400. 2003.
|
|
5
|
Malone PC and Agutter PS: The aetiology of
deep venous thrombosis. QJM. 99:581–593. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schulz C, Engelmann B and Massberg S:
Crossroads of coagulation and innate immunity: the case of deep
vein thrombosis. J Thromb Haemost. 11:233–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watson SP: Platelet activation by
extracellular matrix proteins in haemostasis and thrombosis. Curr
Pharm Des. 15:1358–1372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brill A, Fuchs TA, Chauhan AK, et al: von
Willebrand factor-mediated platelet adhesion is critical for deep
vein thrombosisin mouse models. Blood. 117:1400–1407. 2011.
View Article : Google Scholar :
|
|
9
|
von Brühl ML, Stark K, Steinhart A, et al:
Monocytes, neutrophils, and platelets cooperate to initiate and
propagate venous thrombosis in mice in vivo. J Exp Med.
209:819–835. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jones CI, Moraes LA and Gibbins JM:
Regulation of platelet biology by platelet endothelial cell
adhesion molecule-1. Platelets. 23:331–335. 2012. View Article : Google Scholar
|
|
11
|
Berman ME and Muller WA: Ligation of
platelet/endothelial cell adhesion molecule 1 (PECAM-1/CD31) on
monocytes and neutrophils increases binding capacity of leukocyte
CR3 (CD11b/CD18). J Immunol. 154:299–307. 1995.PubMed/NCBI
|
|
12
|
Cicmil M, Thomas JM, Leduc M, Bon C and
Gibbins JM: Platelet endothelial cell adhesion molecule-1 signaling
inhibits the activation of human platelets. Blood. 99:137–144.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Flynn KM, Michaud M, Canosa S and Madri
JA: CD44 regulates vascular endothelial barrier integrity via a
PECAM-1 dependent mechanism. Angiogenesis. 16:689–705. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kellermair J, Redwan B, Alias S, et al:
Platelet endothelial cell adhesion molecule 1 deficiency misguides
venous thrombus resolution. Blood. 122:3376–3384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Robbins FM and Hartzman RJ: CD31/PECAM-1
genotyping and haplotype analyses show population diversity. Tissue
Antigens. 69:28–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Novinska MS, Pietz BC, Ellis TM, Newman DK
and Newman PJ: The alleles of PECAM-1. Gene. 376:95–101. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Listì F, Caruso C, Di Carlo D, et al:
Association between platelet endothelial cellular adhesion
molecule-1 polymorphisms and atherosclerosis: results of a study on
patients from northern Italy. Rejuvenation Res. 13:237–241. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sahebkar A, Morris DR, Biros E and
Golledge J: Association of single nucleotide polymorphisms in the
gene encoding platelet endothelial cell adhesion molecule-1 with
the risk of myocardial infarction: a systematic review and
meta-analysis. Thromb Res. 132:227–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei YS, Lan Y, Liu YG, Meng LQ, Xu QQ and
Xie HY: Platelet-endothelial cell adhesion molecule-1 gene
polymorphism and its soluble level are associated with ischemic
stroke. DNA Cell Biol. 28:151–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Agnelli G, Gallus A, Goldhaber SZ, et al
ODIXa-DVT Study Investigators: Treatment of proximal deep-vein
thrombosis with the oral direct factor Xa inhibitor rivaroxaban
(BAY 59-7939): the ODIXa-DVT (Oral Direct Factor Xa Inhibitor BAY
59-7939 in Patients With Acute Symptomatic Deep-Vein Thrombosis)
study. Circulation. 116:180–187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wolberg AS, Aleman MM, Leiderman K and
Machlus KR: Procoagulant activity in hemostasis and thrombosis:
Virchow’s triad revisited. Anesth Analg. 114:275–285. 2012.
View Article : Google Scholar :
|
|
22
|
Hasstedt SJ, Bezemer ID, Callas PW, et al:
Cell adhesion molecule 1: a novel risk factor for venous
thrombosis. Blood. 114:3084–3091. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jones CI, Garner SF, Moraes LA, et al:
PECAM-1 expression and activity negatively regulate multiple
platelet signaling pathways. FEBS Lett. 583:3618–3624. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang L, Wei H, Chowdhury SH, et al:
Association of Leu125Val polymorphism of platelet endothelial cell
adhesion molecule-1 (PECAM-1) gene & soluble level of PECAM-1
with coronary artery disease in Asian Indians. Indian J Med Res.
121:92–99. 2005.PubMed/NCBI
|
|
25
|
Goldberger A, Middleton KA, Oliver JA, et
al: Biosynthesis and processing of the cell adhesion molecule
PECAM-1 includes production of a soluble form. J Biol Chem.
269:17183–17191. 1994.PubMed/NCBI
|
|
26
|
Fornasa G, Groyer E, Clement M, et al: TCR
stimulation drives cleavage and shedding of the ITIM receptor CD31.
J Immunol. 184:5485–5492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moraes LA, Vaiyapuri S, Sasikumar P, et
al: Antithrombotic actions of statins involve PECAM-1 signaling.
Blood. 122:3188–3196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eugenin EA, Gamss R, Buckner C, et al:
Shedding of PECAM-1 during HIV infection: a potential role for
soluble PECAM-1 in the pathogenesis of NeuroAIDS. J Leukoc Biol.
79:444–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liao F, Ali J, Greene T and Muller WA:
Soluble domain 1 of platelet-endothelial cell adhesion molecule
(PECAM) is sufficient to block transendothelial migration in vitro
and in vivo. J Exp Med. 185:1349–1357. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pepene CE: Soluble platelet/endothelial
cell adhesion molecule (sPECAM)-1 is increased in polycystic ovary
syndrome and related to endothelial dysfunction. Gynecol
Endocrinol. 28:370–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okoroh EM, Hooper WC, Atrash HK, Yusuf HR
and Boulet SL: Is polycystic ovary syndrome another risk factor for
venous thromboembolism? United States 2003–2008. Am J Obstet
Gynecol. 207:377.e1–e8. 2012. View Article : Google Scholar
|
|
32
|
Falati S, Patil S, Gross PL, et al:
Platelet PECAM-1 inhibits thrombus formation in vivo. Blood.
107:535–541. 2006. View Article : Google Scholar
|
|
33
|
Privratsky JR, Paddock CM, Florey O,
Newman DK, Muller WA and Newman PJ: Relative contribution of
PECAM-1 adhesion and signaling to the maintenance of vascular
integrity. J Cell Sci. 124:1477–1485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kahn SR: Post-thrombotic syndrome after
deep venous thrombosis: risk factors, prevention, and therapeutic
options. Clin Adv Hematol Oncol. 7:433–435. 2009.PubMed/NCBI
|