Introduction
Renal cell carcinoma is the most common cause of
mortality among adult kidney cancers and its global incidence has
been increasing by ~3% per year in Ireland (1). Derived from the renal tubular
epithelial cells, clear cell renal cell carcinoma is the most
common pathology sub-type of renal cancer (2). The prognosis of patients with renal
cell carcinoma remains poor due to limited treatment strategies,
and prognostic methods require to be refined (3). The discovery of novel diagnostic and
prognostic biomarkers for the renal cell carcinoma is required in
order to optimize patient selection for specific treatments.
MicroRNAs (miRNAs/miRs), a class of highly conserved
non-coding and single-stranded RNAs, possess the ability to
regulate gene expression at the post-transcriptional level by
binding primarily to the 3′untranslated region (UTR) of targeted
mRNA. It has been frequently reported that the genesis and
progression of renal cancer is associated with abnormal miRNA
expression (4). Furthermore,
miRNAs are potential tumor biomarkers and are associated with
various signaling pathways, including Von
Hippel-Lindau/hypoxia-inducible factor (5,6),
phosphoinositide-3 kinase/Akt/mammalian target of rapamycin
(7), Wnt/Frizzled (8), Hippo (9), mitogen-activated protein kinase
(MAPK) (10,11) and nuclear factor-κB (12), in renal cancer cells. Among them,
MAPKs have important roles in signal transduction and multiple
biological processes, including cell proliferation, differentiation
and survival. The MAPK family comprises three sub-groups:
Extracellular signal-regulated kinase 1/2, stress-activated protein
kinase (SAPK)/c-Jun N-terminal kinase (JNK) and p38 MAPK (13). Of note, SAPK/JNK signaling has a
dual function by exerting pro- as well as anti-apoptotic effects,
depending on cell type, duration of its activation, the nature of
the death stimulus and the activity of other signaling pathways
(14).
miR-148b was shown to be downregulated in several
types of human cancer, including breast cancer (15), lung cancer (16), hepatocellular carcinoma (17), pancreatic cancer (18), gastric cancer (19) and colorectal cancer (20). However, the role of miR-148b in
renal cell carcinoma as well as the underlying molecular mechanisms
have remained elusive. Therefore, the present study evaluated the
expression of miR-148b in renal cancer tissues and investigated its
roles in the 786-O and OS-RC-2 renal cancer cell lines. The effects
of miR-148b on the proliferation, viability, apoptosis and cell
cycle distribution, as well as MAPK signaling in renal cancer cells
were assessed. Furthermore, as MAP3K9 acts as an upstream activator
of the MAPK kinase (MKK)/JNK signaling pathway (21), a luciferase reporter assay was
performed in order to investigate whether MAP3K9 is a functional
target of miR-148b in human renal cancer cells. The present study
revealed that miR-148b exerts an oncogenic function in human renal
cancer cells by enhancing apoptosis and proliferation through
targeting the MAPK/JNK signaling pathway via MAP3K9.
Materials and methods
Clinical specimens
A total of twelve pairs of renal clear cell
carcinoma and adjacent non-cancerous tissues were collected between
November 2013 and April 2014 from 12 patients (age, 68.80±9.7
years; eight men and four women) who were histologically diagnosed
with renal clear cell carcinoma and who did not suffer from other
severe diseases or kidney disease, and who underwent nephrectomy at
the West China Hospital of Sichuan University (Chengdu, China). All
specimens were immediately frozen in liquid nitrogen, ground into
powder and stored at −80°C until RNA and protein extraction. Prior
written informed consent was obtained from the patients with regard
to experimentation on their tissue specimens and the patients'
privacy rights of human subjects were respected. The protocol of
the present study was approved by the ethics committee of the
College of Laboratory Medicine, Chongqing Medical University
(Chongqing, China).
Cell culture and transfection
The 786-O and OS-RC-2 human renal cancer cell lines
were obtained from the American Type Culture Collection (Manassas,
VA, USA). Cells were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific). The 293T
normal renal cell line was obtained from the Cell Bank of the
Chinese Academy of Sciences (Shanghai, China) and cultured in
Dulbecco's modified Eagle's medium (high glucose; Gibco; Thermo
Fisher Scientific) supplemented with 10% FBS. All cell lines were
cultured at 37°C in a humidified atmosphere containing 5%
CO2.
miR-148b-3p (UCA GUG CAU CAC AGA ACU UUG U) and
negative control (NC) mimics (scrambled miRNA control) were
synthesized by RiboBio Co. (Guangzhou, China). The miR-148b mimics
(50 nM) or NC mimics (50 nM) was transfected into 786-O and OS-RC-2
cells using Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific) according to the manufacturer's
instructions.
Bioinformatics analysis and plasmid
construction
All plasmids and vectors were supplied by the
Department of Laboratory Medicine of the Chongqing Medical
University unless otherwise stated. Target genes of miR-148b were
predicted using the TargetScan prediction software (http://www.targetscan.org/). The 3′UTR of human MAP3K9
was identified to contain a putative target sites for miR-148b (TGC
ACTGA). For dual-luciferase assays, the plasmid containing the
wild-type (WT) target sequence,
pCDNA3.1-lucif-erase-hMAP3K9-3′UTR-WT, was obtained by cloning the
3′-UTR of human MAP3K9 into the BamHI (Thermo Fisher
Scientific) and EcoRI (Thermo Fisher Scientific) sites of
the pCDNA3.1-luciferase vector. The MAP3K9 3′UTR was amplified from
genomic DNA using polymerase chain reaction (PCR) amplification
with the following primers: Forward, 5′-CGC GGA TCC ACA GCC AGC GGA
GAT GAGG-3′ and reverse, 5′-CCG GAA TTC CCA AGT CCC AAT GTC
CAGG-3′. As a negative control, a sequence with a mutation in the
putative miR-148b target site (MUT) was inserted to obtain the
plasmid pCDNA3.1-luciferase-hMAP3K9-3′UTR-MUT. The target site
sequence (TGC ACTGA) was replaced with the MUT sequence (GAT
CAGTG). For this, the mutation method of homologous recombination
was employed by using the following primers: Forward,
5′-TGAGATC-CAGCCCTACTTCT GAT CAG TGT AAT GCA CTT-3′ (MUT) and
reverse, 5′-CTT CAA AGT GCA TTA CAC TGA TCAG AAG TAG GGC TGGA-3′
(MUT).
Dual luciferase reporter assay
To verify the predicted target genes and the
associated signaling pathways of miR-148b, dual luciferase assays
were performed in 293T cells. One day prior to transfection, 293T
cells were cultured in 24-well plates (Corning-Costar, Corning, NY,
USA). As the cells reached 70% confluence, they were co-transfected
with 1.25 μl 50 nM miR-148b mimics/NC mimics together with
0.5 μg target gene plasmids
(pCDNA3.1-luciferase-hMAP3K9-3′UTR-WT or
pCDNA3.1-luciferase-hMAP3K9-3′UTR-MUT) or the following
Wnt/transforming growth factor (TGF)-β/MAPK signaling pathway
reporter plasmids (Agilent Technologies, Inc., Santa Clara, CA,
USA): 0.5 μg TopFlash/0.5 μg (CAGA) 12-LUC/30 ng
pFA-Elk1 (activator plasmid) + 0.5 μg pFR-Luc (reporter
plasmid) with 2.5 μl Lipofectamine® 3000 as the
transfection agent. Normalization was achieved by co-transfection
of 20 ng pRL-SV40 plasmid per well. Following 48 h of incubation,
Renilla and Firefly luciferase activities were measured using a
Dual-Luciferase Reporter Assay kit (cat. no. E1910; Promega Corp.,
Madison, WI, USA) according to the manufacturer's instructions.
Reverse-transcription quantitative
(RT-q)PCR
Following tissue homogenization, total RNA was
extracted from transfected or untransfected cells using TRIzol
reagent (Thermo Fisher Scientific). For the detection of MAP3K9, RT
was performed using the RevertAid First Strand cDNA Synthesis kit
(Thermo Fisher Scientific) according to the manufacturer's
instructions. The mRNA levels were detected using the SYBR-Green
qRT-PCR kit (Takara Bio Inc., Otsu, Japan) with the following
primers: MAP3K9 sense, 5′-TTC CCC AGC AAC TAC GTG AC-3′ and
anti-sense, 5′-TCT AAC AAC TGA ATG GGC GGG-3′; miR-148b sense,
5′-CAC GTC TCA GTG CAT CAC AGA-3′ and anti-sense, 5′-GTG CAG GGT
CCG AGG T-3′. The levels of MAP3K9 mRNA were normalized to 18S
(sense, GTAACCCGTTGAACCCCATT; antisense, CCATCCAATCGGTAGTAGCG;
Invitrogen; Thermo Fisher Scientific) and the expression of
miR-148b was normalized to U6 (sense, CTCGCTTCGGCAGCACA; antisense,
AACGCTTCACGAATTTGCGT; Invitrogen; Thermo Fisher Scientific) using
the ΔΔCt method (22). PCR was
conducted using a CFX96™ Real-Time PCR system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) with a PCR mix containing
cDNA template primers, SYBR® Premix Ex Taq reagent
(Takara Bio Inc.,), and diethylpyrocarbonate-treated water (Takara
Bio Inc.). The following thermocycling conditions were used: 94°C
for 10 sec followed by 40 cycles of 94°C for 10 sec, 60°C for 20
sec and 72°C for 10 sec.
Western blot analysis
Protein was extracted from 786-O and OS-RC-2 cells
using radioimmunoprecipitation assay lysis buffer (Beyotime
Biotechnology Company, Haimen, China). Total protein was quantified
using NanoDrop 2000 (Thermo Fisher Scientific). Equal amounts (100
μg) of protein from each extract were separated by a 12%
SDS-PAGE and then electrotransferred onto a polyvinylidene
difluoride membrane for 1 h at 300 mA. After blocking with 5%
non-fat milk in Tris-buffered saline containing Tween 20 for 1 h at
room temperature, blots were incubated with phosphor (p)-SAPK/JNK
(Thr183/Tyr185) rabbit monoclonal antibody (1:1,000 dilution; cat
no. 4671; Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-MAP3K9 rabbit polyclonal antibody (1:500 dilution; cat no.
bs-10424R; Bioss, Shanghai, China) or anti-β-tubulin mouse
monoclonal antibody (1:1,000 dilution; cat no. HC101; Transgen,
Beijing, China) overnight at 4°C. Following incubation with the
corresponding secondary antibody, horseradish peroxidase-conjugated
goat anti-mouse immunoglobulin (Ig)G (1:2,000 dilution; cat. no.
SA00001-1; Proteintech, Chicago, IL, USA) or goat anti-rabbit IgG
(1:2,000 dilution; cat. no. SA00001-2; Proteintech), respectively,
at room temperature for 1 h, the protein bands were visualized
using an Immobilon Western Chemiluminescent HRP Substrate kit (cat.
no. WBKLS0100; Millipore, Billerica, MA, USA) and a ChemiDoc
XRS+ Imaging system (Bio-Rad Laboratories, Inc.). Grey
value analysis of protein bands was performed for quantification of
the protein levels using ImageJ software version 1 (National
Institutes of Health, Bethesda, MD, USA).
Cell counting kit-8 (CCK-8) assay
786-O and OS-RC-2 cells (2×104 cells per
well) were seeded onto 96-well plates. After 24 h of incubation,
786-O and OS-RC-2 cells were co-transfected with miR-148b mimics or
control mimics. At 48 h after transfection, the proliferation of
786-O and OS-RC-2 cells was determined using the CCK-8 (Beyotime
Institute of Biotechnology) with 10 μl CCK-8 stain added per
well according to the manufacturer's instructions. The apparatus
used for colorimetric analysis was the Bio-Tek Synergy 2 microplate
reader (Bio-Tek Instruments, Inc., Winooski, VT, USA), at a
wavelength of 450 nm.
5-Ethynyl-2′-deoxyuridine (EdU)
assay
The EdU assay, which is based on the determination
of DNA replication activity (23),
was used to quantify cell proliferation in the present study. 786-O
and OS-RC-2 cells (1×105 cells per well) were seeded
onto 24-well plates. After 24 h of incubation, 786-O and OS-RC-2
cells were co-transfected with miR-148b mimics or control mimics.
At 48 h after transfection, the proliferation of 786-O and OS-RC-2
cells was determined using the EdU DNA Proliferation Detection kit
(RiboBio) according to the manufacturer's instructions and DAPI
reagent (Sangon Biotech Co., Ltd., Shanghai, China). The nuclei
were photographed using fluorescence microscopy (Eclipse TE300;
Nikon, Melville, NY, USA).
Cell cycle analysis
786-O and OS-RC-2 cells (5×105 cells per
well) were seeded into six-well plates. Following complete
attachment, cells were transfected with miR-148b mimics or NC
mimics. After 48 h, cells were harvested, washed three times with
cold phosphate-buffered saline and fixed in cold 75% ethanol for at
least 8 h at 4°C. Cells were then treated with RNase A (Sangon
Biotech Co., Ltd.) and stained with propidium iodide (PI; Sangon
Biotech Co., Ltd.) followed by flow cytometric analysis using a
FACSVantage SE flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA).
Apoptosis assay
The FITC Annexin V Apoptosis Detection kit I (BD
Pharmingen, Franklin Lakes, NJ, USA) was used for determination of
apoptotic rates. 786-O and OS-RC-2 cells (5×105 cells
per well) were seeded into six-well plates. Following complete
attachment, cells were transfected with miR-148b mimics (50 nM) or
NC mimics (50 nM). After 48 h of incubation, cells were harvested,
re-suspended in 1X binding buffer and incubated with 5 μl of
fluorescein isothiocyanate-conjugated Annexin V and 5 μl PI
for 15 min at 25°C in the dark. Flow cytometric analysis was
performed within 1 h.
Statistical analysis
All statistical analyses were performed using
Graphpad Prism 5 software (GraphPad Inc., La Jolla, CA, USA). All
quantitative values are expressed as the mean ± standard deviation.
One-way analysis of variance was performed for comparisons between
groups. All other results are representative of three independent
experiments. P<0.05 was considered to indicate a statistically
significant difference between values.
Results
miR-148b is downregulated in renal cancer
tissues and renal cancer cell lines
To determine the potential significance of miR-148b
in renal cancer, the present study determined the expression levels
of miR-148b in renal cancer tissues and cell lines. RT-qPCR
analysis revealed that miR-148b was down-regulated in 12 renal
cancer tissues compared with that in their paired adjacent
non-tumorous tissues (Fig. 1A), as
well as in the 786-O and OS-RC-2 renal cancer cell lines compared
with that in the 293T normal renal cell line (Fig. 1B). These results suggested that
miR148b was downregulated in renal cancer tissues and renal cancer
cell lines.
Overexpression of miR-148b does not
affect the total number of viable renal cancer cells
To explore the effects of miR-148b in renal cell
carcinoma, 786-O and OS-RC-2 cells were transfected with miR-148b
mimics or negative control mimics and following 48 h, cell
viability was assessed using the CCK-8 kit. As shown in Fig. 2A, miR-148b expression was
significantly increased at 48 h post-transfection of miR-148b
mimics However, no significant effect of miR-148b transfection on
the number of viable cells was observed (Fig. 2B). These results suggested that
overexpression of miR-148b had no effect on the total number of
viable renal cancer cells.
Overexpression of miR-148b promotes
proliferation of renal cancer cells
To examine effect of miR-148b on renal cancer cell
proliferation, cells were stained with by EdU and DAPI at 48 h
post-transfection with miR-148b and evaluated by fluorescence
microscopy. As demonstrated in Fig.
3A, overexpression of miR-148b significantly promoted DNA
replication activity in 786-O and OS-RC-2 cells. Furthermore, cell
cycle analysis illustrated that the cell population in G0/G1 phase
cells was decreased, that in S-phase was significantly increased in
786-O and OS-RC-2 cells at 48 h after transfection with miR-148b
mimics (Fig. 3B). These results
indicated that the DNA replication activity of renal cancer cells
was enhanced by miR148b, and that the percentage of cells
undergoing DNA synthesis in S-phase was increased. These results
led to the conclusion that overexpression of miR-148b promoted the
proliferation of 786-O and OS-RC-2 cells.
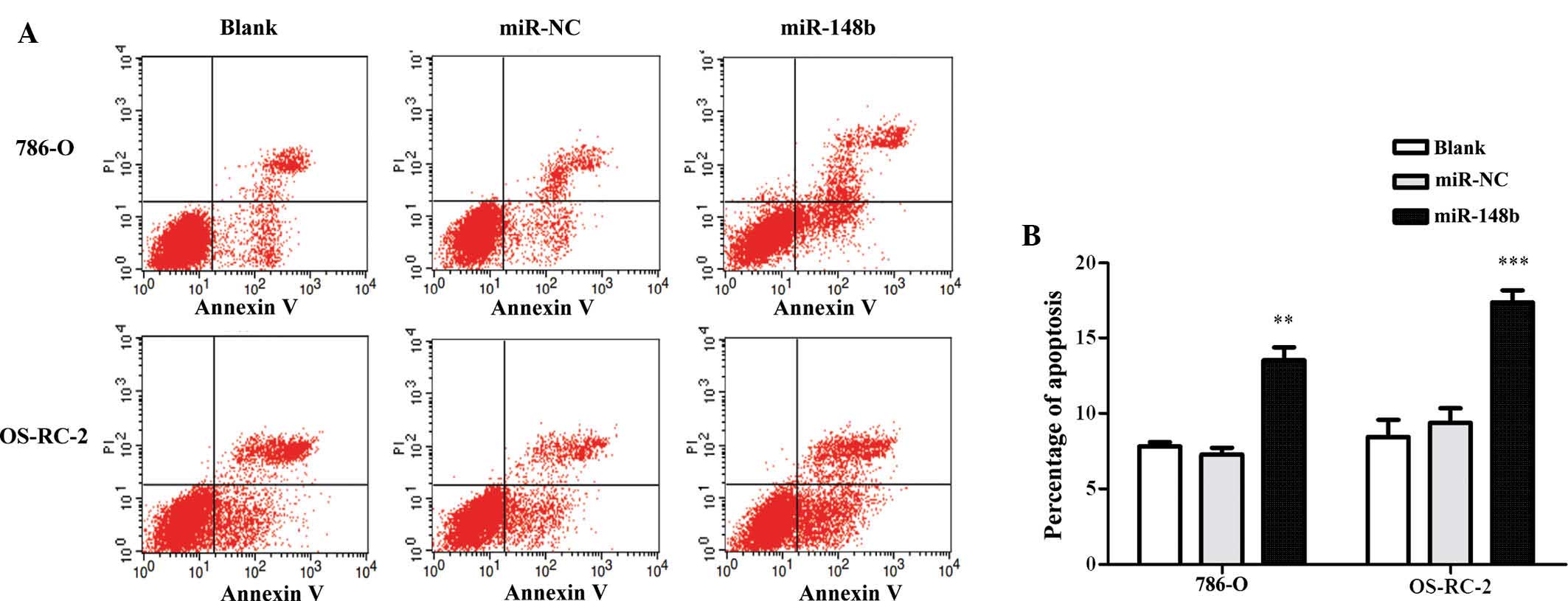
miR148b enhances the apoptotic rate of
renal cancer cells
To estimate whether miR148b can affect the apoptosis
of renal cancer cells, Annexin V/PI double staining and flow
cytometric analysis were performed. It was revealed that miR148b
enhanced the apoptotic rate of renal cancer cells (Fig. 4A and B). While miR-148b promoted
cell proliferation, it also enhanced the apoptotic rate of 786-O
and OS-RC-2, while the total number of viable cells was
retained.
miR-148b inhibits MAPK/JNK signaling by
directly targeting MAP3K9 in renal cancer cells
To identify the signaling pathway via which miR-148b
exerts its effects in renal cell carcinoma, the present study
performed a luciferase reporter assay, which included reporter
plasmids for Wnt, TGF-β and MAPK. The results indicated that the
MAPK signaling pathway was regulated by miR-148b (Fig. 5A). To identify which gene upstream
of the MAPK signaling pathway was regulated by miR-148b,
TargetScan, which is a bioinformatics tool for miRNA target
screening, was used to. The 3′UTR of human MAP3K9, which is an
upstream activator of the MAPK/JNK signaling pathway, was shown to
contain a putative target site for miR-148b, which is highly
conserved across species (Fig.
5B). To experimentally verify that MAP3K9 is a direct target of
miR-148b, a luciferase assay was performed in 293T cells, which
revealed a significant decrease in luciferase expression in the
group co-transfected with the wild-type 3′UTR-containing reporter
plasmid and miR-148b mimics, while the mutation in the putative
binding site in the 3′UTR of MAP3K9 prevented the inhibitory
effects of miR-148b on luciferase expression (Fig. 5C). This result demonstrated that
miR-148b directly bound to the 3′UTR of MAP3K9. Furthermore,
western blot analysis revealed that overexpression of miR-148b
markedly decreased the protein expression of MAP3K9 in renal cancer
cells (Fig. 5D), while the mRNA
levels were not affected according to RT-qPCR (Fig. 5E).
 | Figure 5miR-148b reduces MAPK/JNK signaling by
targeting MAP3K9 in renal cancer cell lines. (A) Dual luciferase
assays were performed in 293T cells at 48 h after transfection with
miR-148b/control mimics and signaling-pathway reporter plasmids
[TopFlash/(CAGA)12-LUC/pFA-Elk1, pFR-Luc]. **P<0.01
vs. miR-NC group. (B) Schematic representation illustrating that
the 3′UTR of MAP3K9 contains a putative target site for miR-148b,
which is highly conserved across species. (C) 293T cells were
transiently transfected with luciferase reporter plasmids
containing the wild-type MAP3K9 3′UTR or the MAP3K9 3′UTR with a
mutation in the predicted miR-148b target sequence, together with
miR-148b or NC mimics. pRL-SV40 was included as an internal
control. Following 48 h of incubation, cells were collected and
luciferase activity was assayed. **P<0.01,
***P<0.001 vs. NC- and MAP3K9 3′UTR-transfected
group. (D) Reverse-transcription polymerase chain reaction analysis
of MAP3K9 mRNA expression in 786-0 and OS-RC-2 cells at 48 h after
transfection with miR-148b or NC mimics, which was quantified using
the 2−ΔΔCT method. (E) Western blot analysis of MAP3K9
protein expression in 786-0 and OS-RC-2 cells at 48 h after
transfection with miR-148b or control mimics. A western blot
representative of three independent experiments is shown. Protein
bands were quantified by densitometric grey value analysis.
*P<0.05, **P<0.01 vs. blank/NC groups.
The blank group was treated with transfection regent and distilled
water at the same volume. Values are expressed as the mean ±
standard deviation. NC, negative control; miR, microRNA; UTR,
untranslated region; MAPK9, mitogen-activated protein kinase kinase
kinase 9; hsa, Homo sapiens; MUT mutated. |
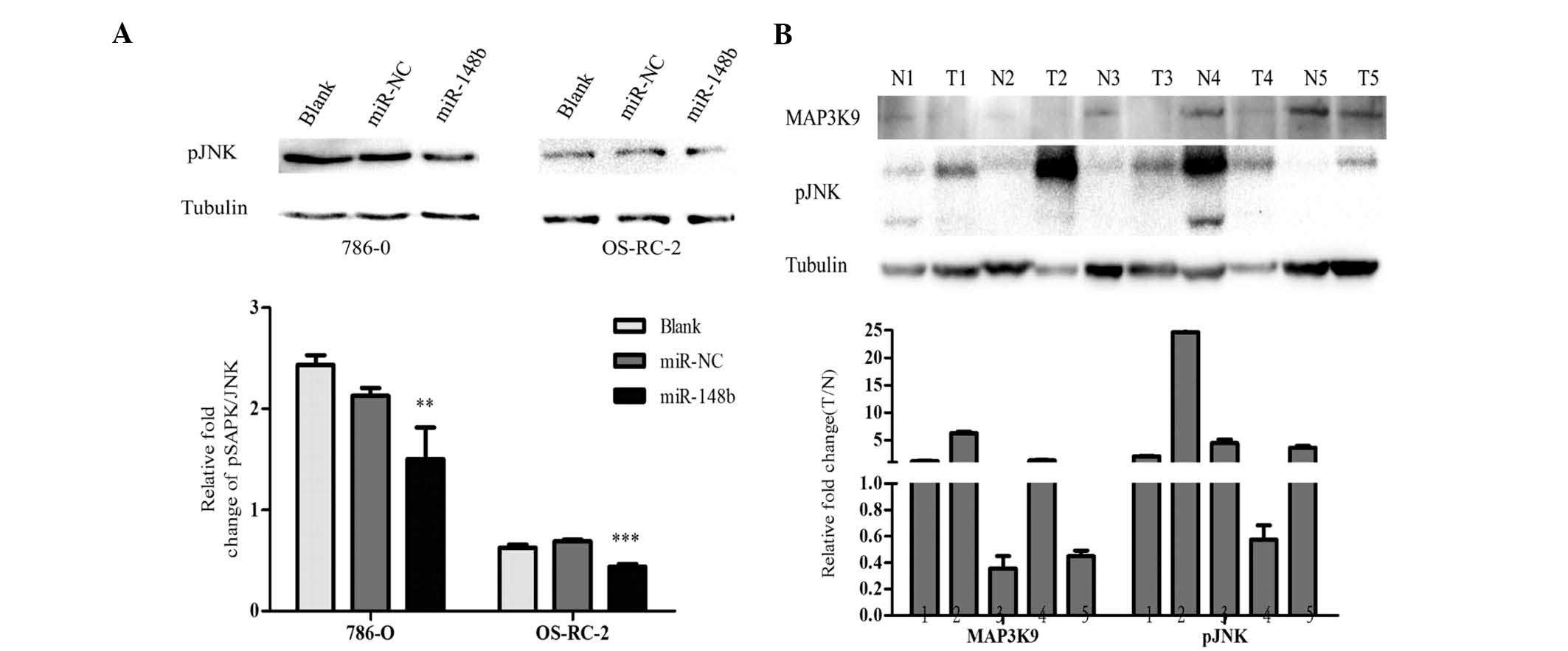
To further elucidate the mechanism by which miR-148
affects the MAPK pathway, the present study examined the protein
expression of p-SAPK/JNK, which is a downstream signaling protein
of MAP3K9, at 48 h after transfection with miR-148b mimics. As
shown in Fig. 6A, the
phosphorylation level of JNK was significantly decreased by
miR-148b. These results indicated that miR-148b regulates MAPK/JNK
signaling via MAP3K9 post-transcriptionally.
miR-148b is upregulated, while MAP3K9 and
pJNK are down- regulated in renal cancer tissues
The present study evaluated the expression of
miR148b, MAP3K9 and pJNK in tissues from five patients with renal
cancer, revealing that miR148b was upregulated in these renal
cancer tissues compared with that in their paired adjacent
non-tumorous tissues, while MAP3K9 and pJNK were downregulated
(Fig. 6B). These results indicated
an inverse correlation between miR-148b and MAP3K9 and a positive
correlation between the expression of MAP3K9 and JNK.
Discussion
Tumorigenesis is a complex process which is
frequently accompanied with the aberrant expression of miRNAs.
miRNAs may serve as diagnostic tumor biomarkers, indicators of
tumor prognosis and targets for therapy (24). Downregulation of miR-148b has been
identified in various types of human cancer and is therefore
considered to be a tumor-suppressive miRNA (15–20).
miR-148b was demonstrated to inhibit cell proliferation and
invasion, suppress the progression of cancer and to induce
apoptosis. However, the potential function of miR-148b in human
renal cell carcinoma has remained elusive. The present study
detected miR-148b in renal cancer tissues and cell lines and
assessed the effects of miR-148b in the 786-O and OS-RC-2 renal
cancer cell lines as well as the underlying mechanisms.
The present study revealed that miR-148b was
significantly downregulated in renal cell carcinoma; furthermore,
overexpression of miR-148b enhanced the proliferation and apoptosis
of renal cancer cells, while not affecting the total number of
viable cells. It was therefore indicated that following 48 h of
transfection, miR-148b mimics enhanced the proliferation and
apoptosis of renal cancer cell growth to a similar extent.
Furthermore, the present study confirmed that miR-148b exerts
effects via inhibiting the MAPK signaling pathway in renal cancer
cells with the upstream MAP3K9 being the direct target of miR-148b
as indicated by a luciferase reporter assay, RT-qPCR and western
blot analysis. MAP3K9, also known as MLK1, is an essential
component of the MAPK/JNK signal transduction pathway (25); once activated, MAP3K9 acts as an
upstream activator of the MKK/JNK signal transduction cascade
through phosphorylating MAP2K4/MKK4 and MAP2K7/MKK7, which in turn
activates the JNKs (26). It was
previously reported that MLK1/MLK2 deficiency did not impair kidney
development and function, and extensive functional redundancy
between MLK1/MLK2 and MLK3 was present (27). The present study indicated that
miR-148b inhibited JNK activation by targeting MLK1. JNK signaling
represents a double-edged sword in the malignant transformation and
tumorigenesis of cells, as it exerts either pro- or anti-apoptotic
effects (28). The present study
demonstrated that miR-148b targeted MAP3K9 and simultaneously
increases apoptosis as well as proliferation and associated DNA
synthesis, thereby indicating that miR-148b has an oncogenic
function by upregulating a variety of cellular processes, while
keeping the number of viable cells in a balance. This result may
enhance the current understanding of miRNA-dependent regulation of
gene expression in regulating proliferation and apoptosis in renal
cancer. However, the present study only assessed the effects of
miR-148b upregulation in renal cancer cells at 48 h following
transfection; further evaluation is required in order to reveal the
time-dependent effects of miR-148b on proliferation and apoptosis
as well as MAPK/JNK signaling in renal cancer cells. In human renal
cancer cells, JNK may have complex roles and crosstalk with other
pathways may exist, and the exact mechanisms require further
elucidation.
In addition, the present study revealed that
detected that miR-148b was overexpressed in renal cancer tissues,
while MAP3K9 and pJNK levels were downregulated compared to those
in normal adjacent tissues. An inverse correlation between the
expression of miR-148b and MAP3K9, and a positive correlation
between the expression of MAP3K9 and JNK activation was
indicated.
In conclusion, the present study revealed that
miR-148b was upregulated in renal cancer cells and upregulates
proliferation and apoptosis. miR-148b significantly decreased the
G0/G1-phase population and increased the S-phase population and
enhanced DNA synthesis in renal cancer cells. miR-148b was revealed
to directly target the 3′UTR of MAP3K9, which inhibits the JNK
pathway. The present study demonstrated that miR-148b exerts an
oncogenic function in human renal cancer and revealed the
underlying mechanisms of its stimulatory effects on proliferation
and apoptosis by inhibiting the MAPK/JNK pathway. miR-148b may be
involved in the progression of renal cancer and represents a
potential biomarker and target for the treatment of renal cancer.
To assess the prognostic value of miR-148b, future studies will
focus on detecting the variety of changes occurring to cellular
processes following knockdown of miR-148b expression, or on
examining the JNK signaling pathway using an inhibitor to
investigate the biological mechanisms underlying renal cancer cell
function.
Acknowledgments
The authors sincerely acknowledge the help of their
laboratory colleagues and thank all members of the Core Facility of
Genetically Engineered Mice, West China Hospital, West China
Medical School, Sichuan University (Chengdu, China) for their
assistance in this study. This work was supported by the National
Basic Research Program of China (grant no. 2011CB944002 to QZ) and
the National Natural Science Foundation of China (grant no.
31271563 to QZ).
References
|
1
|
Falebita OA, Mancini S, Kiely E and Comber
H: Rising incidence of renal cell carcinoma in Ireland. Int Urol
Nephrol. 41:7–12. 2009. View Article : Google Scholar
|
|
2
|
Störkel S and van den Berg E:
Morphological classification of renal cancer. World J Urol.
13:153–158. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bukowski RM, Negrier S and Elson P:
Prognostic factors in patients with advanced renal cell carcinoma:
Development of an international kidney cancer working group. Clin
Cancer Res. 10:S6310–S6314. 2004. View Article : Google Scholar
|
|
4
|
Heinzelmann J, Henning B, Sanjmyatav J,
Posorski N, Steiner T, Wunderlich H, Gajda MR and Junker K:
Specific miRNA signatures are associated with metastasis and poor
prognosis in clear cell renal cell carcinoma. World J Urol.
29:367–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
An J, Liu H, Magyar CE, Guo Y, Veena MS,
Srivatsan ES, Huang J and Rettig MB: Hyperactivated JNK is a
therapeutic target in pVHL-deficient renal cell carcinoma. Cancer
Res. 73:1374–1385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeng L, Bai M, Mittal AK, El-Jouni W, Zhou
J, Cohen DM, Zhou MI and Cohen HT: Candidate tumor suppressor and
pVHL partner Jade-1 binds and inhibits AKT in renal cell carcinoma.
Cancer Res. 73:5371–5380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pal SK, He M, Tong T, Wu H, Liu X, Lau C,
Wang JH, Warden C, Wu X, Signoretti S, et al: RNA-seq reveals
aurora kinase driven-mTOR pathway activation in patients with
sarcomatoid metastatic renal cell carcinoma. Mol Cancer Res.
13:130–137. 2015. View Article : Google Scholar
|
|
8
|
Von Schulz-Hausmann SA, Schmeel LC,
Schmeel FC and Schmidt-Wolf IG: Targeting the Wnt/beta-catenin
pathway in renal cell carcinoma. Anticancer Res. 34:4101–4108.
2014.PubMed/NCBI
|
|
9
|
Schütte U, Bisht S, Heukamp LC, Kebschull
M, Florin A, Haarmann J, Hoffmann P, Bendas G, Buettner R, Brossart
P and Feldmann G: Hippo signaling mediates proliferation,
invasiveness, and metastatic potential of clear cell renal cell
carcinoma. Transl Oncol. 7:309–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang D, Ding Y, Luo WM, Bender S, Qian
CN, Kort E, Zhang ZF, VandenBeldt K, Duesbery NS, Resau JH and Teh
BT: Inhibition of MAPK kinase signaling pathways suppressed renal
cell carcinoma growth and angiogenesis in vivo. Cancer Res.
68:81–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhan YH, Liu J, Qu XJ, Hou KZ, Wang KF,
Liu YP and Wu B: β-Elemene induces apoptosis in human renal-cell
carcinoma 786-0 cells through inhibition of MAPK/ERK and
PI3K/Akt/mTOR signalling pathways. Asian Pac J Cancer Prev.
13:2739–2744. 2012. View Article : Google Scholar
|
|
12
|
Du HF, Ou LP, Song XD, Fan YR, Yang X, Tan
B, Quan Z, Luo CL and Wu XH: Nuclear factor-kB signaling pathway is
involved in phospholipase Cε-regulated proliferation in human renal
cell carcinoma cells. Mol Cell Biochem. 389:265–275. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Munshi A and Ramesh R: Mitogen-activated
protein kinases and their role in radiation response. Genes Cancer.
4:401–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J and Lin A: Role of JNK activation in
apoptosis: A double-edged sword. Cell Res. 15:36–42. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cimino D, De Pittà C, Orso F, Zampini M,
Casara S, Penna E, Quaglino E, Forni M, Damasco C, Pinatel E, et
al: miR148b is a major coordinator of breast cancer progression in
a relapse-associated microRNA signature by targeting ITGA5, ROCK1,
PIK3CA, NRAS and CSF1. FASEB J. 27:1223–1235. 2013. View Article : Google Scholar
|
|
16
|
Liu GL, Liu X, Lv XB, Wang XP, Fang XS and
Sang Y: miR-148b functions as a tumor suppressor in non-small cell
lung cancer by targeting carcinoembryonic antigen (CEA). Int J Clin
Exp Med. 7:1990–1999. 2014.PubMed/NCBI
|
|
17
|
Zhang Z, Zheng W and Hai J: MicroRNA-148b
expression is decreased in hepatocellular carcinoma and associated
with prognosis. Med Oncol. 31:9842014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao G, Zhang JG, Liu Y, Qin Q, Wang B,
Tian K, Liu L, Li X, Niu Y, Deng SC and Wang CY: miR-148b functions
as a tumor suppressor in pancreatic cancer by targeting AMPKα1. Mol
Cancer Ther. 12:83–93. 2013. View Article : Google Scholar
|
|
19
|
Song YX, Yue ZY, Wang ZN, Xu YY, Luo Y, Xu
HM, Zhang X, Jiang L, Xing CZ and Zhang Y: MicroRNA-148b is
frequently down-regulated in gastric cancer and acts as a tumor
suppressor by inhibiting cell proliferation. Mol Cancer. 10:12011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song Y, Xu Y, Wang Z, Chen Y, Yue Z, Gao
P, Xing C and Xu H: MicroRNA-148b suppresses cell growth by
targeting cholecystokinin-2 receptor in colorectal cancer. Int J
Cancer. 131:1042–1051. 2012. View Article : Google Scholar
|
|
21
|
Fawdar S, Trotter EW, Li Y, Stephenson NL,
Hanke F, Marusiak AA, Edwards ZC, Ientile S, Waszkowycz B, Miller
CJ and Brognard J: Targeted genetic dependency screen facilitates
identification of actionable mutations in FGFR4, MAP3K9, and PAK5
in lung cancer. Proc Natl Acad Sci USA. 110:12426–12431. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ribeiro J, Marinho-Dias J, Monteiro P,
Loureiro J, Baldaque I, Medeiros R and Sousa H: miR-34a and
miR-125b expression in HPV infection and cervical cancer
development. Biomed Res Int. 2015:3045842015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lyu Z, Mao Z, Wang H, Fang Y, Chen T, Wan
Q, Wang M, Wang N, Xiao J, Wei H, et al: MiR-181b targets Six2 and
inhibits the proliferation of metanephric mesenchymal cells in
vitro. Biochem Biophys Res Commun. 440:495–501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grange C, Collino F, Tapparo M and Camussi
G: Oncogenic micro-RNAs and renal cell carcinoma. Front Oncol.
4:492014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Durkin JT, Holskin BP, Kopec KK, Reed MS,
Spais CM, Steffy BM, Gessner G, Angeles TS, Pohl J, Ator MA and
Meyer SL: Phosphoregulation of mixed-lineage kinase 1 activity by
multiple phosphorylation in the activation loop. Biochemistry.
43:16348–16355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stronach B, Lennox AL and Garlena RA:
Domain specificity of MAP3K family members, MLK and Tak1, for JNK
signaling in drosophila. Genetics. 197:497–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bisson N, Tremblay M, Robinson F, Kaplan
DR, Trusko SP and Moss T: Mice lacking both mixed-lineage kinase
genes Mlk1 and Mlk2 retain a wild type phenotype. Cell Cycle.
7:909–916. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dhanasekaran DN and Reddy EP: JNK
signaling in apoptosis. Oncogene. 27:6245–6251. 2008. View Article : Google Scholar : PubMed/NCBI
|