Introduction
Embryonic stem cells (ESCs) possess the ability to
differentiate into a variety of cell types, and therefore are a
source of cells for functional studies and regenerative therapies
(1–2). However, the mechanisms of ESC
differentiation in vitro and in vivo remain to be
fully elucidated. Wnt/β-catenin signaling has been demonstrated to
promote differentiation, however, not the self-renewal of human
embryonic stem cells, and is repressed by octamer-binding
transcription factor 4 (OCT4) (3).
Wnt proteins are secreted glycoproteins and are regulators of cell
proliferation and differentiation, functioning as ligands to
stimulate receptor-mediated signal transduction pathways. The
activation of the Wnt signaling pathway has been demonstrated to be
involved in the pathogenesis of various types of human cancer
(4,5). The canonical Wnt (Wnt/β-catenin)
pathway functions by modulating the translocation of β-catenin to
the nucleus, where it controls critical gene expression programs
via interactions with T-cell factor/lymphoid enhancing
factor(TCF/LEF) and additional families of transcription factors
(6). Under normal conditions,
casein kinase 1β (CK1β) and glycogen synthase kinase-3β (GSK-3β)
sequentially phosphorylate β-catenin in the Axin complex, which is
composed of the scaffolding protein Axin, the tumor suppressor
adenomatous polyposis coli gene product, CK1β and GSK-3β (7,8).
Phosphorylated β-catenin is subsequently ubiquitinated, resulting
in its proteasomal degradation (9). This process maintains β-catenin at
low levels in the cytoplasm and prevents it from translocating to
the nucleus, leading to repression of Wnt target genes (9).
The Wnt pathway is composed of two distinct
signaling arms: The canonical and noncanonical pathways. In the
canonical pathway, the Wnt protein binds to the cell surface
receptor Frizzled, which uses the coreceptors low-density
lipoprotein receptor-related protein 5/6 (Lrp5/6) to promote Axin
binding to Dishevelled (6). This
leads to the stabilization of β-catenin, which in turn translocates
to the nucleus, where it interacts with the DNA-binding proteins
from the TCF/LEF family to activate transcription of numerous genes
(10). Dickkopf Wnt signaling
pathway inhibitor 1 (DKK1) is a secreted inhibitor that functions
by binding directly to the Lrp5/6 coreceptors and inhibiting
canonical Wnt signal transduction (11). Previous studies have reported that
secreted inhibitors of the Wnt pathway, such as SFRP1 and DKK1, are
frequently silenced by methylation in numerous types of human
cancer (12).
Wnt/β-catenin signaling has been implicated in the
maintenance of mouse and human ESCs in vitro (13–20).
Additionally, Wnt signaling has been reported to promote the
acquisition of pluripotency during the reprogramming of somatic
cells to induced pluripotent stem cells (21,22).
Numerous studies have demonstrated that activating Wnt/β-catenin
signaling promotes the self-renewal of mouse ESCs (13–20).
Loss-of-function studies have indicated that β-catenin is required
for multilineage differentiation, however, is dispensable for
self-renewal (20,23,24).
β-catenin has additionally been demonstrated to exhibit pleiotropic
effects in ESCs that are essential for ESC differentiation and the
prevention of the acquisition of tumorigenicity (3). These observations demonstrate a role
for β-catenin as a gatekeeper of differentiation and tumorigenesis
in ESCs (23).
The role of Wnt/β-catenin signaling in ESCs remains
unclear, with previous studies reporting contradictory results. In
the present study, the Wnt signaling pathway and its regulation by
DKK1 was investigated in mouse ESCs. The present study aimed to
provide further evidence regarding β-catenin, and the role of DKK1
on the differentiation potential of ESCs in vitro and in
vivo.
Materials and methods
Cells and animal sources
Five C57BL/6 mice used to establish ESC lines were
provided by the Experimental Animal Center of Chongqing Medical
University (Chongqing, China). The Balb/c mice (10 male, 10 female;
HFK Bioscience Co., Ltd., Beijing, China) were kept under standard
conditions and sacrificed at 6–8 weeks old and 18–25 g. This study
was approved by the ethics committee of Chongqing Medical
University (Chongqing, China).
Extraction of the inner cell mass (ICM)
and establishment of ESCs
Uteri were aseptically removed from pregnant mice
(3.5 days pregnant), and the blastocyst was flushed with
phosphate-buffered saline (PBS). The well-developed blastocysts
were collected under a microscope (IX70; Olympus Corporation,
Tokyo, Japan). The ICM was digested in trypsin (HyClone
Laboratories, Inc., Logan, UT, USA) for 3–5 min. The ICM was gently
dispersed using a glass microneedle into small clumps of cells. The
ICM was continuously digested into single cells, which were
inoculated into a 6-well plate.
In vivo transplantation of ESCs
Mouse ESCs at logarithmic phase were prepared,
digested in 0.25% trypsin, and suspended in PBS. The cell density
was adjusted to 1×106 cells/100 µl. The Balb/c
mice were anesthetized and the ESC suspension was injected into the
liver of each mouse (100 µl). Liver nodules >0.5 cm
diameter were classified as tumors. Mice were sacrificed by
intraperitoneal injection with 1% sodium pentobarbital (0.1 ml/10
g; Sigma-Aldrich, St. Louis, MO, USA), following disinfection with
0.2% iodophor (Shandong Lierkang Disinfection Technology Co., Ltd.,
Shandong, China).
Reagents and antibodies
Rabbit anti-mouse OCT4 (cat. no. ab80892), and
rabbit anti-mouse Nanog (cat. no. ab9220) were purchased from Abcam
(Cambridge, MA, USA). Rabbit anti-mouse stage-specific embryonic
antigen 1 (SSEA1; cat. no. sc-101462) was purchased from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Goat anti-rabbit IgG
fluorescein isothiocyanate (FITC)-conjugated antibodies (cat. no.
cw0114) and anti-goat IgG tetramethyl-rhodamine (TRITC)-conjugated
antibodies (cat. no. cw0160) were purchased from Beijing ComWin
Biotech Co., Ltd. (Beijing, China). The anti-rabbit
streptavidin-peroxidase (SP) immunohistochemical kit (SP-0023) and
anti-rat SP immunohistochemical kit (SP-0024) were obtained from
BIOSS (Beijing, China). Rabbit anti-mouse NeuroD (cat. no. ab16508)
and rabbit anti-mouse bone morphogenic protein 4 (BMP4; cat. no.
ab39973) antibodies were from Abcam. Rabbit anti-mouse β-catenin
antibodies (cat. no. 9562) were from Cell Signaling Technology,
Inc. (Danvers, MA, USA), anti-GSK3β (cat. no. YT2082) and
anti-phosphorylated GSK3β (P-GSK3β; cat. no. YP0124) antibodies
were from ImmunoWay Biotechnology Company (Newark, DE, USA), and
rabbit anti-mouse sex determining region Y-box 17 (SOX17; cat. no.
09-038), anti-c-Myc (cat. no. CBL430-KC) and anti-cyclin D1 (cat.
no. ABE52) antibodies were from Merck Millipore (Darmstadt,
Germany).
Cell culture and applying exogenous
DKK1
C57BL/6 ESC culture medium consisted of: Complete
medium (435 ml; Cyagen Biosciences Inc., Guangzhou, China), fetal
bovine serum (50 ml; Gibco Life Technologies, Carlsbad, CA, USA),
glutamine (5 ml; Gibco Life Technologies), 2-hydrophobic based
ethanol (500 µl), non-essential amino acids (5 ml; Gibco
Life Technologies), leukemia inhibitory factor (100 µl;
Gibco Life Technologies) and 105 IU/l penicillin/100 g
streptomycin solution (5 ml; Gibco Life Technologies). ESCs were
added to six-well plates (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) containing fetal mouse fibroblasts which had been treated
with 10 µg/ml mitomycin C (Roche Diagnostics, Basel,
Switzerland) to prevent fibroblast mitosis. Cultures were grown in
an incubator containing 5% CO2 at 37°C. When cells were
adherent and confluent, exogenous recombinant DKK1 (200 ng/ml;
PeproTech, Inc., Rocky Hill, NJ, USA) was applied for 24 h
(19).
Immunofluorescent staining
Mouse fibroblast cells treated with 10 µg/ml
mitomycin C were prepared on 8×8 mm slides with the complete ESC
medium replaced at 24 h, and ESCs were subcultured in the
fibroblast cell layer at a 1:5 ratio. ESCs were cultured for 48–72
h, following which the original culture medium was discarded. Cells
were fixed in 4% formaldehyde (Beyotime Institute of Biotechnology,
Beijing, China) in PBS for 15 min at room temperature, followed by
three washes with 0.1% bovine serum albumin (BSA; Beyotime
Institute of Biotechnology) in PBS. The cells were permea bilized
using 0.1% Triton X-100 (Baisaisi Biotechnology Co., Ltd.,
Tianjing, China) in PBS containing 0.1% BSA and 4% normal goat
serum (Gibco Life Technologies) or 10% donkey serum for SOX17. The
cells were incubated with the primary antibodies (anti-SSEA1,
anti-OCT4 and anti-Nanog, all at 1:100) overnight at 4°C, followed
by a 1 h incubation with the secondary antibodies (goat anti-rabbit
IgG-FITC and anti-goat IgG-TRITC) at room temperature. Subsequent
steps were performed according to the manufacturer's instructions
for the immunofluorescence kit (BIOSS). Images were acquired using
a fluorescence microscope (BX40; Olympus Corporation). Each image
contained >200 cells and totaled >2,000 cells/sample.
Immunohistochemical staining
Feeder layer cells were prepared on 8×8 mm slides
with the complete ESC medium replaced at 24 h, and ESCs were
subcultured in the feeder layer at a 1:5 ratio. ESCs were cultured
for 48–72 h, following which the original culture medium was
discarded. The cells were fixed for 30 min as above. Following
three PBS washes, cells were incubated for 20 min in 0.5% Triton
X-100. Following three further PBS washes, cells were incubated
with 3% H2O2 in deionized water for 10–15 min
to eliminate endogenous peroxidase activity. A further three PBS
washes were followed by 15 min blocking in normal serum (HyClone
Laboratories; GE Healthcare Life Sciences, Logan, UT, USA). The
samples were incubated with primary antibodies (anti-SOX17,
anti-NeuroD and anti-BMP4, all 1:100) at 4°C overnight. Subsequent
steps were performed according to the manufacturer's instructions
for the anti-rabbit and anti-rat SP immunohistochemical kits.
Images were acquired using a confocal microscope (TCS SP8; Leica
Microsystems, Wetzlar, Germany). The sections of tumor tissue were
fixed and blocked by the same method as for the slides mentioned
above. Primary antibodies were used at dilutions of 1:100 and were
incubated at 4°C overnight. The sections were soaked in an avidin
horseradish enzyme tag chain working liquid (ZSGB-BIO Co., Ltd.,
Beijing, China) at 37°C for 15 min and stained with
3,3′-diaminobenzidine chromogenic substrate and hematoxylin (Wuhan
Boster Biological Technology Ltd., Wuhan, China). Images were
captured using a microscope (BX40; Olympus Corporation) and were
analyzed using Image Pro-Plus version 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA). Each image contained >200 cells and totaled
>2,000 cells/sample.
Western blotting for protein
expression
Total protein was extracted from the cells using
radioimmunoprecipitation assay buffer supplemented with protease
(phenylmethylsulfonyl fluoride) and phosphate inhibitors (NaF anf
Na3VO4) (Roche Diagnostics) on ice. Protein
concentration was determined using a Bicinchoninic Acid protein
assay kit (Beyotime Institute of Biotechnology), and loading buffer
(5X) was added to the proteins, which were boiled for 5 min.
Proteins (50 µg/lane) were fractionated by 12% SDS-PAGE and
electrotransferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked for 1 h
using 0.1% Tween-20 (Invitrogen Life Technologies, Carlsbad, CA,
USA) and 5% nonfat milk, prior to incubation with primary
antibodies (anti-OCT4, SSEA1, β-catenin, GSK3β, P-GSK3β, SOX17,
cyclin D1 and c-Myc) at 4°C overnight. The membranes were then
incubated with horseradish peroxidase-conjugated goat
anti-mouse/anti rabbit secondary antibodies (Zhongshan Jinqiao
Biotechnology Co., Ltd., Beijing, China) for 1 h at 37°C, and
antigen antibody complexes were detected by chemiluminescence using
the BeyoECL Plus kit (Beyotime Institute of Biotechnology) for 1–3
min. X-ray film (LCNDT Corporation, Wenzhou, China) was exposed to
membranes in a darkroom, fixed and imaged. Optical density (OD) was
measured using Quantity One software, version 4.4 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA from the cells was isolated using TRIzol
reagent (Invitrogen Life Technologies) according to the
manufacturer's instructions. cDNA was synthesized from 500 ng total
RNA using the Takara PCR AMV 3.0 kit (Takara Bio, Inc., Otsu,
Japan). PCR was conducted with 1 µl cDNA in a 20 µl
reaction volume using a PCR kit (Kangweishiji Biotech Co., Ltd.,
Beijing, China). A negative control was established by using
H2O as the template. PCR products were detected by 1.5%
agarose gel electrophoresis (BD Biosciences, Franklin Lakes, NJ,
USA). The primers used were as follows: GSK-3β, forward
5′-TCCCTCAAATTAAGGCACATC-3′ and reverse
5′-CACGGTCTCCAGTATTAGCATCT-3′. β-actin expression served as a
control, and the primers were as follows: Forward
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse 5′-GAAGATGGTGATGGGATTTC-3′.
PCR was conducted in a thermal cycler (PTC-200; Bio-Rad
Laboratories, Inc.) with 35 cycles of denaturation at 94°C for 45
sec, annealing at 59°C for 45 sec and extension at 72°C for 45 sec
with an initial denaturation of 3 min and a final extension of 5
min. The amplified products were subjected to gel electrophoresis
in 2% agarose and visualized by ethidium bromide (Sigma-Aldrich)
staining under ultraviolet illumination.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using TRIzol
reagent according to the manufacturer's instructions and cDNA was
generated using a reverse transcription kit (Takara Bio, Inc.)
according to the manufacturer's instructions. The PCR primer
sequences were as follows: β-catenin, forward GCTTTCAGTTGAGCTGACCA
and reverse AAGTCCAAGATCAGCAGTCTCA; glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), forward TGCACCACCAACTGCTTAGC and reverse
GGCATGGACTGTGGTCATGAG. The SYBR Premix ExTaq™ II kit (Takara
Biotechnology Co., Ltd., Dalian, China) was used following the
manufacturer's instructions for RT-qPCR, which was performed on an
ABI 7500 system (Applied Biosystems Life Technologies, Foster City,
CA, USA). PCR cycle parameters were as follows: 94°C for 3 min,
followed by 30 cycles of 94°C for 30 sec, 56°C for 30 sec and 72°C
for 30 sec. The relative expression level of β-catenin mRNA was
calculated using the 2−ΔΔCT method. All assays were
performed in triplicate.
Statistical analysis
All data were analyzed using one-way analysis of
variance using SPSS software, version 10.0 (SPSS, Inc., Chicago,
IL, USA). Means were compared using the Student-Newman-Keuls test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
The growth state of ESCs in the
DKK1-treated and control groups
Isolated murine ESCs in the control group exhibited
morphological features characteristic of ESCs: Active
proliferation, closely connected cells, “nest” growth, clear colony
edges with smooth surfaces, dense structure and clear boundaries
between the layers of cells (Fig.
1A). The colony of ESCs in the DKK1-treated group appeared to
be clearer and more rounded compared with those in the control
group (Fig. 1A).
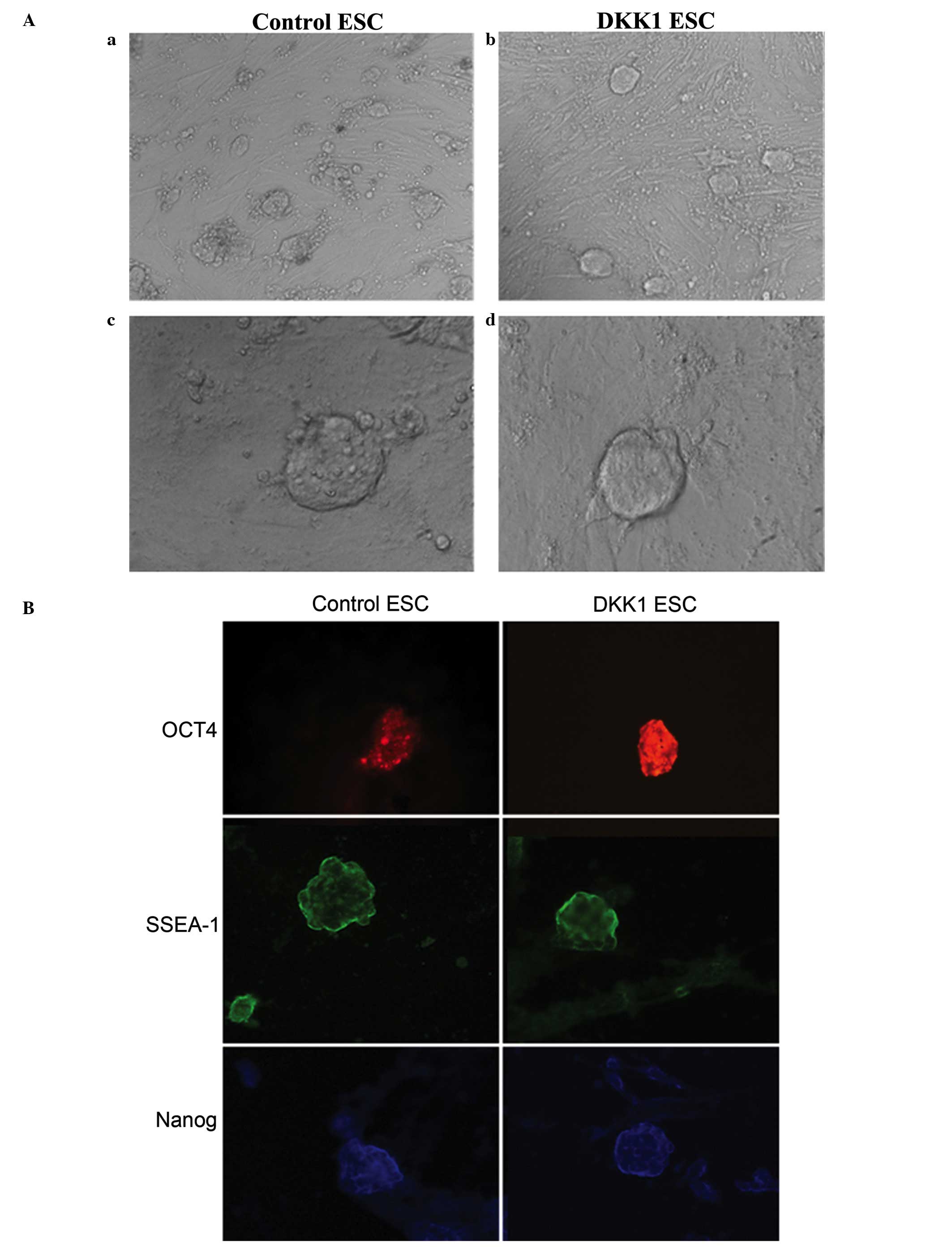 | Figure 1ESC growth state and
immunohistochemical staining for the expression of embryonic
antigens in control ESCs and DKK1-treated ESCs. (A) Growth of
control ESCs and DKK1-treated ESCs: (a) control ESCs
(magnification, ×100); (b) DKK1-treated ESCs (magnification, ×100);
(c) control ESCs (magnification, ×400); (d) DKK1-treated ESCs
(magnification, ×400). (B) OCT4, SSEA1 and Nanog expression in
control ESCs and DKK1-treated ESCs (magnification, x200). ESC,
embryonic stem cell; DKK1, dickkopf Wnt signaling pathway inhibitor
1; OCT4, octamer-binding transcription factor 4; SSEA1,
stage-specific embryonic antigen 1. (C) Western blot analysis of
OCT4, SSEA1 and Nanog expression in control ESCs and DKK1-treated
ESCs: Lane 1, control ESC group; lane 2, DKK1-treated ESCs. (D)
Histogram for western blot analysis results for OCT4, SSEA1 and
Nanog in control ESCs and DKK1-treated ESCs. *P<0.05
vs. control. The abundance of GAPDH was determined as a control.
Values are presented as the mean ± standard deviation (n=3). OCT4,
octamer-binding transcription factor 4; SSEA1, stage-specific
embryonic antigen 1; ESC, embryonic stem cell; DKK1, dickkopf Wnt
signaling pathway inhibitor 1; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
OCT4, SSEA1 and Nanog expression in
ESCs
Immunofluorescent staining was conducted for OCT4,
SSEA1 and Nanog, which indicated postive expression in the control
ESCs for OCT4, SSEA1 and Nanog (Fig.
1B). In the DKK1-treated ESC group, the expression of OCT4
appeared greater compared with the control group, however, the
expression levels of SSEA1 and Nanog were comparable to the levels
observed in the control group.
Western blot analysis of OCT4, SSEA1 and
Nanog expression in ESCs
The protein expression levels of OCT4, SSEA1 and
Nanog in ESCs were analyzed by western blotting and were quantified
(Fig. 1C and D). This indicated
positive expression of OCT4 protein in the control ESCs, however,
significantly greater expression of OCT4 was observed in
DKK1-treated ESCs (P<0.05; Fig
1D). The expression levels of SSEA1 in control ESCs were not
significantly different compared with the DKK1-treated ESCs. In
addition, the expression levels of Nanog in control ESCs were not
significantly different compared with the DKK1-treated ESCs.
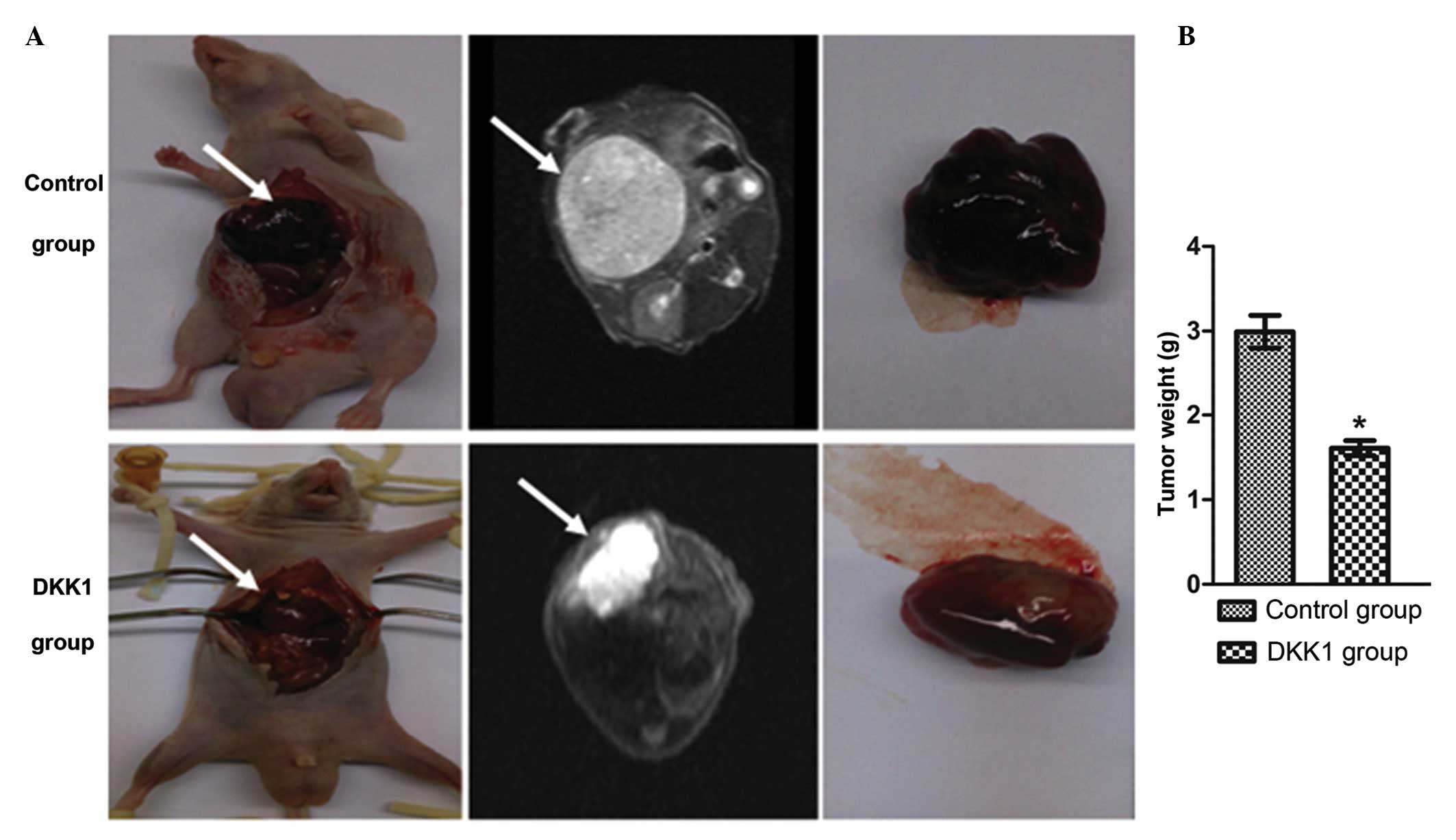
Histological pathology of liver
teratomas
The teratomas in the control group and the
DKK1-treated group were collected 4 weeks following
transplantation, and the tumor weight was measured. The weight of
the tumors from the DKK1-treated group was significantly reduced
compared with the control group (Fig.
2A and B). Sections of tumor tissue from each group were
stained with hematoxylin and eosin to investigate the presence of
the three germ layers (Fig. 2C).
In the control ESC group, the liver teratomas exhibited primarily
glandular epithelial structure, with an atypical cell and tissue
structure, disordered cell size, hyperchromatic nuclei, increased
nuclear fission, irregular mitosis and sections of necrosis.
Histopathological evaluation indicated that the lesion area had
formed a mature teratoma of epithelial cancer (Fig. 2C). In the DKK1-treated ESC group,
the liver teratomas had a primarily glandular epithelial structure,
with atypical cell and tissue structure. However, the ganglia were
reduced in number compared with the control group, and the
cartilage tissue was observed to be less mature compared with the
control group. Furthermore, tissue from the digestive gland
demonstrated increased numbers of cells compared with the control
group, with a clearer structure than that of the control group
(Fig. 2C).
NeuroD, SOX17 and BMP4 expression in
ESCs
Staining for the markers of the three germ layers
indicated that DKK1-treatment resulted in altered expression in
ESCs compared with the control (Fig.
3A and B). Expression of NeuroD was reduced in the DKK1-treated
group compared with the control group (P<0.05; Fig. 3B). SOX17 staining demonstrated
increased expression in the DKK1-treated ESCs compared with the
control group (P<0.05; Fig.
3B). Expression of BMP4 was reduced in the DKK1-treated ESCs
compared with the control group (P<0.05; Fig. 3B).
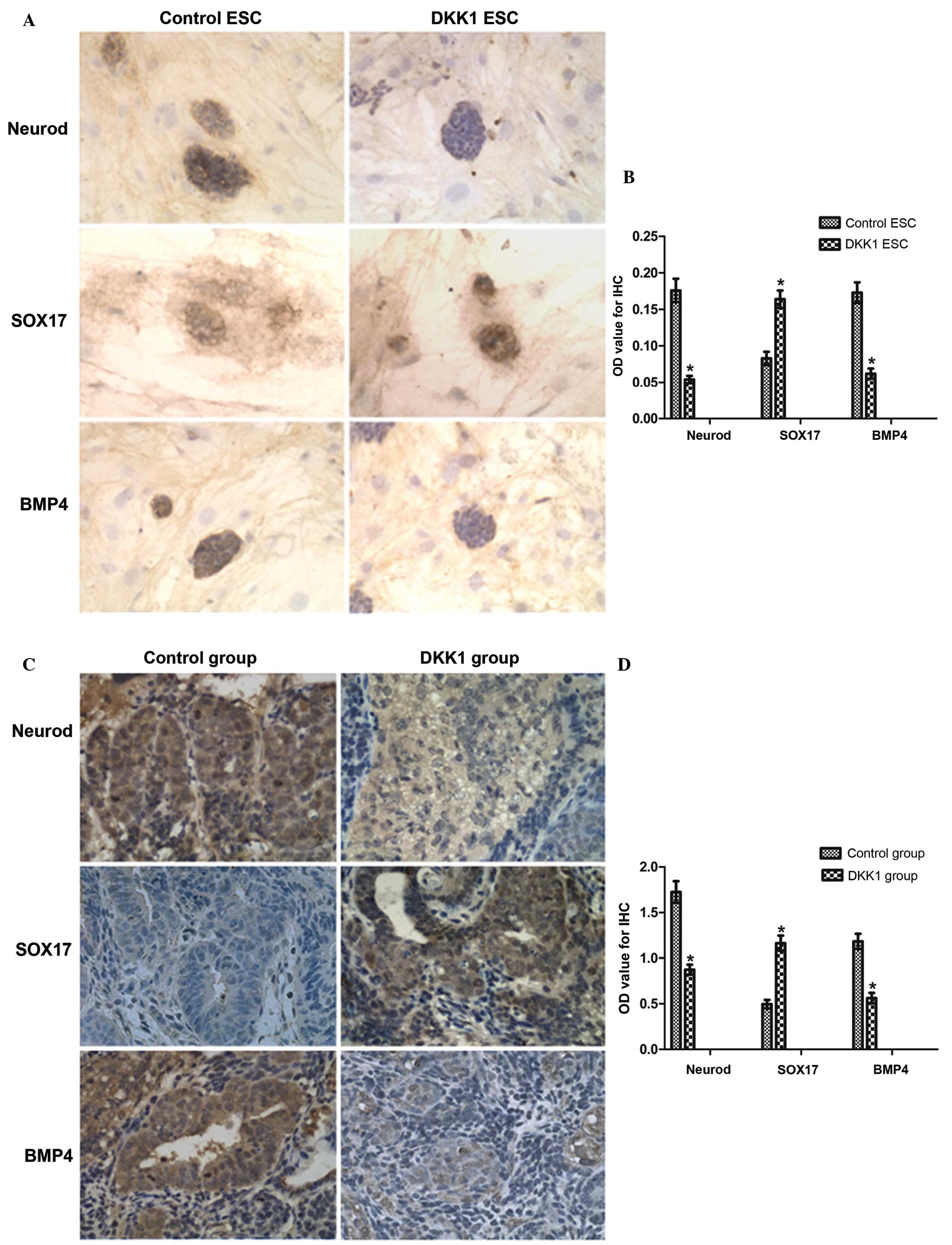 | Figure 3IHC staining for NeuroD, SOX17 and
BMP4 expression in ESCs and derived teratomas. (A) NeuroD, SOX17
and BMP4 expression in ESC groups by IHC staining (magnification,
x200). (B) Quantification of IHC staining for NeuroD, SOX17 and
BMP4. (C) NeuroD, SOX17 and BMP4 expression in teratomas derived
from control ESCs and DKK1-treated ESCs (magnification, ×400). (D)
Quantification of IHC staining for NeuroD, SOX17 and BMP4.
*P<0.05 vs. control. IHC, immunohistochemical; SOX17,
sex determining region Y-box 17; BMP4, bone morphogenic factor 4;
ESC, embryonic stem cell; DKK1, dickkopf Wnt signaling pathway
inhibitor 1; OD, optical density. |
NeuroD, BMP4 and SOX17 expression in the
teratomas
As presented above, the markers of the three germ
layers additionally exhibited alterations in expression between the
teratomas derived from the control and DKK1-treated ESCs (Fig. 3C and D). Staining for NeuroD
expression in tumors derived from control ESCs is presented in
Fig. 3C, and this indicated that
in the DKK1-treated group, NeuroD exhibited reduced expression
compared with the control group (P<0.05; Fig. 3D). Staining for BMP4 expression
indicated reduced expression in tumors from the DKK1-treated group
compared with the control group (P<0.05; Fig. 3D). However, staining for SOX17
expression indicated increased expression in the DKK1-treated group
compared with the control group (P<0.05; Fig. 3D).
RT-PCR analysis for GSK-3β mRNA
expression in ESCs and teratoma tissues
Expression levels of GSK-3β mRNA were analyzed by
RT-PCR. This indicated that there was no significant difference in
the expression of GSK-3β mRNA between the control ESCs,
DKK1-treated ESCs, control teratomas or DKK1-treated teratomas
(P>0.05; Fig. 4B).
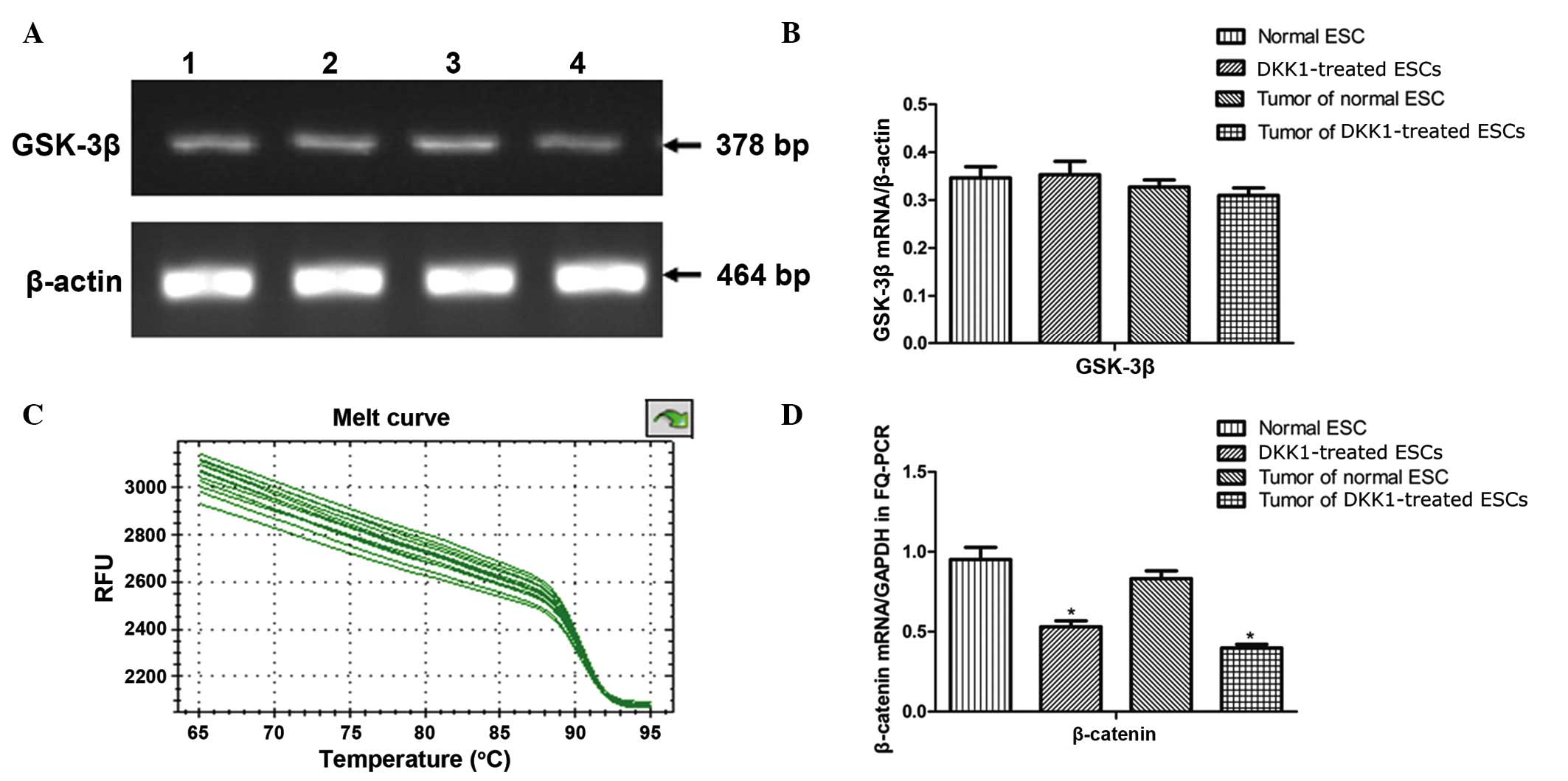 | Figure 4RT-PCR analysis for GSK-3β mRNA and
RT-qPCR for β-catenin mRNA. (A) Following 35 cycles, 5 µl
samples were separated electrophoretically through the agarose gel,
and the expected product at 378 base pairs for GSK-3 β were stained
with ethidium bromide. Lane 1, control ESC; lane 2, DKK1-treated
ESCs; lane 3, teratoma tissue derived from control ESCs; lane 4,
teratoma tissue derived from DKK1-treated ESCs. (B) Histogram of
GSK-3β mRNA expression by RT-PCR. (C) Melt curve of RT-qPCR for
β-catenin. (D) Histogram for β-catenin by RT-qPCR.
*P<0.05 vs. control. The abundance of β-actin or
GAPDH was used as a control. Values are presented as the mean ±
standard deviation (n=3). RT-PCR, reverse transcription-polymerase
chain reaction; GSK-3β, glycogen synthase kinase 3β; RT-qPCR,
RT-quantitative PCR; ESC, embryonic stem cells; DKK1, dickkopf Wnt
signaling pathway inhibitor 1; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; RFU, relative fluorescence units. |
RT-qPCR analysis of β-catenin mRNA in
ESCs and teratomas
To gain further insight into the molecular
mechanisms underlying the differential expression of the markers,
the mRNA levels of β-catenin were measured in ESCs and the derived
teratomas. RT-qPCR was used to examine the mRNA expression of
β-catenin, which is associated with the Wnt signaling pathway. This
demonstrated that β-catenin expression was significantly reduced in
DKK1-treated ESCs and teratomas derived from DKK1-treated ESCs when
compared with the control group (P<0.05; Fig. 4C and D).
Western blotting of β-catenin, GSK-3β and
P-GSK-3β expression in ESCs and teratomas
The expression levels of key proteins in the Wnt
signaling pathway were measured in ESCs and derived tumor tissues
using western blotting. This indicated clear expression of
β-catenin in control ESCs and teratomas derived from control ESCs
whilst expression was significantly reduced in DKK1-treated ESCs
and teratomas derived from DKK1-treated ESCs compared with controls
(Fig. 5A and B). The expression of
P-GSK-3β was significantly increased in DKK1-treated ESCs and
derived teratomas compared with control ESCs and teratomas
(P<0.05, Fig. 5A and B). Whilst
the expression levels of P-GSK-3β were altered by DKK1 treatment,
the expression levels of GSK-3β were not significantly different
between the groups (P>0.05, Fig. 5A
and B).
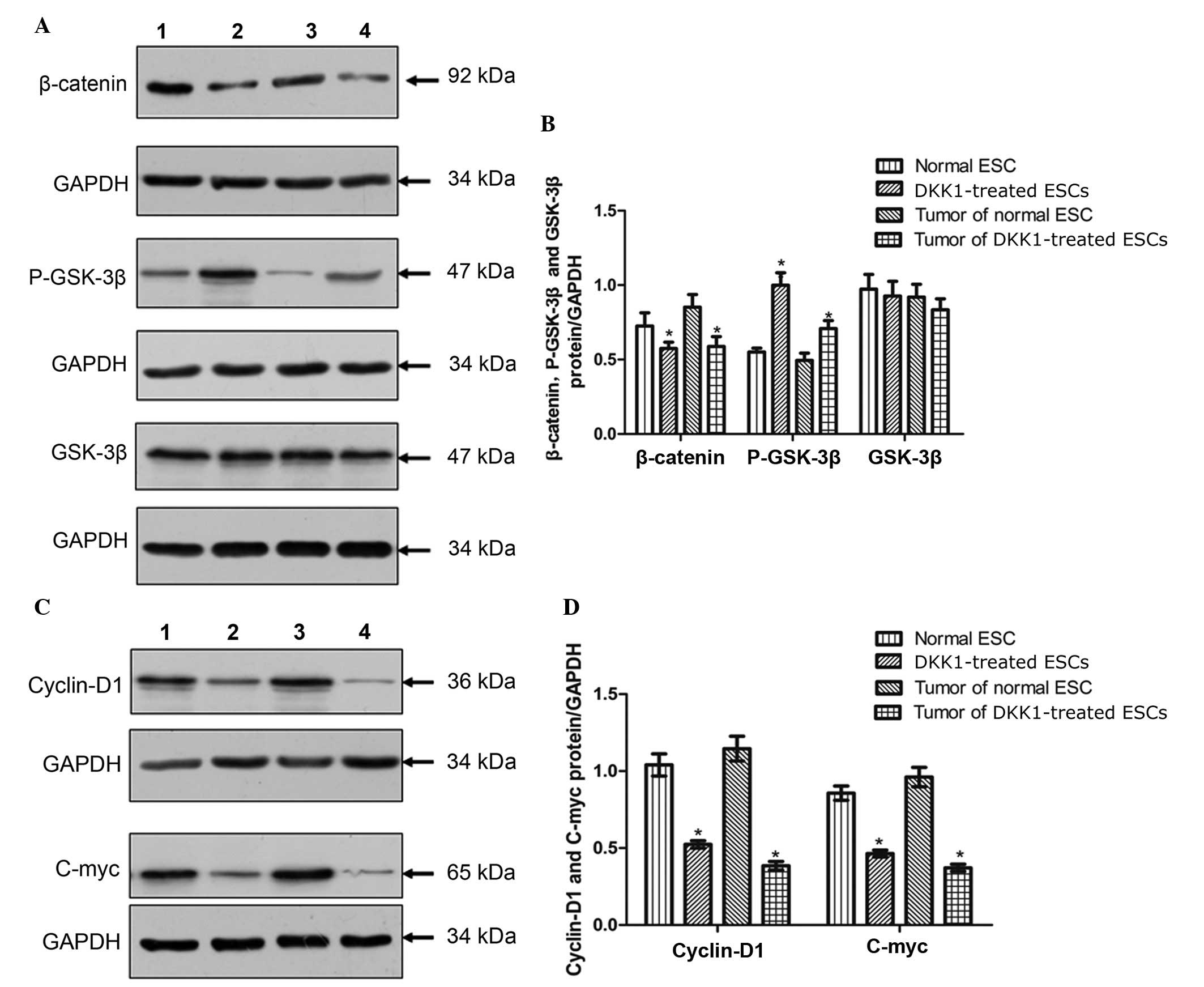 | Figure 5Western blot analysis for the
expression of molecules in the Wnt/β-catenin signaling pathway. (A)
Western blotting images of β-catenin, P-GSK-3β and GSK-3β: Lane 1,
control ESCs; lane 2, DKK1-treated ESCs; lane 3, teratoma derived
from control ESCs, lane 4, teratoma derived from DKK1-treated ESCs.
(B) Quantification of western blotting for β-catenin, P-GSK-3β and
GSK-3β. (C) Western blotting images of cyclin D1 and c-Myc. (D)
Quantification of western blotting for cyclin D1 and c-Myc.
*P<0.05 vs. control. GAPDH was used as a control.
Values are presented as the mean ± standard deviation (n=3).
P-GSK-3β, phosphorylated glycogen synthase kinase 3β; ESC,
embryonic stem cell; DKK1, dickkopf Wnt signaling pathway inhibitor
1; GAPDH, glyceralde-hyde 3-phosphate dehydrogenase. |
Western-blotting for cyclin D1 and c-Myc
expression
The expression levels of additional key proteins in
the Wnt signaling pathway were measured in ESCs and tumor tissues.
This demonstrated that the expression levels of cyclin D1 were
reduced in DKK1-treated ESCs and tumors derived from DKK1-treated
ESCs compared with control ESCs and the corresponding tumors
(P<0.05; Fig. 5C and D). In
addition, the expression levels of c-Myc in DKK1-treated ESCs and
tumors derived from DKK1-treated ESCs were significantly reduced
compared with the expression in control ESCs and tumors derived
from control ESCs (P<0.05; Fig. 5C
and D).
Discussion
In the current study, the staining of typical ESC
antigens was used to characterize ESCs. The expression of OCT4,
SSEA1 and Nanog was observed, verifying the identity of the cells
as ESCs. It was observed that the treatment with DKK1 resulted in
improved cellular morphology and an increase in OCT4 expression.
Variation in the inhibitory effects of Wnt/β-catenin signaling in
ESCs has been observed in previous studies, and a potential
explanation for this may involve Wnt repression. A previous study
supported a model whereby Wnt/β-catenin signaling is repressed by
OCT4 in the context of self-renewing human ESCs (hESCs) and is
derepressed when hESCs differentiate (3). In the current study, liver teratomas
derived from control ESCs in vivo, HE staining indicated
that tumor tissue consisted predominantly of glandular epithelial
structures that formed a mature teratoma. Neoplastic cells assumed
a nest-like morphology and pathological tests confirmed epithelioid
cancerous progression. The current study identified differential
alterations in the markers of the three germ layers when
DKK1-treated ESCs were injected into mice. The NeuroD staining of
the ectoderm and the BMP4 staining of the mesoderm exhibited
reduced expression in the DKK1-treated ESC groups in vitro
and in vivo, whilst SOX17 staining of the endoderm indicated
increased expression compared with the control group. These results
suggest that the inhibition of Wnt/β-catenin signaling may have
promoted endoderm differentiation.
In addition, Wnt signaling has been reported to
promote pluripotency during the reprogramming of somatic cells to
induce pluripotent stem cells (25,26).
However, studies have reported that inhibiting Wnt3A or GSK3
results in the differentiation of hESCs towards the primitive
streak and definitive endoderm lineages (27,28).
Davidson et al (3) reported
a primary role for Wnt/β-catenin signaling in the differentiation,
rather than the self-renewal, of hESCs in vitro. This was
demonstrated through sustained inhibition of the pathway, which did
not prevent the expansion of hESCs, suggesting that endogenous
Wnt/β-catenin signaling is not required for the self-renewal of
undifferentiated hESCs. Furthermore, the activation of β-catenin
signaling resulted in an induction of mesoderm lineage transcripts
and an eventual loss of self-renewal following several passages
(3). In the current study,
Wnt/β-catenin signaling was not required for the self-renewal of
undifferentiated ESCs however, was required for the differentiation
of ESCs. The expression levels of β-catenin were significantly
reduced in DKK1-treated ESCs and teratomas derived from
DKK1-treated ESCs compared with the control groups. It was
demonstrated that P-GSK-3β expression was significantly greater in
the DKK1 treatment groups, with no effect on total GSK-3β levels.
The expression levels of cyclin D1 and c-Myc were significantly
reduced in the DKK1 treatment groups compared with the control
groups. These results indicate that the Wnt/β-catenin signaling
pathway regulated the differentiation of ESCs through OCT4 and
β-catenin. Cyclin D1 serves a critical role in the regulation of
cell cycle progression, and a TCF4 binding site is located in its
promoter region, suggesting the involvement of the β-catenin/TCF4
pathway in the regulation of cyclin D1 expression (29). The current study additionally
indicated that the levels of cyclin D1 and c-Myc protein expression
were significantly reduced in teratomas derived from DKK1-treated
ESCs compared with teratomas derived from control ESCs. The tumor
weight in the DKK1-treated ESC group was significantly reduced
compared with the control ESC group. This indicated that the
Wnt/β-catenin signaling pathway may additionally be associated with
the proliferation and tumorigenicity of ESCs.
In conclusion, the current study demonstrated that
the Wnt signaling pathways were regulated by the inhibitor DKK1 in
ESCs. DKK1 was demonstrated to antagonize the Wnt/β-catenin pathway
via a reduction in β-catenin and an increase in OCT4 expression. In
addition, the present study suggests that the Wnt/β-catenin
signaling pathway was associated with the direction of
differentiation of ESCs in vitro and in vivo.
Furthermore, the current study identified a novel molecular
interaction between DKK1 and the Wnt/β-catenin pathway during ESC
development.
Acknowledgments
The current study was supported by a project grant
from the National Basic Research Program of China (973 program;
grant no. 2011CB707900).
References
|
1
|
Doetschman TC, Eistetter H, Katz M,
Schmidt W and Kemler R: The in vitro development of
blastocyst-derived embryonic stem cell lines: Formation of visceral
yolk sac, blood islands and myocardium. J Embryol Exp Morphol.
87:27–45. 1985.PubMed/NCBI
|
|
2
|
Keller G: Embryonic stem cell
differentiation: Emergence of a new era in biology and medicine.
Genes Dev. 19:1129–1155. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davidson KC, Adams AM, Goodson JM,
McDonald CE, Potter JC, Berndt JD, Biechele TL, Taylor RJ and Moon
RT: Wnt/β-catenin signaling promotes differentiation, not
self-renewal, of human embryonic stem cells and is repressed by
Oct4. Proc Natl Acad Sci USA. 109:4485–4490. 2012. View Article : Google Scholar
|
|
4
|
Karim R, Tse G, Putti T, Scolyer R and Lee
S: The significance of the Wnt pathway in the pathology of human
cancers. Pathology. 36:120–128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and β-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Daniels DL and Weis WI: β-catenin directly
displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated
transcription activation. Nat Struct Mol Biol. 12:364–371. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang H and He X: Wnt/β-catenin signaling:
New (and old) players and new insights. Curr Opin Cell Biol.
20:119–125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
MacDonald BT, Tamai K and He X:
Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev
Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Amerongen R and Nusse R: Towards an
integrated view of Wnt signaling in development. Development.
136:3205–3214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gadue P, Huber TL, Paddison PJ and Keller
GM: Wnt and TGF-beta signaling are required for the induction of an
in vitro model of primitive streak formation using embryonic stem
cells. Proc Natl Acad Sci USA. 103:16806–16811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura T, Sano M, Songyang Z and
Schneider MD: A Wnt- and beta -catenin-dependent pathway for
mammalian cardiac myogenesis. Proc Natl Acad Sci USA.
100:5834–5839. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Monzen K, Shiojima I, Hiroi Y, Kudoh S,
Oka T, Takimoto E, Hayashi D, Hosoda T, Habara-Ohkubo A, Nakaoka T,
et al: Bone morphogenetic proteins induce cardiomyocyte
differentiation through the mitogen-activated protein kinase kinase
kinase TAK1 and cardiac transcription factors Csx/Nkx-2.5 and
GATA-4. Mol Cell Biol. 19:7096–7105. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naito AT, Akazawa H, Takano H, Minamino T,
Nagai T, Aburatani H and Komuro I: Phosphatidylinositol
3-kinase-Akt pathway plays a critical role in early
cardiomyogenesis by regulating canonical Wnt signaling. Circ Res.
97:144–151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng CF, Wei Y, Levsky JM, McDonald TV,
Childs G and Kitsis RN: Microarray analysis of global changes in
gene expression during cardiac myocyte differentiation. Physiol
Genomics. 9:145–155. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alexander J and Stainier DY: A molecular
pathway leading to endoderm formation in zebrafish. Curr Biol.
9:1147–1157. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sinner D, Rankin S, Lee M and Zorn AM:
Sox17 and beta-catenin cooperate to regulate the transcription of
endodermal genes. Development. 131:3069–3080. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yasunaga M, Tada S, Torikai-Nishikawa S,
Nakano Y, Okada M, Jakt LM and Nishikawa S, Chiba T, Era T and
Nishikawa S: Induction and monitoring of definitive and visceral
endoderm differentiation of mouse ES cells. Nat Biotechnol.
23:1542–1550. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kitajima S, Takagi A, Inoue T and Saga Y:
MesP1 and MesP2 are essential for the development of cardiac
mesoderm. Development. 127:3215–3226. 2000.PubMed/NCBI
|
|
19
|
Semënov MV, Tamai K, Brott BK, Kühl M,
Sokol S and He X: Head inducer Dickkopf-1 is a ligand for Wnt
coreceptor LRP6. Curr Biol. 11:951–961. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato N, Meijer L, Skaltsounis L, Greengard
P and Brivanlou AH: Maintenance of pluripotency in human and mouse
embryonic stem cells through activation of Wnt signaling by a
pharmacological GSK-3-specific inhibitor. Nat Med. 10:55–63. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
von Both I, Silvestri C, Erdemir T,
Lickert H, Walls JR, Henkelman RM, Rossant J, Harvey RP, Attisano L
and Wrana JL: Foxh1 is essential for development of the anterior
heart field. Dev Cell. 7:331–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ng ES, Azzola L, Sourris K, Robb L,
Stanley EG and Elefanty AG: The primitive streak gene Mixl1 is
required for efficient haematopoiesis and BMP4-induced ventral
mesoderm patterning in differentiating ES cells. Development.
132:873–884. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okumura N, Akutsu H, Sugawara T, Miura T,
Takezawa Y, Hosoda A, Yoshida K, Ichida JK, Yamada M, Hamatani T,
et al: β-catenin functions pleiotropically in differentiation and
tumorigenesis in mouse embryo-derived stem cells. PLoS One.
8:e632652013. View Article : Google Scholar
|
|
24
|
Gubbay J, Collignon J, Koopman P, Capel B,
Economou A, Münsterberg A, Vivian N, Goodfellow P and Lovell-Badge
R: A gene mapping to the sex-determining region of the mouse Y
chromosome is a member of a novel family of embryonically expressed
genes. Nature. 346:245–250. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marson A, Foreman R, Chevalier B, Bilodeau
S, Kahn M, Young RA and Jaenisch R: Wnt signaling promotes
reprogramming of somatic cells to pluripotency. Cell Stem Cell.
3:132–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lluis F, Pedone E, Pepe S and Cosma MP:
Periodic activation of Wnt/beta-catenin signaling enhances somatic
cell reprogramming mediated by cell fusion. Cell Stem Cell.
3:493–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bone HK, Nelson AS, Goldring CE, Tosh D
and Welham MJ: A novel chemically directed route for the generation
of definitive endoderm from human embryonic stem cells based on
inhibition of GSK-3. J Cell Sci. 124:1992–2000. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakanishi M, Kurisaki A, Hayashi Y,
Warashina M, Ishiura S, Kusuda-Furue M and Asashima M: Directed
induction of anterior and posterior primitive streak by Wnt from
embryonic stem cells cultured in a chemically defined serum-free
medium. FASEB J. 23:114–122. 2009. View Article : Google Scholar
|
|
29
|
Lin SY, Xia W, Wang JC, Kwong KY, Spohn B,
Wen Y, Pestell RG and Hung MC: Beta-catenin, a novel prognostic
marker for breast cancer: Its roles in cyclin D1 expression and
cancer progression. Proc Natl Acad Sci USA. 97:4262–4266. 2000.
View Article : Google Scholar : PubMed/NCBI
|