Introduction
A large proportion of patients with thoracic
carcinomas receive thoracic radiotherapy (TRT) as a part of their
treatment. A number of these patients exhibit radiation-induced
toxicity, such as acute radiation-induced esophagitis (ARIE), which
is the primary dose-limiting complication of radiotherapy. The
incidence rate of ARIE in a clinical setting is relatively high and
the common symptoms are non-specific, mainly comprising dysphagia,
odynophagia and a substernal burning sensation (1). As demonstrated in Table I, the degrees of ARIE defined by
the National Cancer Institute Common Toxicity Criteria (NCI-CTC)
(2) are essentially based on the
symptoms patients have experienced. Clinically, patients often
complain of dysphagia, odynophagia and a substernal burning
sensation 2–3 weeks after initiation of radiotherapy (2). The course of radiation treatment is
delayed if complications are serious.
 | Table IGrade of acute radiation-induced
esophagitis. |
Table I
Grade of acute radiation-induced
esophagitis.
| Grade | Description |
|---|
| 0 | None |
| 1 | Mild dysphagia, but
is able to eat regular diet |
| 2 | Dysphagia, requiring
predominantly pureed, soft or liquid diet |
| 3 | Dysphagia, requiring
feeding tube, i.v. hydration or hyperalimentation |
| 4 | Complete obstruction
(cannot swallow saliva); ulceration with bleeding not induced by
minor trauma, abrasion or perforation |
Cell death and defluxion of the esophageal mucous
epithelium in the radiation fields have been observed, along with
bleeding or ulceration when the dose of radiation was sufficiently
high (3). Inflammatory cell
infiltration in the esophageal tissue is another prominent
histopathological change that occurs in ARIE (3,4).
These types of damage may evoke symptoms of pain, and substance P
(SP) secretion may be increased, playing an important role in the
inflammatory procedure. SP is an 11 amino acid polypeptide released
by sensory nerves. The biological function of SP is induced by its
binding to the receptor, neurokinin-1 (5), which is expressed on a limited number
of cell types, including epithelial cells, fibroblasts and
inflammatory cells (6). As a
neurotransmitter and a neuromodulator, SP is associated with
inflammatory processes and pain. It participates in the process of
nociception by transmitting information regarding damage from the
peripheral receptors to the central nervous system, thus resulting
in the generation of symptoms of pain (7).
In the present study, we established the animal
model of ARIE and demonstrated the therapeutic effect of the
compound of white peony root oral liquid (cWPROL) on this model
(8). We elucidated a molecular
mechanism via which the symptoms of pain are relieved; cWPROL is
able to relieve symptoms of pain in the ARIE animal model by
decreasing the expression level of SP in esophageal tissues.
Materials and methods
Subjects
Adult male Wistar rats with an average weight of
180–220 g were used. Animals were housed with a 12-hour light/dark
cycle, and were given access to food and water ad libitum.
All experimental animal techniques and handling procedures were
approved by the Institutional Animal Care and Use Committee of
Hebei Medical University. The certification number of the animals
was DK0512053.
Grouping and irradiation
Twenty-four subjects were randomly divided into four
groups (n=6), including the cWPROL treatment (CT) group; the
mixture of lidocaine, dexamethasone and gentamycin (mLDG) treatment
(MT) group; the radiation (R) group; and the non-intervention (NI)
group. In the CT group, rats were administered 0.475 g/ml cWPROL by
intra-esophageal perfusion following irradiation; while in the MT
group, rats were treated with mLDG, which was also administered
following irradiation. By contrast, in the R group, rats were not
administered any treatment following irradiation; while in the NI
group, rats did not receive irradiation or treatment. Animals
receiving treatment were administered 2 ml of the agent, 3 times a
day, with treatment initiated on the seventh day following
irradiation and continuing for 7 days. Rats were deprived of food
and water for 30 min following administration.
Conscious rats received a single dose of gamma
irradiation (total dose, 43 Gy) to the chest. The irradiation
procedure was performed with a 60Co therapy apparatus
(Xinhua Factory, Shandong, China) at a dose rate of 0.111 Gy/min.
The irradiation field was 3×30 cm, with a center dose point on the
back of the rats located 1 cm beneath the body surface, and an
irradiation range of 3 cm on the upper esophagus; the remaining
parts of the body were covered. Rats in the NI group were not
irradiated, but were otherwise treated as irradiated rats.
Detection of the general condition of the
rats
Before and after the experiment, rat body weights in
each group were recorded, in addition to the daily weight of food
and volume of water. These data were regarded as indirect evidence
reflecting symptoms of pain.
Immunohistochemical staining
Rats were anesthetized with 2% pentobarbital sodium
administered by intraperitoneal injection (45 mg/kg). Esophageal
samples were fixed with 4% paraformaldehyde for 24 h, embedded in
paraffin and sectioned at 4 μm. Tissue sections were deparaffinized
with xylene, rehydrated through an ethanol series and with TBST and
immersed in 3% formaldehyde hydrogen peroxide liquid to block
endogenous peroxidase. Antigen retrieval was performed by microwave
treatment in the presence of antigen retrieval solution. The
sections were incubated with the primary antibody at 4°C overnight,
and then treated with biotin-labeled secondary antibody (Zhongshan
Jinqiao Bio-technology Limited Company, Beijing, China) at 37°C for
20 min. This was followed by the addition of a
peroxidase-conjugated streptavidin-labeled tertiary antibody
(Zhongshan Jinqiao Bio-technology Limited Company) at 37°C for 20
min. We used antibodies against SP (1:50) as the primary antibodies
(Boshide Program Limited Company, Wuhan, China). The sections were
counterstained with hematoxylin, dehydrated, transparentized and
then sealed with neutral gum. The blank control and replacing
control were treated with PBS and normal rabbit serum,
respectively. The appearance of brown particles in the stained
parts was regarded as positive. Five successive visual fields
centered on the lesion area of each section were observed under a
microscope (x400) and the average of their integral optical density
(IOD) was regarded as the representative value.
Statistical analysis
Experimental results were analyzed for their
statistical significance using SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA). Groups were compared by a one-way ANOVA. The
Student-Newman-Keuls test was utilized when the variance was equal,
while the Kruskal-Wallis H test was used if the variance was
unequal. Results are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
The effect of treatment on rat body
weight
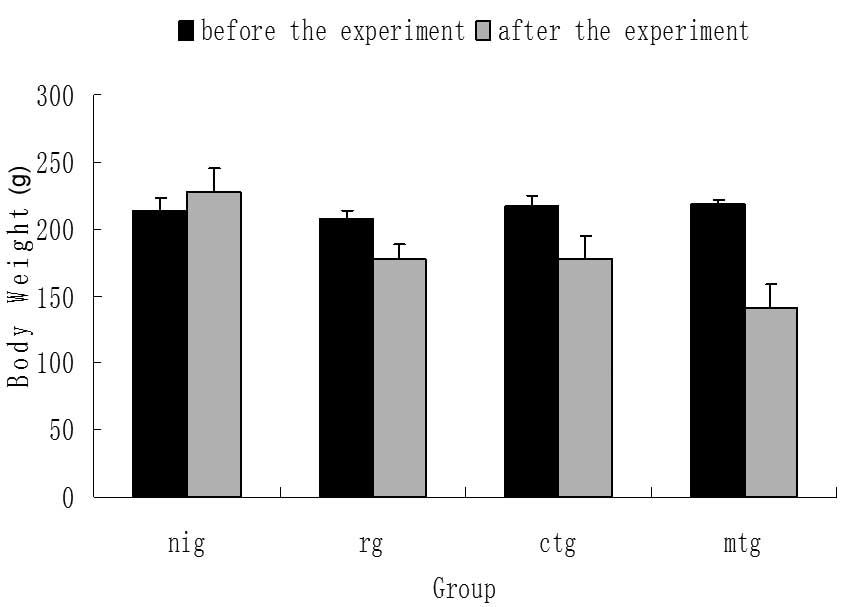
As demonstrated in Fig.
1, there was no significant difference in body weight between
groups prior to the experiment (P>0.05). Following the
experiment, the body weight of rats in the NI group significantly
increased (P<0.05); while that of the remaining experimental
groups significantly decreased (P<0.05), particularly in the MT
group (P<0.01). Additionally, compared with the R group, there
was no significant difference in the body weight of rats in the CT
group, while the body weight significantly decreased in the MT
group (P<0.05). Furthermore, the body weight of rats in the MT
group was significantly lower than that of the CT group
(P<0.05).
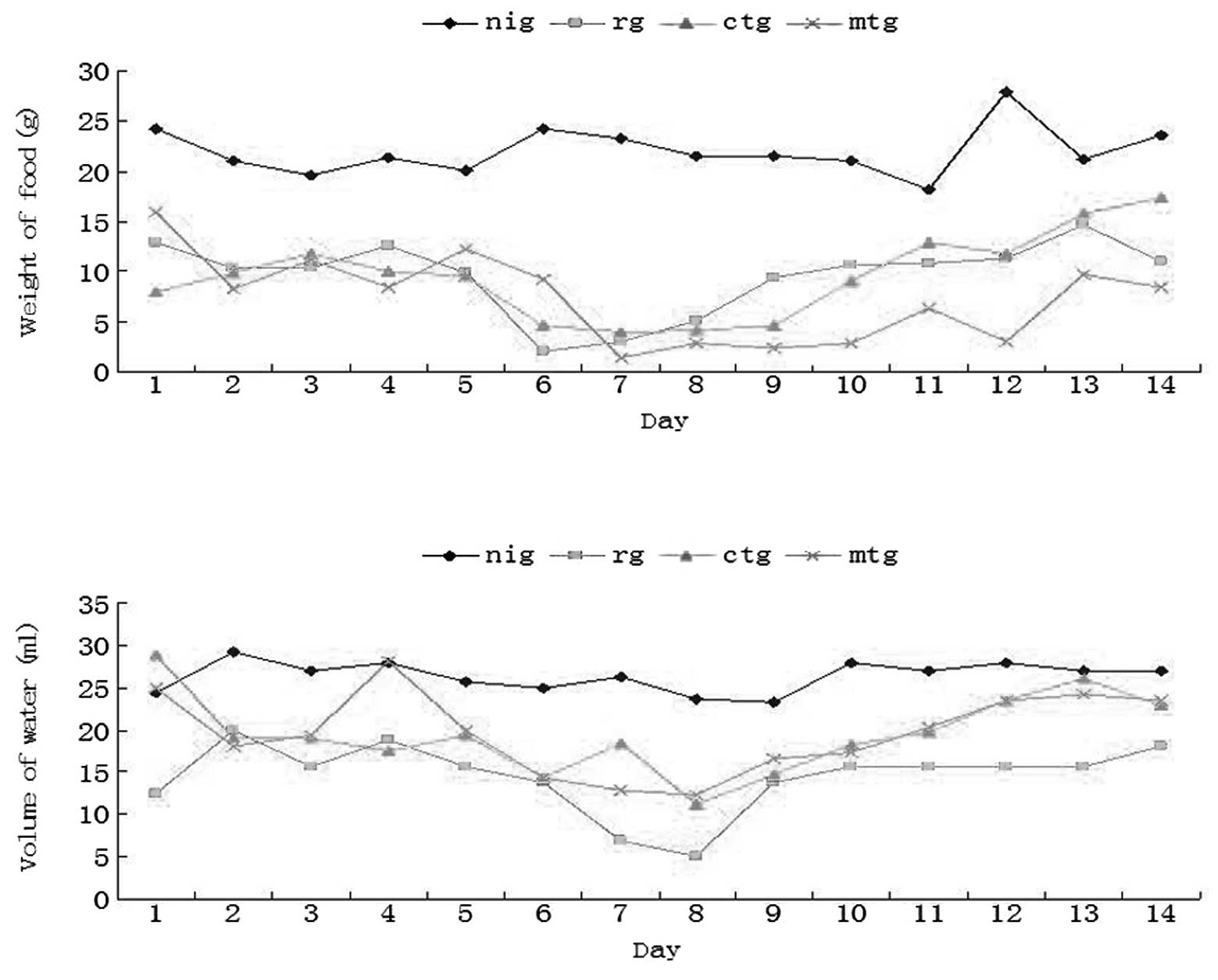
Changes in the weight of food and volume
of water following treatment
We detected the daily changes in the weight of food
and volume of water, and these were assumed to indirectly reflect
the symptoms of pain. As demonstrated in Fig. 2, the weight of food and volume of
water remained stable in the NI group, while those of the remaining
experimental groups gradually decreased and reached the lowest
point on the seventh day following irradiation, before slowly
increasing. We found that following administration of cWPROL or
mLDG, the volume of water was increased to a certain extent
compared with that of the R group. Notably, compared with the R
group, the weight of food rats consumed in the MT group did not
increase, thus demonstrating that the symptoms of pain in this
group were not effectively reduced.
The level of SP expression in each
group
We observed the variation in the expression levels
of SP in the esophageal tissues by immunohistochemistry, before and
after irradiation and treatment administration (Fig. 3). It was revealed that the
expression level of SP was increased following exposure to
radiation; in the R group, the expression of SP was significantly
increased compared with the NI group (P<0.01). A low level of SP
expression was observed in normal rat esophageal tissues, and was
mainly distributed in the mucosal epithelium and nerve fiber
peripheries (Fig. 3A). Following
irradiation in the R group, marked SP expression was detected in
the epithelium of the area surrounding ulcers, the fibrocytes and
fibroblasts of the lamina propria and the submucosa in the inflamed
area (Fig. 3B). Following the
administration of cWPROL, the expression of SP was lower than that
of the R group (P<0.01), but higher than that of the NI group
(P<0.05); while the location of SP had no clear variance
(Fig. 3C). The result of the MT
group was as predicted; the expression level of SP in the MT group
was significantly different compared with that of the NI
(P<0.01) and R (P<0.05) groups (Fig. 3D). With regard to the comparison
between the CT and MT groups, although the difference in SP
expression levels was not significantly different, the effect of
reducing the expression level of SP by administration of cWPROL was
greater than that of mLDG (P>0.05).
Discussion
SP is a member of the tachykinin family that is
secreted by neurons and other types of cells. SP is also detected
in endothelial cells, inflammatory cells, fibroblasts and other
types of cells (9). It has a wide
range of functions, which include inducing cancer cell
proliferation, such as in malignant melanomas (10). In the present study, we focused on
the mediating function of SP, which involves delivering nociceptive
information to the nervous system. It is commonly known that SP
acts through the tachykinin NK1 receptor, which is a member of the
G-protein-coupled receptor superfamily. Additionally, Bie and Zhao
(11), along with Holzer (12), have demonstrated that the
tachykinin NK1 receptor may contribute to the development and
maintenance of inflammatory pain as a nociceptor. It has been
demonstrated that SP is upregulated in response to irradiation and
is involved in the process of inflammation (13). Therefore, we hypothesized that SP
may also influence the symptoms of pain caused by radiation-induced
inflammation. In addition, secreted SP may promote the inflammatory
procedures caused by radiation, through facilitating inflammatory
cell infiltration and activation (14).
The aim of radiation treatment is to administer an
effective dose of radiation to the tumor, with an acceptable dose
delivered to the neighboring normal tissues; however, the
radiotherapy of thoracic neoplasms is likely to expose the
esophagus to a high dose of ionizing radiation. In clinical
settings, ARIE is a common complication in cancer patients
receiving radiotherapy. Among these patients, a large proportion
suffer from serious symptoms of pain and their quality of life is
reduced. Furthermore, certain patients are forced to suspend their
irradiation treatment when they are not able to tolerate any
further treatment, resulting in a decreased tumor control rate. As
for the treatment of ARIE, there are currently no clinically
effective drugs or strategies. Minimizing the extent of the
esophagus that is irradiated is an effective means to prevent ARIE;
however, this would reduce the control of thoracic malignancies.
Adrenocorticotropic hormones and certain antibiotics are the main
drugs used in the treatment of ARIE; however, few efficacies have
been demonstrated in a wide range of patients (4,15).
Furthermore, the adverse effects of these drugs, including an
increased risk of osteoporosis and antibiotic resistance,
negatively limit their usage. Studies have also investigated the
effect of sucralfate on preventing ARIE; however, 58% of patients
resigned from the study, due to the adverse effects of nausea and
vomiting (16). Another drug,
amifostine, regarded to be the most effective radioprotective
compound screened by the U.S. Army, did not effectively relieve the
symptoms of ARIE in a large clinical trial (RTOG 9801) (17). Therefore, there is a requirement to
investigate a novel method with effective functions and without
obvious side-effects.
Drugs of a herbal origin with few side effects are
of high interest amongst alternative treatments. Furthermore,
traditional Chinese herb medicine (tChm) may provide a novel
therapy that is not only able to relieve clinical symptoms, but
also improve the general condition, including eating, sleeping and
immune function (18,19). We have previously investigated the
effect of cWPROL in treating the animal model of ARIE, and
demonstrated that this prescription formula was able to repair the
damaged esophageal tissues (20).
The present study was designed to demonstrate that cWPROL is able
to relieve the symptoms of pain caused by irradiation, and to
clarify the underlying mechanism. We observed the general
conditions, including body weight, and the weight of food and
volume of water, to indirectly reflect symptoms of pain. Meanwhile,
the expression level of SP in tissues within the radiation field
was detected using immunohistochemical staining. As demonstrated by
the results, cWPROL was able to decrease the level of SP
expression, which was hypothesized to be correlated with a decrease
in the symptoms of pain. Correspondingly, in the CT group, the
weight of food and volume of water increased to a certain extent,
after the lowest point of weight of food on the seventh day and the
lowest point of volume of water on the eighth day. However, in the
MT group, the weight of food and volume of water did not increase,
although decreased SP expression was observed. It may be concluded
that the pain relieving effect of mLDG is not as strong as that of
cWPROL.
According to the outcomes of the present study, a
partial mechanism for the pain-relieving effect of cWPROL has been
elucidated. SP plays a key role in delivering pain information to
the nervous system when tissues respond to injury (9). Our study suggests that the expression
level of SP may be reduced by administration of cWPROL, and that
the general condition, including body weight and the intake of food
or drinking water, may be improved to a certain extent. However,
other detailed molecular mechanisms should also be investigated in
more detail in the future. For example, the critical receptor of
SP, the tachykinin NK1 receptor, may exert a simultaneous
influence.
Acknowledgements
This study was supported by the Foundation Science
Research Program of Hebei Province Science and Technology Office
(no. 04236101D-252004-2005).
References
|
1
|
Byhardt RW, Scott C, Sause WT, et al:
Response, toxicity, failure patterns, and survival in five
Radiation Therapy Oncology Group (RTOG) trails of sequential and/or
concurrent chemotherapy and radiotherapy for locally advanced
non-small-cell carcinoma of the lung. Int J Radiat Oncol Biol Phys.
42:469–478. 1998. View Article : Google Scholar
|
|
2
|
Bradley J and Movsas B: Radiation
esophagitis: Predictive factors and preventive strategies. Semin
Radiat Oncol. 14:280–286. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abdel-Latif MM, Duggan S, Reynolds JV and
Kelleher D: Inflammation and esophageal carcinogenesis. Curr Opin
Pharmacol. 9:396–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y, Yu H, Zhang C, Cheng Y, Hu L, Meng
X and Zhao Y: Protective effects of berberine on radiation-induced
lung injury via intercellular adhesion molecular-1 and transforming
growth factor-beta-1 in patients with lung cancer. Eur J Cancer.
44:2425–2432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamoto K, Kureyama N, Asano K, Ikeda T
and Yamatodani A: Involvement of substance P and the neurokinin-1
receptor in radiation-induced hair loss in mice. J Pharmacol Sci.
112:118–120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J and Hauer-Jensen M: Neuroimmune
interactions: potential target for mitigating or treating
intestinal radiation injury. Br J Radiol. 80:S41–S48. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yasui Y, Saper CB and Cechetto DF:
Calcitonin gene-related peptide immunoreactivity in the visceral
sensory cortex, thalamus, and related pathways in the rat. J Comp
Neurol. 290:487–501. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li S, Baoen S, Li Z, et al: Establishment
of animal model of radiation esophagitis. Chin J Cancer Prev Treat.
14:13–16. 2007.
|
|
9
|
Nessler S, Stadelmann C, Bittner A, et al:
Suppression of autoimmune encephalomyelitis by a neurokinin-1
receptor antagonist - a putative role for substance P in CNS
inflammation. J Neuroimmunol. 179:1–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Korcum AF, Sanlioglu S, Aksu G, Tuncel N
and Erin N: Radiotherapy-induced decreases in substance P levels
may potentiate melanoma growth. Mol Med Rep. 2:319–326.
2009.PubMed/NCBI
|
|
11
|
Bie BH and Zhao ZQ: Peripheral
inflammation alters desensitization of substance P-evoked current
in rat dorsal root ganglion neurons. Eur J Pharmacol. 670:495–499.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holzer P: Implications of tachykinins and
calcitonin gene-related peptide in inflammatory bowel disease.
Digestion. 59:269–283. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Christensen HD and Haley TJ: Distribution
of substance P in the central nervous system and small intestine of
the rat after x-irradiation. Radiat Res. 33:588–595. 1968.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Foster AP and Cunningham FM: Substance P
induces activation, adherence and migration of equine eosinophils.
J Vet Pharmacol Ther. 26:131–138. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kosaka Y, Mitsumori M, Araki N, et al:
Avascular necrosis of bilateral femoral head as a result of
long-term steroid administration for radiation pneumonitis after
tangential irradiation of the breast. Int J Clin Oncol. 11:482–486.
2006. View Article : Google Scholar
|
|
16
|
McGinnis WL, Loprinzi CL, Buskirk SJ, et
al: Placebo-controlled trail of sucralfate for inhibiting
radiation-induced esophagitis. J Clin Oncol. 15:1239–1243.
1997.PubMed/NCBI
|
|
17
|
Movsas B, Scott C, Langer C, et al: Phase
III study of amifostine in patients with locally advanced non-small
cell lung cancer (NSCLC) receiving chemotherapy and
hyperfractionated radiation (chemo/HFxRT): Radiation Therapy
Oncology Group (RTOG) 98–01. Proc Am Soc Clin Oncol. 22(abstr
2559): 6362003.
|
|
18
|
Hou W and Zhou Y: Function of traditional
Chinese medicine in cancer radiotherapy and its prospect. World Sci
Tech. 11:742–746. 2009. View Article : Google Scholar
|
|
19
|
Zhang P and Hu PL: TCM VVM Therapy's
influence on tumor patients' survival. Chin J Oncol.
25:3022003.
|
|
20
|
Shen L, Shan BE, Zhang L, et al: Study on
preventive and therapeutic function of compound white pony root
oral liquid in treating radiation-induced esophagitis. Radiat
Protect. 27:119–227. 2007.
|