Introduction
Tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL) is a member of the TNF
superfamily, and has a strong antitumor activity in a wide range of
cancer cell types with minimal cytotoxity to most normal cells and
tissues (1). TRAIL induces cell
apoptosis by interacting with cell-surface receptors, five of which
have been discovered thus far, including the two agonistic
receptors, death receptor (DR)4 and DR5, and the three antagonistic
decoy receptors, DcR1, DcR2 and osteoprotegerin (2,3). DR4
and DR5 contain a cytoplasmic region designated as the ‘death
domain’ (DD), and, following ligation of TRAIL, they recruit and
activate an adaptor protein known as Fas-associated death domain
(FADD) through interactions between the DD on them and FADD. FADD,
in turn, recruits and activates caspase-8 via its death effector
domain (DED), leading to the formation of the death-inducing
signaling complex (DISC). Activation of caspase-8 subsequently
initiates proteolytic activation of downstream effector caspases
such as caspase-3, -6 and -7 and finally induces apoptosis.
Alternatively, caspase-8 can also trigger a mitochondria-dependent
apoptotic amplification loop by activating Bid, which induces the
release of cytochrome c from mitochondria, the activation of
caspase-9, caspase-3, caspase-7 and finally apoptosis (4). Recent studies have shown that a number
of cancer cells are resistant to apoptosis induction by TRAIL
(5). The mechanisms involved in
TRAIL resistance include the loss of DR4 or DR5, upregulation of
decoy receptors, alternations in expression of proteins that affect
caspase activation (inactivation of pro-apoptotic molecules: Bax,
Bak, Bad, Bim or Bid; overexpression of anti-apoptotic molecules:
survivin, cFLIP, FAP-1, Bcl-2, Bcl-xL) (5–7).
However, TRAIL-resistant cancer cells can be sensitized by combined
treatment with chemotherapeutic drugs and TRAIL (8–11),
suggesting that TRAIL resistance may be overcome by combination
treatment, which may be a promising novel approach in cancer
therapy.
Casticin, a flavonoid isolated from Vitex
rotundifolia, is widely used as an anti-inflammatory agent in
Chinese traditional medicine. Recent studies have shown that
casticin has a wide range of actions, including anti-oxidant,
anti-inflammatory, immunomodulatory, pro-apoptotic and
anti-proliferative properties, and has antitumor activity in a
variety of cancers, including breast, lung and colon cancer and
leukemia (12–18). Yang et al recently reported
that casticin induces apoptosis of hepatocellular carcinoma cells
and the mechanisms involve depletion of intracellular glutathione
content and upregulation of DR5 (19). In the present study, we investigated
whether casticin can potentiate TRAIL-induced apoptosis and its
mechanisms. We found that casticin can indeed enhance TRAIL-induced
apoptosis through downregulation of cell survival proteins and
upregulation of DR5 mediated by reactive oxygen species (ROS).
Materials and methods
Drugs and reagents
Casticin was purchased from Chengdu Biopurify
Phytochemicals Ltd., (Chengdu, China), and was prepared in
dimethylsulfoxide (DMSO) at a concentration of 10 mmol/l and
aliquots were stored at −80°C. Soluble recombinant human TRAIL was
purchased from PeproTech (Rocky Hill, NJ, USA). DMEM medium and
fetal bovine serum (FBS) were obtained from Invitrogen; Tris,
glycine, NaCl, SDS, bovine serum albumin, N-acetylcysteine,
fluorouracil (5-FU), paclitaxel (TAX), and antibodies against TRAF1
and β-actin were from Sigma. 2′,7′-dichlorofluorescein diacetate
(DCFH-DA) was purchased from Molecular Probes Inc.; antibody
against DR5 from ProSci Inc.; anti-XIAP antibody from Cell
Signaling Technology (Danvers, MA, USA). Antibodies against DR4,
poly(ADP-ribose) polymerase (PARP), Bcl-2, Bcl-xL, Bax, Bid,
survivin and caspase-3 were obtained from Santa Cruz
Biotechnology.
Cell lines
Human colon cancer HT-29, HCT-116, SW480 cell lines
were purchased from the American Type Culture Collection. Cells
were cultured in DMEM with 10% FBS, 100 U/ml penicillin and 100
mg/ml streptomycin.
MTT assay
Cells were seeded in a 96-well plate at a density of
0.5×104 cells/well and incubated for 24 h, followed by
treatment with various concentrations of casticin and TRAIL alone
or in combination for 24 h. 3-[4,5-dimethylthiazol-2-yl]-2,5
diphenyl tetrazolium bromide (MTT) colorimetric analysis was
performed as described by Cao et al(20). The IC50 value, at which
50% of the cell growth inhibition compared with DMSO control, was
calculated by non-linear regression analysis using GraphPad Prism
software (San Diego, CA, USA).
Apoptosis detection by morphological
observation following AO/EB staining
Cells were treated with various concentrations of
casticin and TRAIL alone or in combination for 24 h, and then
harvested with 0.25% trypsin and resuspended in DMEM medium. After
staining for 10 min with 4 ml of an AO (100 mg/ml)/EB (100 mg/ml)
dye mixture, cells were visualized immediately under a fluorescence
microscope. Specific apoptotic cell death was calculated as
(percentage of experimental apoptosis - percentage of spontaneous
apoptosis)/(100 - percentage of spontaneous apoptosis) × 100.
Flow cytometry using propidium iodide
(PI) staining
Cells were seeded at a density of 4×106
cells/ml in 100 ml culture flasks for 24 h and then treated with
the medium containing various concentrations of casticin or TRAIL
or both for the indicated times. PI staining for DNA content
analysis was performed as described by Yang et al(21).
Analysis of cell-surface expressions of
DR4 and DR5
Cells were treated with 3 μmol/l casticin for 24 h,
and then stained with phycoerythrin-conjugated mouse anti-human DR4
or DR5 monoclonal antibody (R&D Systems) for 45 min at 4°C
according to the manufacturer’s instructions. The cells were then
resuspended and analyzed by flow cytometry with
phycoerythrin-conjugated mouse IgG2B as an isotype control.
Determination of ROS
Intracellular ROS accumulation was measured by flow
cytometry using the fluorescent probe DCFH-DA. Cells were incubated
with 10 μmol/l of DCFH-DA for 30 min at 37°C in the dark. Following
incubation, the cells were washed with PBS and analyzed within 30
min using FACScan (Becton-Dickinson, San Jose, CA, USA) equipped
with an air-cooled argon laser tuned to 488 nm. The specific
fluorescence signals corresponding to DCFH-DA were collected with a
525-nm band pass filter. As a rule, 104 cells were
counted in each determination.
Western blot analysis
Total cell extracts were obtained as described by
Yang et al(21). Cell lysate
containing 50 μg of protein was separated on 7.5–12%
SDS-polyacrylamide gel for electrophoresis and then
electro-transferred to polyvinylidene difluoride (PVDF) membranes
(Millipore, Bedford, MA, USA), blotted with each antibody and
detected using an ECL kit (Amersham Pharmacia Biotech, Piscataway,
NJ, USA).
Transfection with siRNA
The 25-nucleotide small interfering RNA (siRNA)
duplexes used in this study were purchased from Invitrogen and had
the following sequences: DR5, AUC AGC AUC GUG UAC AAG GUG UCC C;
the scrambled control, AAG ACC CGC GCC GAG GUG AAG. HT-29 cells
were plated in each well of 6-well plates and allowed to adhere for
24 h. On the day of transfection, 12 μl of Lipofectamine 2000
(Invitrogen) was added to 50 nmol/l siRNA in a final volume of 100
μl of culture medium. After 48 h of transfection, cells were
treated with casticin for 12 h and then exposed to TRAIL for 24
h.
Statistical analysis
Data are presented as the means ± standard deviation
(SD) unless otherwise indicated. Differences between groups were
analyzed using one-way analysis of variance (ANOVA) or t-test when
appropriate. All the statistical analyses were performed with the
SPSS 15.0 software package (SPSS Inc., Chicago, IL, USA). P<0.05
was considered to indicate statistically significant
differences.
Results
Casticin enhances TRAIL-induced apoptosis
in HT-29 cells
The cytotoxicity of TRAIL or casticin in HT-29 cells
was determined by MTT assay. TRAIL or casticin alone inhibited the
proliferation of HT-29 cells in a concentration-dependent manner,
and their IC50 values were 19.9 μmol/l and 318 ng/ml,
respectively. Only a slight cytotoxic activity was observed when
the cells were treated with TRAIL at 25–50 ng/ml or casticin at
1.0–3.0 μmol/l for 24 h. However, combined treatment with TRAIL and
casticin at the above subtoxic concentrations resulted in marked
cytotoxicity compared with TRAIL or casticin alone, suggesting that
casticin enhances TRAIL-induced cytotoxicity in HT-29 cells.
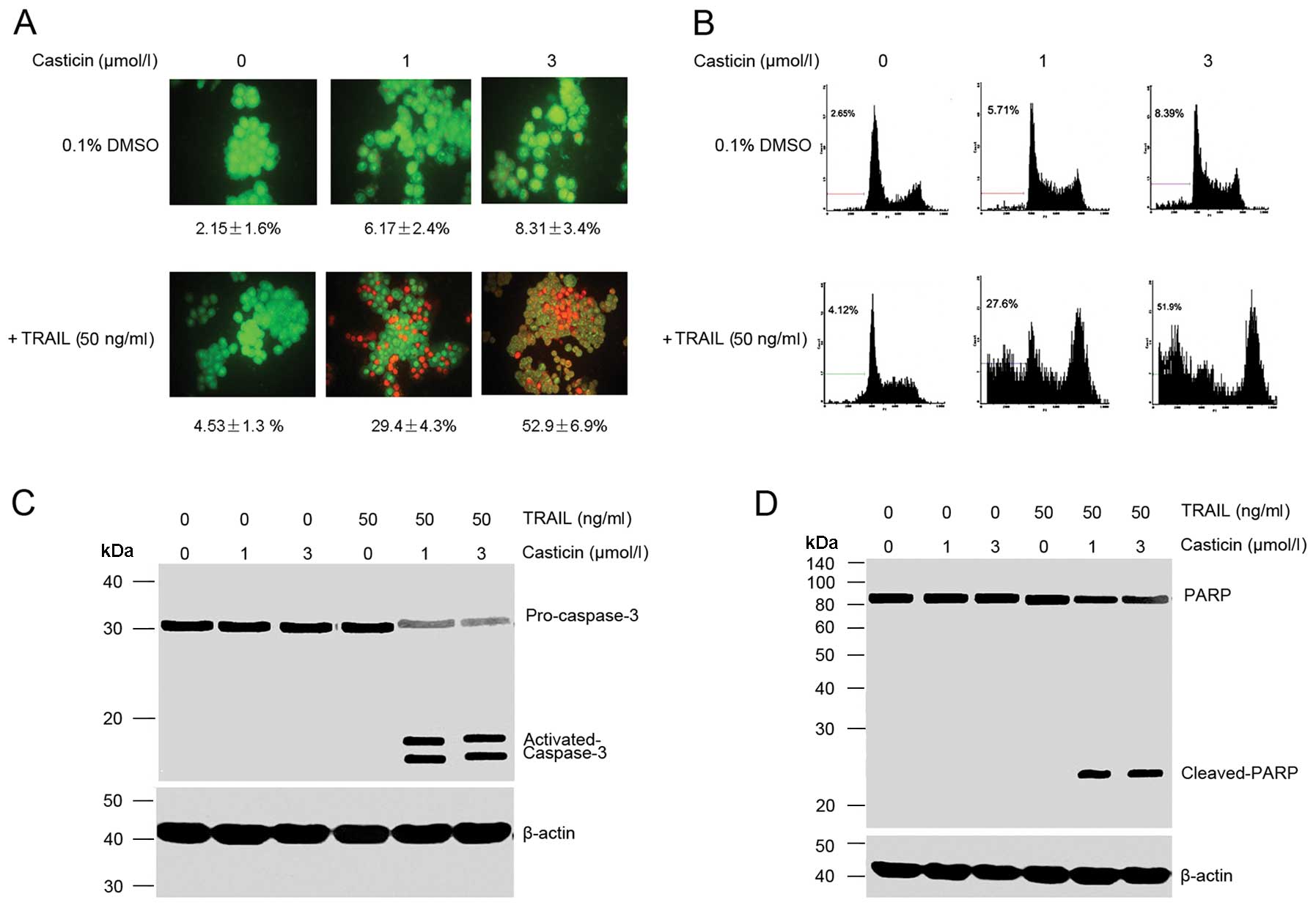
Apoptosis was determined by morphological
observation and flow cytometric analysis. Our observation using
AO/EB staining showed that casticin (1 and 3 μmol/l) or TRAIL (50
ng/ml) alone induced ~10% apoptosis in HT-29 cell. However, the
combination treatment with casticin and TRAIL enhanced apoptosis to
52.9±6.9% (Fig. 1A). Flow
cytometric analysis also showed that casticin potentiated
TRAIL-induced apoptosis, from 6.1±3.7 and 5.38±3.3% with casticin
(3 μmol/l) and TRAIL (50 ng/ml) alone, respectively, to 53.8±6.9%
when used in combination (Fig.
1B).
Next, we examined the effect of casticin, TRAIL and
their combination on the activation of caspase-3 and PARP cleavage,
and found that casticin or TRAIL alone had little effect on the
activation of caspase-3 and PARP cleavage, but the combined
treatment was highly effective (Fig. 1C
and D), which further demonstrates that casticin enhances
TRAIL-induced apoptosis.
Casticin downregulates the expression of
cell survival proteins in HT-29 cells
Numerous anti-apoptotic proteins are involved in
resistance to TRAIL-induced apoptosis (22–25).
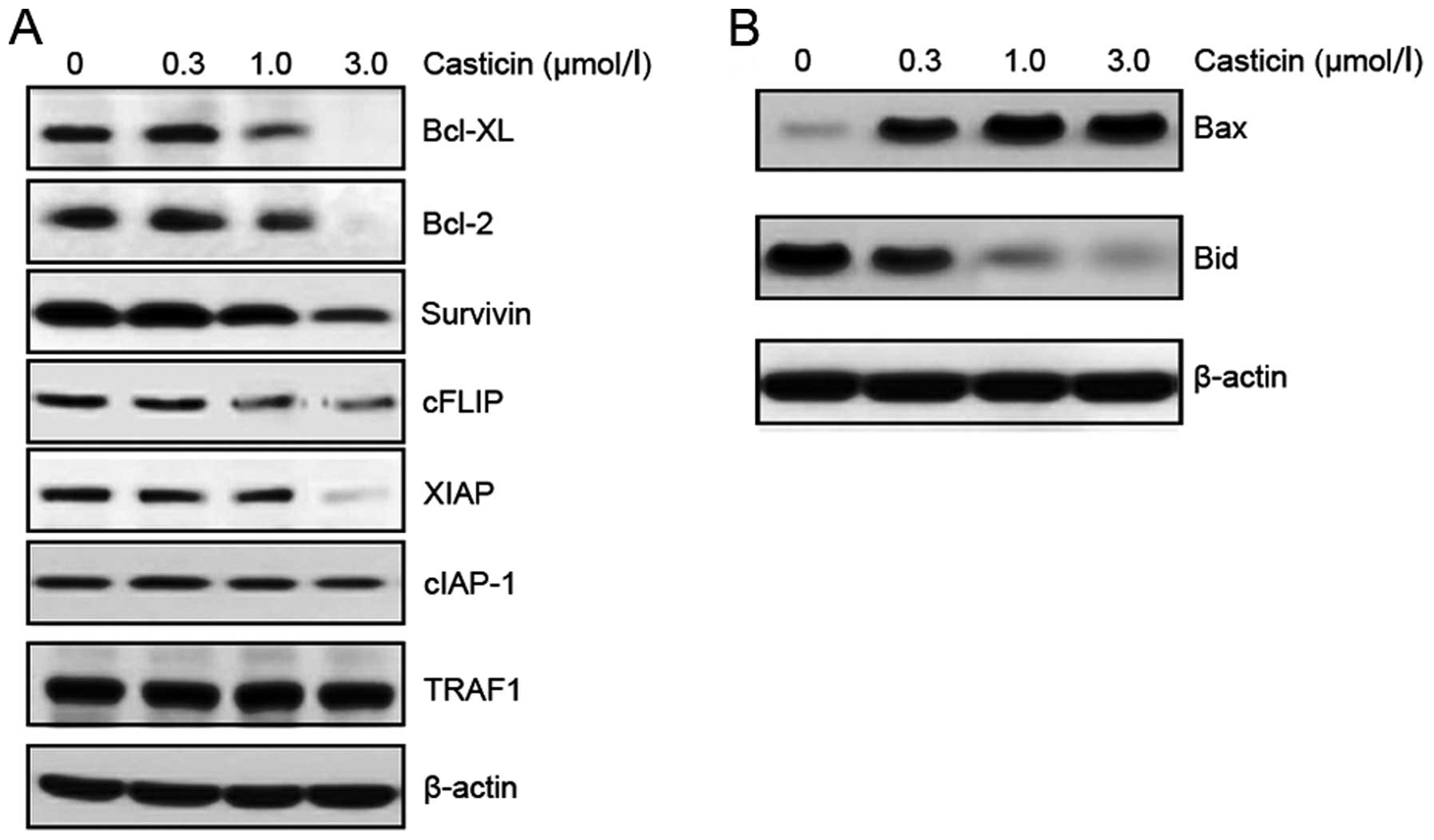
The effect of casticin on the expressions of these anti-apoptotic
proteins was examined. HT-29 cells were exposed to different
concentrations of casticin for 24 h, and the expressions of Bcl-xL,
Bcl-2, survivin, XIAP, cFLIP, cIAP-1 and TRAF1 were analyzed by
western blotting. Casticin inhibited the expressions of Bcl-xL,
Bcl-2, survivin, XIAP, cFLIP, but had no effect on the expressions
of cIAP-1 and TRAF1 (Fig. 2A).
In addition, the effect of casticin on the
expressions of pro-apoptotic proteins was also examined. Casticin
upregulated the expression of Bax and caused the cleavage of Bid
protein in a dose-dependent manner (Fig. 2B). The above results suggest that
downregulation of anti-apoptotic proteins and upregulation of
pro-apoptotic proteins may be the mechanism by which casticin
potentiates TRAIL-induced apoptosis.
Casticin induces the expression of DR5 in
colon cancer cells
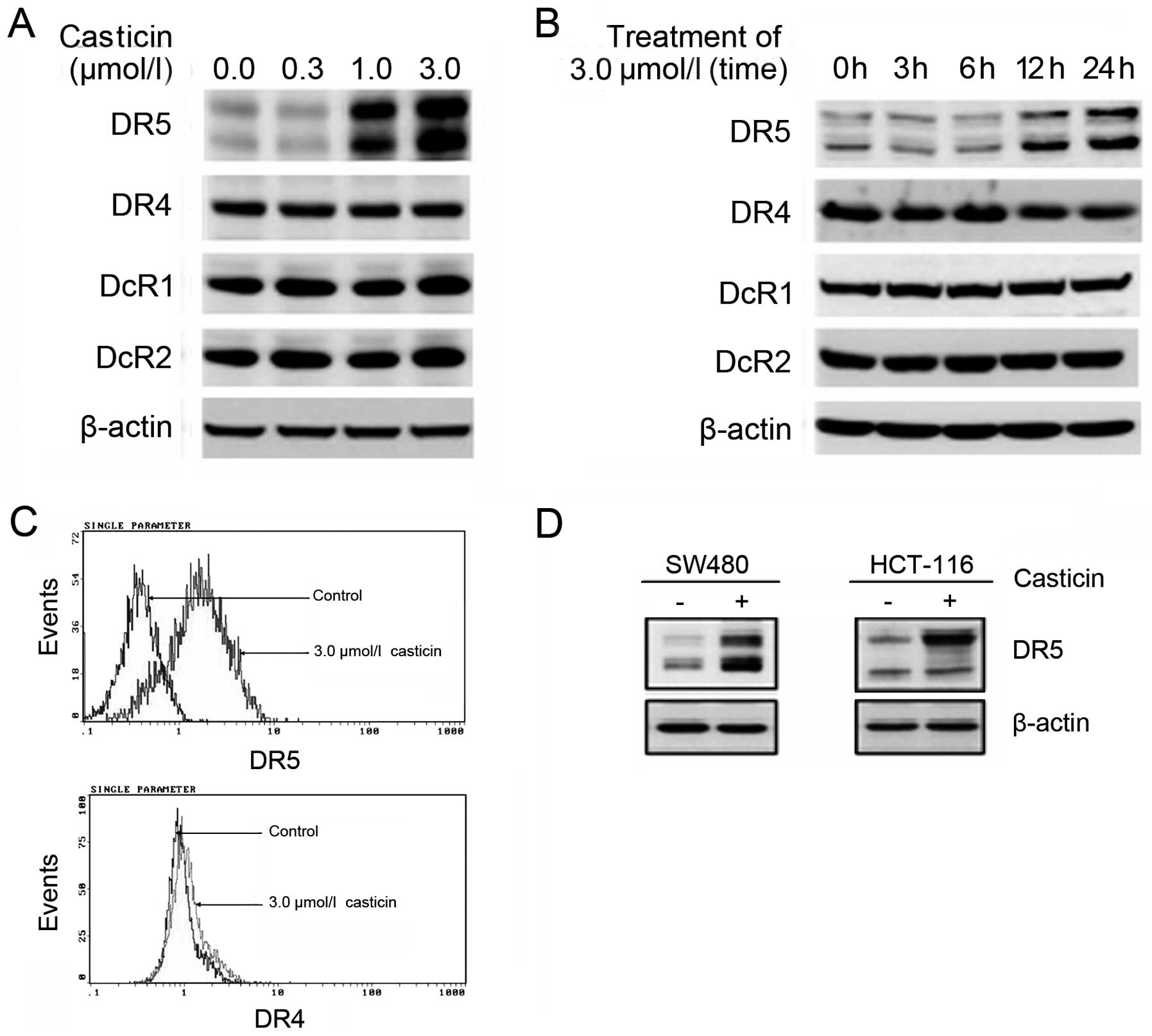
The effect of casticin on the expressions of TRAIL
receptors was examined with western blotting or flow cytometry.
Casticin increased the expression of DR5, but had no effect on the
expressions of DR4, DcR1 and DcR2 in HT-29 cells. Furthermore,
casticin increased the expression of DR5 in a dose-and
time-dependent manner (Fig.
3A–C).
To investigate whether the upregulation of DR5 by
casticin was specific to HCT-116 or whether it also occurs in other
cell types, we examined the expression of DR5 in SW480 and HCT-116
after treatment with casticin. Casticin induced the expression of
DR5 in both cell types (Fig.
3D).
DR5 induction is required for the
enhancement of TRAIL-induced apoptosis by casticin in HT-29
cells
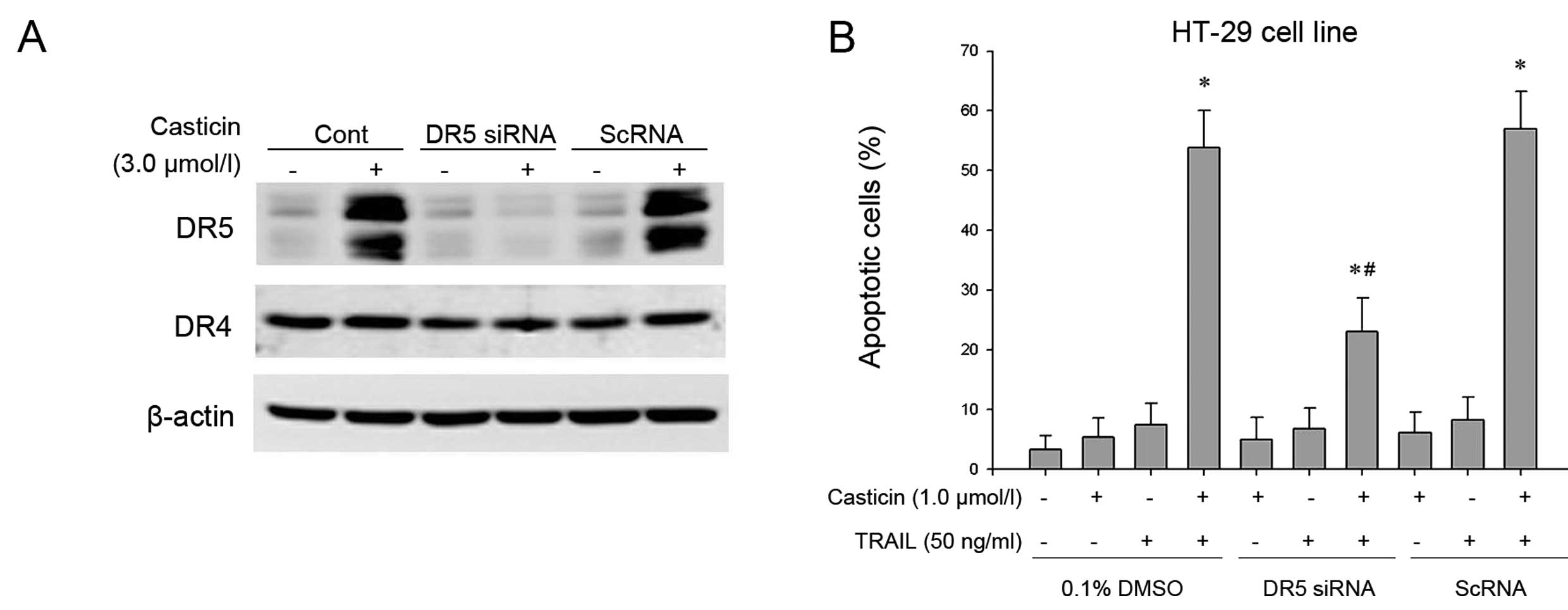
To determine the role of DR5 in TRAIL-induced
apoptosis, we used siRNA specific to DR5 to downregulate its
expression in HT-29 cells. Transfection of cells with siRNA for DR5
but not with the control siRNA reduced casticin-induced DR5
expression. DR5 siRNA had a minimal effect on the expression of DR4
(Fig. 4A).
Subsequently, the effect of DR5 siRNA on the
enhancement of TRAIL-induced apoptosis by casticin was examined
using morphological observation following AO/EB staining. Our
results showed that the effect of casticin on TRAIL-induced
apoptosis was effectively abolished in cells transfected with DR5
siRNA, but treatment with control siRNA had no effect (Fig. 4B). These facts suggest that DR5
plays a critical role in the enhancement of TRAIL-induced apoptosis
by casticin.
DR5 induction and enhancement of
TRAIL-induced apoptosis by casticin are ROS-dependent in HT-29
cells
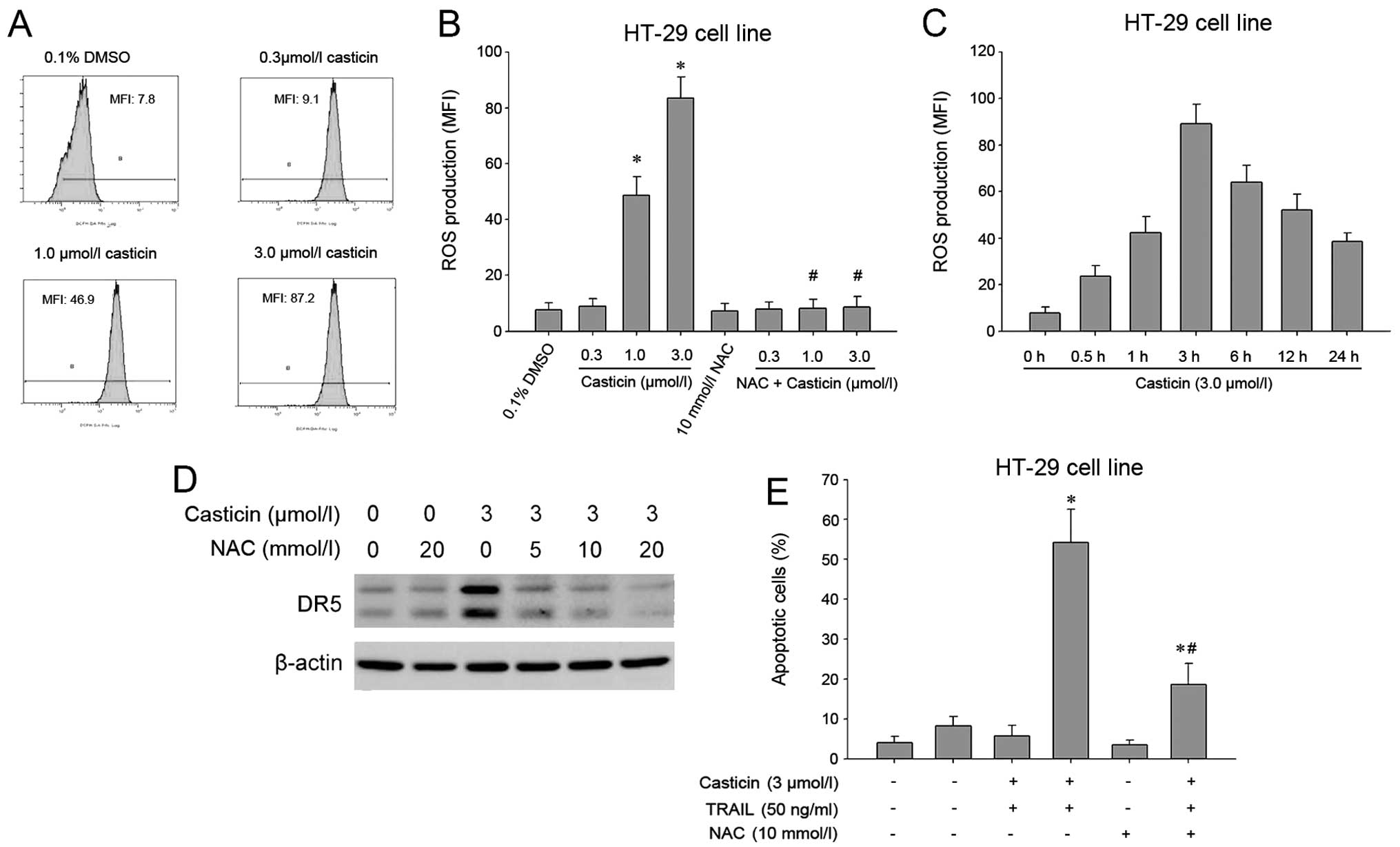
Numerous studies have shown that ROS are implicated
in TRAIL receptor induction (8,26,27).
Therefore, we investigated whether casticin mediates its effects
through ROS. Intracellular ROS was measured by flow cytometry using
the fluorescent probe DCFH-DA, and the results showed that casticin
induced ROS generation in a dose-dependent manner (Fig. 5A and B). Furthermore, ROS levels
increased initially at 0.5 h, reached the peak at 3 h and persisted
for up to 24 h after treatment with 3.0 μM casticin (Fig. 5C). Next, the effect of ROS on
casticin-induced DR5 was examined. We found that pretreatment of
cells with N-acetylcysteine reduced the casticin-induced
upregulation of DR5 expression (Fig.
5D). In addition, we investigated the effect of ROS on the
potentiation of TRAIL-induced apoptosis by casticin. As shown in
Fig. 5E, pretreatment with
N-acetylcysteine markedly reduced the enhancement of TRAIL-induced
apoptosis by casticin in HT-29 cells from 54.2±8.4 to 18.7±5.3%.
The above findings suggest that ROS play a critical role in
mediating the effects of casticin on DR5 expression and
TRAIL-induced apoptosis.
Discussion
TRAIL is a potential anticancer agent that can
selectively induce apoptosis in a wide variety of cancer cells
(1). However, resistance of cancer
cells to TRAIL-induced apoptosis limits its therapeutic
application. Recent studies have shown that several
chemotherapeutic drugs, i.e., curcumin, garcinol, gossypol,
kaempferol, can modulate TRAIL-induced apoptosis in cancer cells
(8–11), suggesting that TRAIL resistance can
be overcome by combination treatment, which may be a new strategy
for cancer therapy. Casticin, a flavonoid isolated from Vitex
rotundifolia, has been demonstrated to have antitumor
activities (i.e., inhibition of proliferation, induction of
apoptosis) against breast, lung, liver and colon cancer, and
leukemia (15–17, 20).
In the present study, we found that casticin enhances TRAIL-induced
apoptosis in colon cancer cells, and the mechanisms involve
downregulation of cell survival proteins and upregulation of
DR5.
Bcl-2 family proteins are central regulators of
apoptosis and act primarily on the mitochondria. On the basis of
the structural and functional characteristics, they are divided
into anti-apoptotic and pro-apoptotic proteins. The former have 4
Bcl-2 homology domains (BH1, -2, -3 and -4) and include Bcl-2,
Bcl-xL, Mcl-1, Bcl-w and A1/Bfl-1 and the latter include Bax, Bak,
and Box that contain 3 BH domains (BH1, -2 and -3) and Bid, Bim and
Bad that contain only the BH3 domain (28). Several studies have shown that Bcl-2
and Bcl-xL are involved in TRAIL resistance in tumor cells
(22,23). In the present study, we found that
casticin inhibits the expressions of Bcl-xL and Bcl-2; furthermore,
casticin upregulates the expression of Bax and causes the cleavage
of Bid protein in a dose-dependent manner, which may be one of its
mechanisms of potentiation of TRAIL-induced apoptosis.
Inhibitors of apoptosis (IAPs) are a family of
anti-apoptotic proteins that bind and inhibit caspases-3, -7 and
-9, but not caspase-8, thereby inhibiting their activation and
preventing apoptosis. Eight IAPs have been identified thus far,
including survivin, X-chromosome-linked IAP (XIAP), cellular IAP1
(c-IAP1), and c-IAP2 (29).
Survivin and XIAP have also been shown to be associated with tumor
cell resistance to TRAIL. Azuhata et al reported that
survivin can inhibit apoptosis induced by TRAIL, and that
inhibiting survivin expression by antisense oligonucleotides
enhances TRAIL sensitivity in human NB cells (24). Overexpression of XIAP is shown to
confer resistance against TRAIL in cancer cells (22). Furthermore, Siegelin et al
have shown that the XIAP inhibitor Embelin enhances TRAIL-mediated
apoptosis in malignant glioma cells, and its mechanism involves
downregulation of the short isoform of FLIP (25). FLICE (FADD-like IL-1β-converting
enzyme) inhibitory protein (FLIP) is a critical anti-apoptotic
regulator that inhibits TNF-α, Fas-L and TRAIL-induced apoptosis as
well as chemotherapy-triggered apoptosis in malignant cells. Three
isoforms of human cytosolic FLIP (c-FLIP) have been identified,
long (c-FLIPL), short (c-FLIPS) and c-FLIPR. c-FLIP binds to FADD
and/or caspase-8 or -10 in a ligand-dependent and-independent
fashion, thereby preventing the activation of procaspase-8
(30). In the present study, we
found that casticin inhibits the expressions of survivin, XIAP and
cFLIP, but has no effect on the expressions of cIAP-1 and TRAF1
[tumor necrosis factor receptor-associated factor 1, thought to be
a regulator of cell death and cellular responses to stress
(31)]. Inhibition of survivin,
XIAP and cFLIP may also be one of the mechanisms of potentiation of
TRAIL-induced apoptosis by casticin.
Disregulation of TRAIL receptors is involved in
TRAIL resistance in tumor cells (6,7). Five
TRAIL receptors have been discovered so far, including DR4, DR5,
DcR1, DcR2 and osteoprotegerin. DR4 and DR5 contain a cytoplasmic
region designated ‘death domain’ (DD) that transduces the death
signal. However, DcR1 and DcR2 lack a functional death domain and
cannot transduce a pro-apoptotic signal; instead, they compete with
DR4 and DR5 for TRAIL binding, and inhibit DR4- and DR5-mediated
apoptosis by TRAIL (2,3). In the present study, we found that
casticin increases the expression of DR5 in a dose-and
time-dependent manner, but it has no effect on the expressions of
DR4, DcR1 and DcR2 in colon cancer cells. Consistent with our
study, casticin has been shown to upregulate DR5 in hepatocellular
carcinoma cells (18). In addition,
we found that DR5 upregulation is critical for the enhancement of
TRAIL-induced apoptosis by casticin, which is evidenced by the fact
that gene silencing of DR5 abolished the effect of casticin on
TRAIL-induced apoptosis.
Reactive oxygen species (ROS) are chemically
reactive molecules containing oxygen, including oxygen ions
(O2•), hydrogen peroxide
(H2O2), and play an important role in a
variety of physiological and pathological processes. Kwon et
al showed that H2O2 can upregulate the
expression of DR5, thereby enhancing TRAIL-induced cell death in
human astrocytic cells (26). There
are several chemotherapeutic agents that induce DR5 through ROS
dependent pathways and sensitize TRAIL induced-apoptosis, including
curcumin, zerumbone, sulforaphane (8,26,32),
demonstrating that ROS play a major role in the modulation of DR5.
In the present study, we found that casticin induces ROS generation
in a dose-dependent manner in colon cancer cells, which, however,
is in contrast to another report that casticin has no effect on
intracellular ROS in hepatocellular carcinoma cells (18). The explanation for this discrepancy
may be due to different cell types. Similar to those previous
studies, our study also showed that ROS play a critical role in
mediating the effects of casticin on DR5 expression and
TRAIL-induced apoptosis, as evidenced by the fact that quenching of
ROS by N-acetylcysteine abolished the above effects.
In summary, the present study demonstrated that
casticin can enhance TRAIL-induced apoptosis through downregulation
of cell survival proteins (Bcl-xL, Bcl-2, survivin, XIAP and cFLIP)
and induction of DR5 mediated by ROS. Casticin is a potential
chemotherapeutic agent that can overcome TRAIL resistance when used
in combination with TRAIL; therefore, further studies are
warranted, particularly in clinical trials.
Acknowledgements
This study was supported by grants from the Project
Item of Scientific Research of the Administration Bureau of
Traditional Chinese Medicine of Hunan Province (no. 2010081), the
Project Item of Scientific Research of the Department of Education
of Hunan Province (no. 10C0975), and the Major Project Item of
Scientific Research of the Department of Education of Hunan
Province (no. 09A054).
References
|
1
|
MacFarlane M: TRAIL-induced signalling and
apoptosis. Toxicol Lett. 139:89–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pan G, Ni J, Wei YF, Yu G, Gentz R and
Dixit VM: An antagonist decoy receptor and a death
domain-containing receptor for TRAIL. Science. 277:815–818. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Emery JG, McDonnell P, Burke MB, et al:
Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J
Biol Chem. 273:14363–14367. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mellier G, Huang S, Shenoy K and Pervaiz
S: TRAILing death in cancer. Mol Aspects Med. 31:93–112. 2010.
View Article : Google Scholar
|
|
5
|
Zhang L and Fang B: Mechanisms of
resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther.
12:228–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rieger J, Frank B, Weller M and Wick W:
Mechanisms of resistance of human glioma cells to Apo2
ligand/TNF-related apoptosis-inducing ligand. Cell Physiol Biochem.
20:23–34. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kurbanov BM, Fecker LF, Geilen CC, Sterry
W and Eberle J: Resistance of melanoma cells to TRAIL does not
result from upregulation of antiapoptotic proteins by NF-kappaB but
is related to downregulation of initiator caspases and DR4.
Oncogene. 26:3364–3377. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jung EM, Lim JH, Lee TJ, Park JW, Choi KS
and Kwon TK: Curcumin sensitizes tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)-induced apoptosis through
reactive oxygen species-mediated upregulation of death receptor 5
(DR5). Carcinogenesis. 26:1905–1913. 2005. View Article : Google Scholar
|
|
9
|
Prasad S, Ravindran J, Sung B, Pandey MK
and Aggarwal BB: Garcinol potentiates TRAIL-induced apoptosis
through modulation of death receptors and antiapoptotic proteins.
Mol Cancer Ther. 9:856–868. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sung B, Ravindran J, Prasad S, Pandey MK
and Aggarwal BB: Gossypol induces death receptor-5 through
activation of the ROS-ERK-CHOP pathway and sensitizes colon cancer
cells to TRAIL. J Biol Chem. 285:35418–35427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siegelin MD, Reuss DE, Habel A,
Herold-Mende C and von Deimling A: The flavonoid kaempferol
sensitizes human glioma cells to TRAIL-mediated apoptosis by
proteasomal degradation of survivin. Mol Cancer Ther. 7:3566–3574.
2008. View Article : Google Scholar
|
|
12
|
Koh DJ, Ahn HS, Chung HS, et al:
Inhibitory effects of casticin on migration of eosinophil and
expression of chemokines and adhesion molecules in A549 lung
epithelial cells via NF-kappaB inactivation. J Ethnopharmacol.
136:399–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choudhary MI, Jalil S, Nawaz SA, Khan KM
and Tareen RB: Antiinflammatory and lipoxygenase inhibitory
compounds from Vitex agnus-castus. Phytother Res.
23:1336–1339. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mesaik MA, Murad S, Khan KM, Tareen RB,
Ahmed A and Choudhary MI: Isolation and immunomodulatory properties
of a flavonoid, casticin from Vitex agnus-castus. Phytother
Res. 23:1516–1520. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Csupor-Loffler B, Hajdu Z, Zupko I, et al:
Antiproliferative effect of flavonoids and sesquiterpenoids from
Achillea millefolium sl on cultured human tumour cell lines.
Phytother Res. 23:672–676. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haidara K, Zamir L, Shi QW and Batist G:
The flavonoid Casticin has multiple mechanisms of tumor
cytotoxicity action. Cancer Lett. 242:180–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kobayakawa J, Sato-Nishimori F, Moriyasu M
and Matsukawa Y: G2-M arrest and antimitotic activity mediated by
casticin, a flavonoid isolated from Viticis Fructus
(Vitex rotundifolia Linne file). Cancer Lett. 208:59–64.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen JK, Du HP, Yang M, Wang YG and Jin J:
Casticin induces leukemic cell death through apoptosis and mitotic
catastrophe. Ann Hematol. 88:743–752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J, Yang Y, Tian L, Sheng XF, Liu F
and Cao JG: Casticin-induced apoptosis involves death receptor 5
upregulation in hepatocellular carcinoma cells. World J
Gastroenterol. 17:4298–4307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao JG, Peng SP, Sun L, Li H, Wang L and
Deng HW: Vascular basement membrane-derived multifunctional
peptide, a novel inhibitor of angiogenesis and tumor growth. Acta
Biochim Biophys Sin. 38:514–521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang XH, Zheng X, Cao JG, Xiang HL, Liu F
and Lv Y: 8-Bromo-7-methoxychrysin-induced apoptosis of
hepatocellular carcinoma cells involves ROS and JNK. World J
Gastroenterol. 16:3385–3393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fulda S, Meyer E and Debatin KM:
Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression.
Oncogene. 21:2283–2294. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song JJ, An JY, Kwon YT and Lee YJ:
Evidence for two modes of development of acquired tumor necrosis
factor-related apoptosis-inducing ligand resistance. Involvement of
Bcl-xL. J Biol Chem. 282:319–328. 2007. View Article : Google Scholar
|
|
24
|
Azuhata T, Scott D, Griffith TS, Miller M
and Sandler AD: Survivin inhibits apoptosis induced by TRAIL, and
the ratio between survivin and TRAIL receptors is predictive of
recurrent disease in neuroblastoma. J Pediatr Surg. 41:1431–1440.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siegelin MD, Gaiser T and Siegelin Y: The
XIAP inhibitor Embelin enhances TRAIL-mediated apoptosis in
malignant glioma cells by down-regulation of the short isoform of
FLIP. Neurochem Int. 55:423–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kwon D, Choi K, Choi C and Benveniste EN:
Hydrogen peroxide enhances TRAIL-induced cell death through
up-regulation of DR5 in human astrocytic cells. Biochem Biophys Res
Commun. 372:870–874. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yodkeeree S, Sung B, Limtrakul P and
Aggarwal BB: Zerumbone enhances TRAIL-induced apoptosis through the
induction of death receptors in human colon cancer cells: evidence
for an essential role of reactive oxygen species. Cancer Res.
69:6581–6589. 2009. View Article : Google Scholar
|
|
28
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Altieri DC: Survivin and IAP proteins in
cell-death mechanisms. Biochem J. 430:199–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Safa AR and Pollok KE: Targeting the
anti-apoptotic protein c-FLIP for cancer therapy. Cancers.
3:1639–1671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bradley JR and Pober JS: Tumor necrosis
factor receptor-associated factors (TRAFs). Oncogene. 20:6482–6491.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim H, Kim EH, Eom YW, et al: Sulforaphane
sensitizes tumor necrosis factor-related apoptosis-inducing ligand
(TRAIL)-resistant hepatoma cells to TRAIL-induced apoptosis through
reactive oxygen species-mediated up-regulation of DR5. Cancer Res.
66:1740–1750. 2006. View Article : Google Scholar
|