Introduction
Consumption of carbonated soft drinks is high in
Saudi Arabia, particularly in middle-aged individuals aged between
35–50 years old. The effects of these products on health are
unclear, although epidemiological studies have suggested their
association with obesity, kidney disease, liver disease and
osteoporosis (1,2). They predominantly consist of water
but also commonly contain phosphoric acid, caffeine, sugar and
chemicals in the form of colorings, flavors, preservatives and
sweeteners. The rate of consumption of these drinks is particularly
high in affluent countries (1).
The majority of individuals view carbonated soft
drink consumption as fairly innocuous (1). However, there are a number of serious
health issues associated with regular consumption of carbonated
soft drinks, for example, previous peer-reviewed studies have
reported 25 separate harmful effects, including osteoporosis, and
kidney and liver disease (2,3).
Carbonated soft drinks contain several compounds including
caffeine; which is the most widely consumed behaviorally active
substance worldwide. Almost all caffeine comes from dietary sources
(4). Acute and chronic caffeine
intake appear to have only minor negative consequences on health
(5). For this reason and because
few caffeine users report loss of control over their caffeine
intake, governmental regulatory agencies impose no restrictions on
its use. In the majority of carbonated beverages, caffeine is
deliberately added to make it addictive. However, caffeine in
carbonated drinks is more readily absorbed than that from other
non-carbonated beverages. The majority of carbonated soft drinks
also contain phosphoric acid, caffeine, sugar or aspartame or
saccharin, caramel coloring, carbon dioxide, and aluminum. Each of
which have been demonstrated to have negative effects on human
health (5).
Caffeine is known to be an addictive drug that has
the ability to stimulate mental alertness, overcome fatigue and
enhance endurance. Caffeine acts by blocking adenosine
(neurotransmitter) receptor sites in the central nervous system,
and adenosine generally exhibits a depressant action in the brain,
heart and kidneys. The resultant stimulation is accompanied by
constriction of the cerebral arteries, elevated heartbeat, high
blood pressure and excessive excretion of urine. Cases of
caffeine-associated fatalities and seizures have previously been
identified (4,5) due to a combination of excess caffeine
intake and cardiovascular disorders. Moreover, several studies have
reported a weaker compensatory response after consumption of
caloric liquids (6,7). Previous studies have examined the
effect of energy intake on brain histology and activity (6–9). It
has been reported that soda exhibited an adverse effect on the
cerebellum, whereas non-diet soda exhibited harmful effects
(10).
In the Middle East, particularly in Saudi Arabia, it
is common for individuals to consume carbonated soft drinks 3 times
per day with each meal. Therefore, the current study was conducted
to examine the effect of chronic consumption of three common drinks
in Saudi Arabia (Cola, Pepsi and 7-UP) on oxidative stress,
antioxidant levels, aggression markers, and histopathology of the
brain to outline their potential effects on the brains of Wistar
rats. In addition, the effect of soft beverages on the expression
and activity of certain genes associated with anxiety, violence
and/or aggression, such as monoamine oxidase (MAO) and dopamine D2
receptors (DD2R) were examined.
Materials and methods
Chemicals and kits
Ethidium bromide, agarose, Mayer's hematoxylin and
eosin (H&E) and Tris-Borate-EDTA (TBE) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The Wistar albino rats were
purchased from the King Fahd Center for Scientific Research, King
Abdel-Aziz University (Jeddah, Saudi Arabia). Serologic kits for
catalase, malondialdehyde (MDA), glutathione reductase (GR) and
glutathione peroxidase (GPx) were purchased from Bio-diagnostic
Co., (Giza, Egypt). Cola (Atlanta, GA, Pepsi (PepsiCo, Purchase,
NY, USA) and 7-UP (Dr Pepper Snapple Group, Inc., Plano, TX, USA)
were used. DNA 100 bp ladder was purchased from MBI, Fermentas,
Thermo Fisher Scientific. Inc. (Waltham, MA, USA). Qiazol for RNA
extraction and oligo dT primers were purchased from Qiagen, Inc.,
(Valencia, CA, USA).
Animals, experimental design and
sampling
All animal procedures were approved by the Ethical
Committee Office of Taif University (Taif, Saudi Arabia). Forty
male Wistar rats (age, 3 months; weight, 200–280 g) were used for
this study. For acclimatization, animals were handled daily and
kept under observation for 1 week prior to the onset of the
experiment. The animals were kept under a 12-h light-dark cycle and
had ad libitum access to food and water. Animals were
divided into the following 4 groups: Control group (CNT) without
any treatment; Cola group; Pepsi group and 7-UP group. Groups 2–4
received free access to food and only carbonated soft drinks for 3
consecutive months. At the end of the 3 months, all rats were
anesthetized using diethyl ether inhalation and sacrificed via
decapitation. Blood was collected in vacuteiner tubes from
retro-orbital venous plexuses following anesthetization. Brain
tissues from the right hemisphere were harvested for gene
expression and left hemisphere tissues were used for
histopathological analyses. Serum was extracted after blood
centrifugation for 10 min at 4,000 × g. For gene expression
analysis, brain tissues were kept in QIAzol reagent at −80°C for
RNA extraction and in 10% neutral buffered formalin (NBF) at room
temperature for 24 h for histopathological and immunohistochemical
analysis.
Serum chemistry assays
Catalase, GR, GP and MDA were measured using
commercial spectrophotometric analysis kits (Bio-Diagnostic
Company, Giza, Egypt). MAO and acetylcholine esterase (AChE) levels
were measured using commercial enzyme-linked immunosorbent assay
kits obtained from MyBioSource, Co. (San Diego, CA, USA). All
procedures were conducted according to the manufacturer's protocol.
mRNA expression levels of glutathione-S-transferase (GST) and
5-hydroxy tryptamine transporter (5-HTT) were assessed using
reverse transcription-polymerase chain reaction (RT-PCR)
analysis.
Gene expression analysis
Total RNA was extracted from the brain tissue
samples as previously described (11). RNA concentration and purity were
determined spectrophoto-metrically after measuring the optical
density at 260 and 280 nm using a SmartSpec Plus spectrophotometer
(Bio-Rad, Hercules, CA, USA). The RNA integrity was confirmed after
running in 1.5% denatured agarose gel stained with ethidium
bromide. A mixture of 3 µg total RNA and 0.5 ng oligo dT
primer (Qiagen Inc., Valencia, CA, USA) were used for cDNA
synthesis in a total volume of 11 µl sterilized
diethylpyrocarbonate (DEPC) water and was incubated in the Bio-Rad
T100 Thermal cycler (Bio-Rad) at 65°C for 10 min for denaturation.
Then, 2 µl of 10X RT-buffer, 2 µl of 10 mM dNTPs and
100 units Moloney Murine Leukemia Virus Reverse Transcriptase
(SibEnzyme. Ak, Novosibirsk, Russia) were added and made up to a
total volume of 20 µl with DEPC water. The mixture was then
re-incubated in the thermal cycler at 37°C for 1 h, then at 90°C
for 10 min to inactivate the enzyme. For semi-quantitative RT-PCR
analysis, specific primers for examined genes (Table I) were designed using the Oligo-4
computer program (version 7; Molecular Biology Insights, Colorado
Springs, CO, USA) and synthesized by Macrogen (Macrogen Inc.,
Gasadong, Korea). PCR was conducted in a final volume of 25
µl consisting of 1 µl cDNA, 1 µl of 10 pM of
each primer (forward and reverse), and 12.5 µl PCR master
mix (Promega Corporation, Madison, WI, USA), the volume was made up
to 25/µl using sterilized deionized water. PCR was conducted
using the Bio-Rad T100 Thermal Cycler with the following cycle
sequence: 94°C for 5 min for one cycle, followed by 27–31 cycles
(Table I) each of which consisted
of denaturation at 94°C for 1 min, annealing at the specific
temperature corresponding to each primer (Table I) and extension at 72°C for 1 min
with an additional final extension at 72°C for 7 min. As a
reference, expression of glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) mRNA was examined (Table
I). PCR products were visualized under UV light after
electrophoresis on 1.5% agarose (Bio Basic Int., Markham, ON,
Canada) gel stained with ethidium bromide in TBE buffer. PCR
products were confirmed using a 100 bp DNA ladder and were
subsequently photographed using an InGenius 3.0 gel documentation
system (Syngene, Frederick, MD, USA). The intensities of the bands
were quantified densitometrically using Image J software version
1.47 (http://imagej.en.softonic.com/).
 | Table IPolymerase chain reaction conditions
and primers sequence of examined genes. |
Table I
Polymerase chain reaction conditions
and primers sequence of examined genes.
| Gene | Product size
(bp) | Annealing | Direction | Sequence
(5′-3′) |
|---|
| GST | 570 | 55 | Forward |
GCTGGAGTGGAGTTTGAAGAA |
| | | Reverse |
GTCCTGACCACGTCAACATAG |
| GPx | 406 | 57 | Forward |
AAGGTGCTGCTCATTGAGAATG |
| | | Reverse |
CGTCTGGACCTACCAGGAACTT |
| MAOA | 492 | 60 | Forward |
ATGGATGAAATGGGAAAAGAGAT |
| | | Reverse |
TAATTGGTTTCTCTCAGGTGGAA |
| AChE | 785 | 55 | Forward |
GACTGCCTTTATCTTAATGTG |
| | | Reverse |
CGGCTGATGAGAGATTCATTG |
| DD2R | 488 | 55 | Forward |
CCTGAGGACATGAAACTCTGC |
| | | Reverse |
TAGAGGACTGGTGGGATGTTG |
| 5-HTT | 448 | 55 | Forward |
CTCTGGCTTCGTCATCTTCAC |
| | | Reverse |
GCTGCAGAACTGAGTGATTCC |
| GAPDH | 309 | 52 | Forward |
AGATCCACAACGGATACATT |
| | | Reverse |
TCCCTCAAGATTGTCAGCAA |
Brain histopathology
Brain was removed following diethyl ether inhalation
and sacrifice of the rats and fixed overnight in a 10% NBF
solution. Fixed brain tissues were processed routinely, washed and
preserved in 70% ethanol, dehydrated in ascending grades of ethanol
solution, cleared in xylene, embedded in paraffin wax, pressed and
cut into 5-µm sections. Subsequently, the sections were
placed on top of glass slides. The slides were stained with Mayer's
H&E (12). Tissue slides were
visualized using a Wolfe S9–0982 microscope (Carolina Biological
Supply Co., Burlington, NC, USA) and photos were captured using a
Canon Power-Shot SX500 IS digital camera (Canon, Tokyo, Japan).
Statistical analysis
Results are presented as the mean ± standard error
of mean. Data were analyzed using analysis of variance and Fisher
post hoc descriptive tests using SPSS software version 11.5 (SPSS,
Inc., Chicago, IL, USA). Regression analysis was performed using
the same software. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of carbonated soft drink
consumption for 3 months on serum levels of MDA, GR, GPx and
catalase in Wistar rats
Consumption of Cola, Pepsi and 7-UP for 3 months
showed a significant increase in MDA levels (Fig. 1A; P<0.05) with the greatest
increase in the rats from the Pepsi group. In parallel, the levels
of antioxidants GR, GPx and catalase in the rats were decreased
significantly in all groups administered carbonated soft drinks
compared with the control. Notably, the greatest changes were
observed in the rats from the Pepsi group (Fig. 1B–D).
Effect of carbonated soft drink
consumption for 3 months on mRNA expression of GST and GPx in the
brain tissues of Wistar rats
As shown in Fig. 2,
carbonated soft drink consumption for 3 months downregulated the
mRNA expression of GST and GPx. Expression levels were
significantly decreased by 50 and 40% in the Cola and Pepsi groups
for GST and GPx, respectively (P<0.05). Although still
significantly reduced when compared with the control (P<0.05),
rats in the 7-UP group demonstrated increased levels of GST and
GPx, as compared with the Cola and Pepsi groups.
Effect of carbonated soft drink
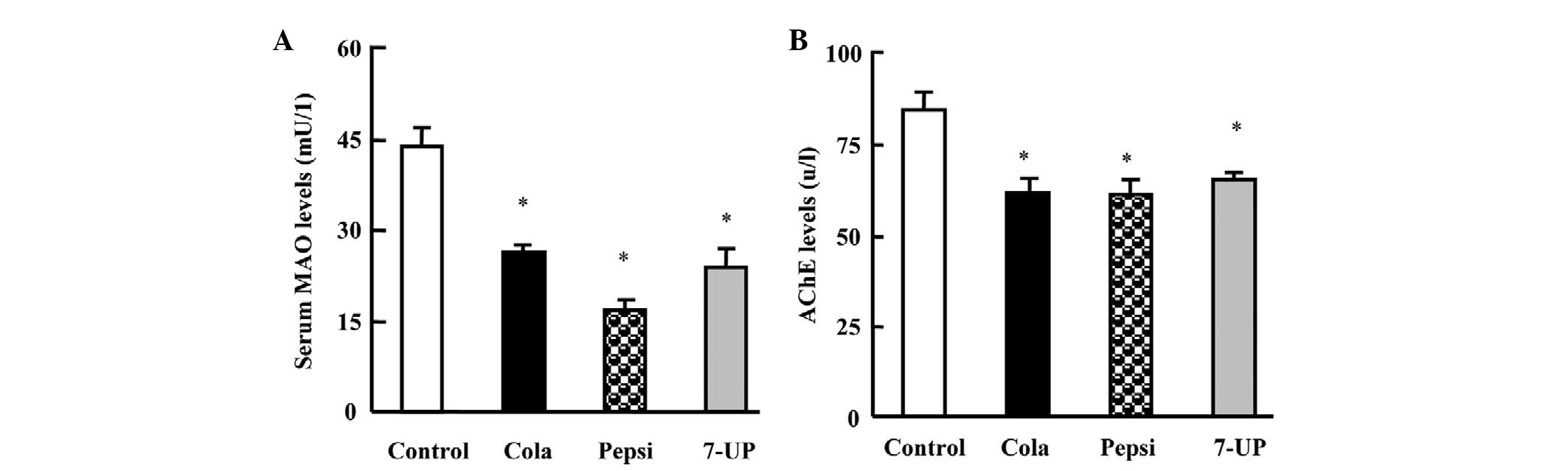
consumption for 3 months on serum levels of MAO-A and AChE in
Wistar rats
Next, the changes in MAO-A and AChE levels (Fig. 3) were examined. Cola, Pepsi and
7-UP consumption for 3 months resulted in a significant decrease in
MAO and AChE levels (P<0.05). Rats in the Pepsi group exhibited
the greatest decreases in MAO-A and AChE levels, as compared with
the other groups.
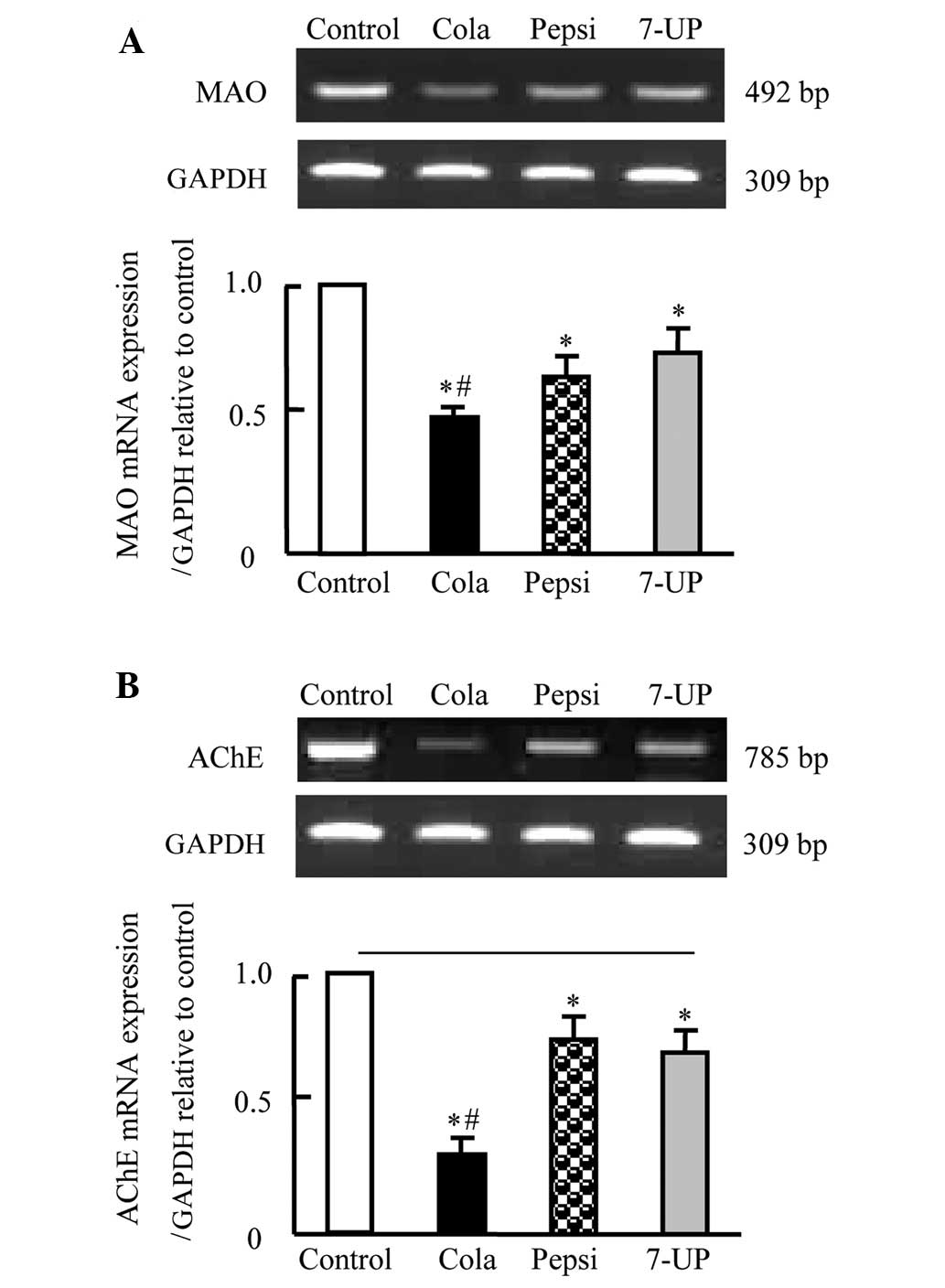
Effect of carbonated soft drink
consumption for 3 months on mRNA expression of MAO-A and AChE in
brain tissues of Wistar rats
Fig. 4 shows that,
consistent with serum changes of MAO-A and AChE, the mRNA
expression of MAO-A and AChE was significantly downregulated in the
brain tissues of rats administered carbonated soft drinks
(P<0.05). Rats in the Cola group exhibited the greatest decrease
in MAO-A and AChE expression, followed by Pepsi and 7-UP,
respectively. The decrease was not identified to be significantly
different between the Pepsi and 7-UP groups (Fig. 4).
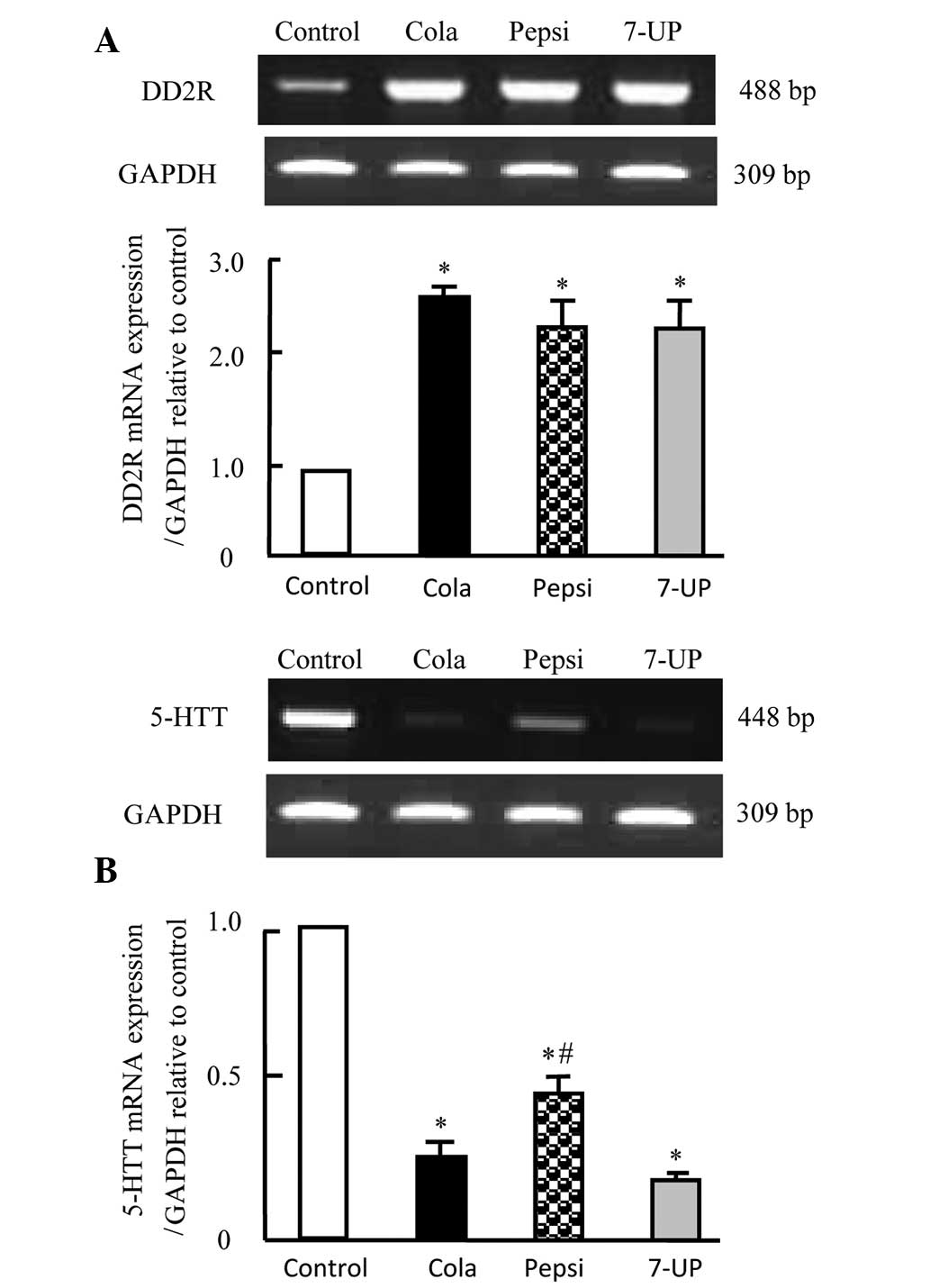
Effect of carbonated soft drink
consumption for 3 months on mRNA expression of DD2R and 5-HTT in
brain tissues of Wistar rats
The effects of carbonated soft drink consumption on
the expression of certain genes that have been shown to be
associated with aggression were investigated. As shown in Fig. 5A, expression of DD2R was
significantly upregulated in rats in the Cola, Pepsi and 7-UP
groups (P<0.05). By contrast, the rats in these groups exhibited
significant downregulation of 5-HTT mRNA expression (Fig. 5B; P<0.05). Furthermore, rats in
the Cola and 7-UP groups exhibited significantly decreased
expression of 5-HTT, as compared with rats in the Pepsi group
(P<0.05).
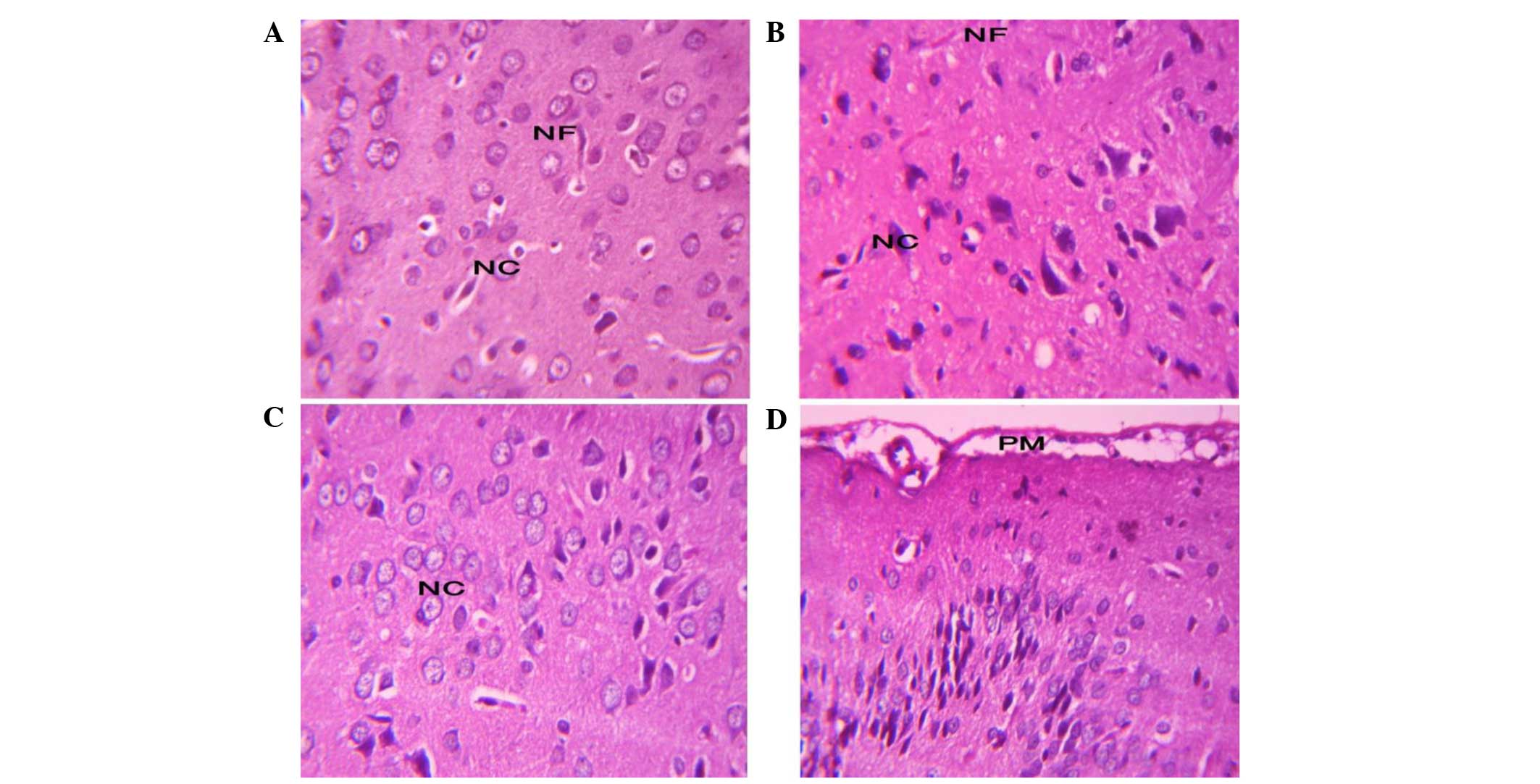
Effect of carbonated soft drink
consumption for 3 months on brain histopathology
Although changes induced by carbonated soft drinks
were observed at the biochemical and molecular levels, brain
histopathology analysis showed normal brain architecture in all
groups (Fig. 6). The gray matter
of the rats appeared normal with its well-organized regularly
arranged six layers and different size and shape nerve cells. The
normal pattern of the white matter is formed of homogeneously
stained nerve fibers running down the cortex was also
identified.
Discussion
The results of current study confirmed that chronic
consumption of carbonated soft drinks induced oxidative stress and
changes in antioxidant expression in the brains of Wistar rats.
Moreover, soft drink consumption decreased the serum levels and
mRNA expression of MAO and AChE in the brain. Notably, it was also
demonstrated that the levels of DD2R were downregulated and the
levels of 5-HTT expression were upregulated in the brain, while
brain histopathology remained unaffected.
Oxidative stress has been associated with the
etiopatho-genesis of several chronic diseases and exhibits a key
role in the aging process (13).
One of the consequences of uncontrolled oxidative stress (imbalance
between the prooxidant and antioxidant levels in favor of
prooxidants) is injury to cells, tissues and organs by oxidative
damage. It has long been recognized that high levels of free
radicals or reactive oxygen species (ROS) can directly damage
lipids. The primary sources of endogenous ROS production are the
mitochondria, plasma membrane, endoplasmic reticulum and
peroxisomes (14). ROS are
produced through a variety of mechanisms, including enzymatic
reactions and/or auto-oxidation of several compounds, such as
catecholamines and hydroquinone. In this study, chronic carbonated
soft drink consumption induced oxidative stress in the brain as
indicated by the increase in MDA levels in addition to the decrease
in the expression of antioxidants, GR, GPx and catalase.
Carbonated soft drinks contain caffeine and
phosphoric acid. Caffeine causes the release of adrenaline and an
accompanying increase in blood sugar levels to produce the required
energy. Caffeine reaches its peak level in the blood within 1 h of
consumption and remains in the body for 4–6 h (4,5).
Caffeine in soft drinks causes an increases in the release of acid
into the stomach, which may lead to an upset stomach or heartburn.
Moreover, caffeine has been reported that chronic exposure to the
various components of energy and soft drinks may result in
significant alterations in the cardiovascular system and brain
activity (15,16). The results of the present study
demonstrated an alteration in MAO-A and AChE at the serum and mRNA
levels. MAO has 2 isozymes, A and B. MAO-A in humans is encoded by
the MAOA gene (17,18). It preferentially deaminates
norepinephrine, epinephrine, serotonin and dopamine (all are
equally deaminated by MAO-A and MAO-B). Inhibition of both MAO-A
and MAO-B using MAO inhibitor is used in the treatment of clinical
depression, erectile dysfunction and anxiety. MAO-A has been shown
to be increased in patients with depression (19) and an association has been
demonstrated between low-activity forms of the MAO-A gene and
autism (20). A dysfunctional
MAO-A gene has also been correlated with increased aggression
levels in mice (21,22), as well as with heightened levels of
aggression in humans (23). The
results of the present study demonstrated that the consumption of
carbonated soft drinks decreased MAO-A gene expression and were
associated with oversensitivity, as MAO-A decrease is correlated
with aggression and violence, this may suggest consumption of
carbonated soft drinks is correlated with increased aggression and
violence but this requires further investigation.
It is well-established that cholinergic neurons are
involved in several neuropsychic functions, such as learning,
memory and sleep. Acetylcholine exhibits a key role in modulating
these functions (24). A central
cholinergic deficit is strongly associated with certain
neurodegenerative diseases, such as Alzheimer's disease and
Parkinson's disease (25).
Evidence of autism due to dysfunction of the cholinergic system has
recently been reported (26). AChE
is a specific cholinergic marker protein for the functional state
of cholinergic neurons. It is key in the maintenance of
acetylcholine levels at the cholinergic neurons (27) as it is responsible for degradation
of acetylcholine to acetate and choline in the synaptic cleft.
Notably, AChE was observed to be decreased in the serum and AChE
mRNA expression was observed to be decreased in the brain. It has
previously been suggested that acetylcholine disruption may be a
primary cause of depression and/or aggression (28).
Dopamine is a neurotransmitter of the catecholamine
and phenethylamine families that exhibits a number of roles in the
human brain and body. There are 5 isoforms of the dopamine
receptor, dopamine D1–5 receptors. DD2R is the most common receptor
in the mammalian brain. DD2R antagonists have been used for decades
to treat aggressive behavior in psychotic patients (29). In addition, in a preclinical study
the role of dopamine D1, D2 and D3 receptors in the modulation of
aggression has been documented (30). Several studies have indicated that
the mesocorticolimbic dopamine system is involved in the
preparation, execution and consequences of aggressive acts
(31–33). Pharmacologically induced dopamine
increases are associated with increased aggressive behavior under
certain conditions (32,33). The results of the present study
demonstrated that DD2R levels increased following chronic
carbonated soft drink consumption. This was probably due to the
increase in dopamine levels resulting from the downregulation of
MAO-A expression.
Two major enzymes are responsible for catecholamine
catabolism in the brain: Catechol-O-methyltransferase (COMT) and
monoamine oxidase A (MAO-A). If aggressive behavior is enhanced by
catecholaminergic activity, then decreased activity of COMT and
MAO-A should indirectly increase levels of aggression (31,34).
5-HTT expression was shown to be downregulated
following chronic carbonated soft drink consumption. A number of
studies have shown that elevated serotonin levels lead to decreased
aggression in a number of species (35), including humans (36). Blocking serotonin transporter
molecules is effective in reducing and preventing aggressive
behavior in humans and other animals, presumably due to increased
brain 5-HT levels (35).
Clinically, blocking 5-HTT with the administration of selective
serotonin reuptake inhibitors (SSRIs), reduces aggressive outbursts
and violent behavior in psychiatric patients (37–39).
In addition, in animal models, acute and chronic treatment with
SSRIs can dose-dependently reduce aggressive behavior (40). Acute administration of several
SSRIs reduced aggression in different contexts and species,
including rodents and non-human primates (40,41).
5-HTT is a type of monoamine transporter protein that transports
serotonin from the synaptic cleft to the pre-synaptic neuron. This
transport of serotonin by the 5-HTT protein terminates the action
of serotonin and recycles it in a sodium-dependent manner. This
protein is the target of numerous antidepressant agents, including
those of the SSRI class (42). A
repeat length polymorphism in the promoter of this gene has been
shown to affect the rate of serotonin uptake and may exhibit a role
in sudden infant death syndrome, aggressive behavior in Alzheimer
disease patients, post-traumatic stress disorder and
depression-susceptibility in individuals experiencing emotional
trauma (43). It has also been
suggested that alterations in 5-HTT expression levels following the
consumption of carbonated soft drinks may be a predisposing factor
for depression.
Histological examination of the brain revealed that
the brain exhibited normal histology and cell distribution
following chronic carbonated soft drink consumption. This finding
was also reported in another study (10) for regular soft drinks; however,
previously diet soft drinks were shown to exhibit adverse effects
on the cerebellum of albino rats (10).
In conclusion, chronic term carbonated soft drink
consumption induced oxidative stress and alterations in
antioxidants and the expression levels of certain genes associated
with brain function. Therefore, the results of the present study
suggested that the consumption of carbonated soft drinks may induce
adverse effects, thus these drinks must be consumed with
caution.
Acknowledgments
The authors would like to thank Al-Saedan Research
Chair for Genetic Behavioral Disorders, Taif University, Kingdom of
Saudi Arabia, for their financial support.
References
|
1
|
Adjene JO, Ezeoke JC and Nwose EU:
Histological effects of chronic consumption of soda pop drinks on
kidney of adult Wister rats. N Am J Med Sci. 2:215–217.
2010.PubMed/NCBI
|
|
2
|
Amato D, Maravilla A, García-Contreras F
and Paniagua R: Soft-drinks and health. Rev Invest Clin.
49:387–395. 1997.
|
|
3
|
Amato D, Maravilla A, Montoya C, Gaja O,
Revilla C, Guerra R and Paniagua R: Acute effects of soft drink
intake on calcium and phosphate metabolism in immature and adults
rats. Rev Invest Clin. 50:185–189. 1998.PubMed/NCBI
|
|
4
|
Clauson KA, Shields KM, McQueen CE and
Persad N: Safety issues associated with commercially available
energy drinks. J Am Pharm Assoc (2003). 48:e55–e67. 2008.
View Article : Google Scholar
|
|
5
|
Iyadurai SJ and Chung SS: New-onset
seizures in adults: Possible association with consumption of
popular energy drinks. Epilepsy Behav. 10:504–508. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DiMeglio DP and Mattes RD: Liquid versus
solid carbohydrate: Effects on food intake and body weight. Int J
Obes Relat Metab Disord. 24:794–800. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mattes RD: Fluid energy-Where's the
problem? J Am Diet Assoc. 106:1956–1961. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Frank GK, Oberndorfer TA, Simmons AN,
Paulus MP, Fudge JL, Yang TT and Kaye WH: Sucrose activates human
taste pathways differently from artificial sweetener. Neuroimage.
39:1559–1569. 2008. View Article : Google Scholar
|
|
9
|
Smeets PA, de Graaf C, Stafleu A, van Osch
MJ and van der Grond J: Functional magnetic resonance imaging of
human hypothalamic responses to sweet taste and calories. Am J Clin
Nutr. 82:1011–1016. 2005.PubMed/NCBI
|
|
10
|
Eluwa MA, Inyangmme II, Akpantah AO,
Ekanem TB, Ekong MB, Asuquo OR and Nwakanma AA: A comparative study
of the effect of diet and soda carbonated drinks on the histology
of the cerebellum of adult female albino Wistar rats. Afr Health
Sci. 13:541–545. 2013.PubMed/NCBI
|
|
11
|
Soliman MM, Abdo Nassan M and Ismail TA:
Immunohistochemical and molecular study on the protective effect of
curcumin against hepatic toxicity induced by paracetamol in Wistar
rats. BMC Complement Altern Med. 14:4572014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bancroft JD and Gamble M: Theory and
practice of histological techniques. 6th ed. Churchill Livingstone
Elsevier; Philadelphia: pp. 126–127. 2008
|
|
13
|
Ceconi C, Boraso A, Cargnoni A and Ferrari
R: Oxidative stress in cardiovascular disease: Myth or fact? Arch
Biochem Biophys. 420:217–221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moldovan L and Moldovan NI: Oxygen free
radicals and redox biology of organelles. Histochem Cell Biol.
122:395–412. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Higgins JP, Tuttle TD and Higgins CL:
Energy beverages: Content and safety. Mayo Clin Proc. 85:1033–1041.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Usman A and Jawaid A: Hypertension in a
young boy: An energy drink effect. BMC Res Notes. 5:5912012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hotamisligil GS and Breakefield XO: Human
monoamine oxidase A gene determines levels of enzyme activity. Am J
Hum Genet. 49:383–392. 1991.PubMed/NCBI
|
|
18
|
De Colibus L, Li M, Binda C, Lustig A,
Edmondson DE and Mattevi A: Three-dimensional structure of human
monoamine oxidase A (MAO A): Relation to the structures of rat MAO
A and human MAO B. Proc Natl Acad Sci USA. 102:12684–12689. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meyer JH, Ginovart N, Boovariwala A,
Sagrati S, Hussey D, Garcia A, Young T, Praschak-Rieder N, Wilson
AA and Houle S: Elevated monoamine oxidase a levels in the brain:
An explanation for the monoamine imbalance of major depression.
Arch Gen Psychiatry. 63:1209–1216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cohen IL, Liu X, Lewis ME, Chudley A,
Forster-Gibson C, Gonzalez M, Jenkins EC, Brown WT and Holden JJ:
Autism severity is associated with child and maternal MAOA
genotypes. Clin Genet. 79:355–362. 2011. View Article : Google Scholar
|
|
21
|
Scott AL, Bortolato M, Chen K and Shih JC:
Novel monoamine oxidase A knock out mice with human-like
spontaneous mutation. Neuroreport. 19:739–743. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vishnivetskaya GB, Skrinskaya JA, Seif I
and Popova NK: Effect of MAO A deficiency on different kinds of
aggression and social investigation in mice. Aggress Behav. 33:1–6.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brunner HG, Nelen M, Breakefield XO,
Ropers HH and van Oost BA: Abnormal behavior associated with a
point mutation in the structural gene for monoamine oxidase A.
Science. 262:578–580. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mohapel P, Leanza G, Kokaia M and Lindvall
O: Forebrain acetylcholine regulates adult hippocampal neurogenesis
and learning. Neurobiol Aging. 26:939–946. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oda Y: Choline acetyltransferase: The
structure, distribution and pathologic changes in the central
nervous system. Pathol Int. 49:921–937. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lam KS, Aman MG and Arnold LE:
Neurochemical correlates of autistic disorder: A review of the
literature. Res Dev Disabil. 27:254–289. 2006. View Article : Google Scholar
|
|
27
|
Eckenstein F and Sofroniew MV:
Identification of central cholinergic neurons containing both
choline acetyltransferase and acetylcholinesterase and of central
neurons containing only acetylcholinesterase. J Neurosci.
3:2286–2291. 1983.PubMed/NCBI
|
|
28
|
Shytle RD, Silver AA, Lukas RJ, Newman MB,
Sheehan DV and Sanberg PR: Nicotinic acetylcholine receptors as
targets for antidepressants. Mol Psychiatry. 7:525–535. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Glazer WM and Dickson RA: Clozapine
reduces violence and persistent aggression in schizophrenia. J Clin
Psychiatry. 59(Suppl 3): 8–14. 1998.PubMed/NCBI
|
|
30
|
Miczek KA, Covington HE III, Nikulina EM
Jr and Hammer RP: Aggression and defeat: Persistent effects on
cocaine self-administration and gene expression in peptidergic and
aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev.
27:787–802. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Almeida RM, Ferrari PF, Parmigiani S
and Miczek KA: Escalated aggressive behavior: Dopamine, serotonin
and GABA. Eur J Pharmacol. 526:51–64. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ferrari PF, van Erp AM, Tornatzky W and
Miczek KA: Accumbal dopamine and serotonin in anticipation of the
next aggressive episode in rats. Eur J Neurosci. 17:371–378. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ferrari PF, Palanza P, Parmigiani S, de
Almeida RM and Miczek KA: Serotonin and aggressive behavior in
rodents and nonhuman primates: Predispositions and plasticity. Eur
J Pharmacol. 526:259–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gogos JA, Morgan M, Luine V, Santha M,
Ogawa S, Pfaff D and Karayiorgou M:
Catechol-O-methyltransferase-deficient mice exhibit sexually
dimorphic changes in catecholamine levels and behavior. Proc Natl
Acad Sci USA. 95:9991–9996. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chiavegatto S and Nelson RJ: Interaction
of nitric oxide and serotonin in aggressive behavior. Horm Behav.
44:233–241. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Coccaro EF and Kavoussi RJ:
Neuropsychopharmacologic challenge in biological psychiatry. Clin
Chem. 40:319–327. 1994.PubMed/NCBI
|
|
37
|
Barkan T, Peled A, Modai I, Barak P,
Weizman A and Rehavi M: Serotonin transporter characteristics in
lymphocytes and platelets of male aggressive schizophrenia patients
compared to non-aggressive schizophrenia patients. Eur
Neuropsychopharmacol. 16:572–579. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Blader JC: Pharmacotherapy and
postdischarge outcomes of child inpatients admitted for aggressive
behavior. J Clin Psychopharmacol. 26:419–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bond AJ: Antidepressant treatments and
human aggression. Eur J Pharmacol. 526:218–225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Carrillo M, Ricci LA, Coppersmith GA and
Melloni RH Jr: The effect of increased serotonergic
neurotransmission on aggression: A critical meta-analytical review
of preclinical studies. Psychopharmacology (Berl). 205:349–368.
2009. View Article : Google Scholar
|
|
41
|
Caldwell EE and Miczek KA: Long-term
citalopram maintenance in mice: Selective reduction of
alcohol-heightened aggression. Psychopharmacology (Berl).
196:407–416. 2008. View Article : Google Scholar
|
|
42
|
Deutch AY and Roth RH: Neurotransmitters.
Fundamental Neuroscience. Squire L, Berg S, Bloom F, du Lac S,
Ghosh A and Spitzer N: 3rd edition. Elsevier/Academic Press;
Amsterdam: pp. 1432008
|
|
43
|
Holmes A, Murphy DL and Crawley JN:
Reduced aggression in mice lacking the serotonin transporter.
Psychopharmacology (Berl). 161:160–167. 2002. View Article : Google Scholar
|