Introduction
Gestational choriocarcinoma, one type of gestational
trophoblastic neoplasia, is a malignant epithelial trophoblastic
tumor that can develop in the uterus after pregnancy (1). Half of the cases are preceded by a
hydatidiform mole, while others occur after spontaneous abortion or
normal term pregnancy (1).
Treatment of a hydatidiform mole using a clinical marker, human
chorionic gonadotropin (hCG), has significantly reduced the
incidence of choriocarcinoma and improved survival (2). hCG is a glycoprotein that is produced
by fused and differentiated placental syncytiotrophoblast cells and
serves as a marker for placental function and assessment of
treatment for malignant trophoblastic disease (3). The function and structure of hCG are
well-established and hCG variants are known to be heavily
glycosylated and sialylated glycoproteins (3).
Glycosylation is a common and versatile co- and
post-translational modification that involves attachment of glycans
to proteins, lipids or other organic molecules by
glycosyltransferases encoded by glycogenes (4,5).
Altered glycan structure by glycosylation is a common feature in
cancer cells and promotes tumor cell invasion and metastasis.
Certain glycans are markers for tumor progression (6,7), and
thus it is important to analyze the structure and binding
mechanisms of these structures in the context of cancer diagnosis
and treatment. However, comprehensive glycan analysis has not been
performed in choriocarcinoma although several molecular studies
have revealed the expression of tumor-related proteins in
choriocarcinoma (1). Indeed,
conventional glycan profiling tools, such as capillary
electrophoresis, liquid chromatography and mass spectrometry, may
not be suitable for the initial detection of glycan alterations
between samples because of the time-consuming, low-throughput and
requirements for complex equipment and the generation of complex
data (8,9). Conversely, lectin microarray has
emerged in recent years and could be used for rapid and
high-throughput analysis for protein glycosylation (8,9).
We previously established a new choriocarcinoma cell
line, induced choriocarcinoma cell-1 (iC3-1), that
mimics the clinical pathohistology in vivo, and we used
these cells to examine the tumorigenesis and pathogenesis of
choriocarcinoma (10,11). The goal of the present study was to
investigate alterations in glycan structure in the development of
choriocarcinoma using comprehensive glycan profiling in clinical
samples and in iC3-1 cells using a conventional
microarray and the recently developed lectin microarray. Herein, we
report the initial data from a lectin microarray in choriocarcinoma
tissue.
Materials and methods
Patients and samples
Formalin-fixed, paraffin-embedded tissues from 4
cases were used in the study. First trimester villi were obtained
from induced-abortion cases (n=2) and choriocarcinoma samples were
obtained from pregnancies that had gone to term and in which
hysterectomy was performed piror to other treatment (n=2). The
samples were obtained during surgery or delivery and were
immediately fixed in formalin. All patients had been treated
between 2008 and 2011 at Keio University Hospital. The samples were
examined and diagnosed by two independent pathologists. This study
was approved by the Institutional Review Board of Keio University
School of Medicine (No. 20130115).
Cell culture
HTR8/SVneo, a human extravillous trophoblast cell
line immortalized using SV40 T antigen (SV40Tag), was kindly
provided by Dr C.H. Graham (Queen’s University, Kingston, ON,
Canada) (12). HTR8/SVneo/EGFP and
iC3-1 cells were obtained as previously described
(10). Before culture, cell sorting
was performed with a FACSVantage SE (BD Biosciences, Franklin
Lakes, NJ, USA) (10) after
staining with propidium iodide (P4170; Sigma-Aldrich, St. Louis,
MO, USA). Sorting based on GFP and propidium iodide fluorescence
with gating on forward and side scatter was used to obtain
transfected cells and exclude non-viable cells. Sorted
HTR8/SVneo/EGFP and iC3-1 cells were cultured in
Dulbecco’s modified Eagle’s medium (Gibco-BRL, Burlington, ON,
Canada) supplemented with 10% heat-inactivated fetal calf serum,
penicillin (100 U/ml) and streptomycin (100 mg/ml) at 37°C in a 5%
CO2 atmosphere (10).
Microarray analysis
Microarray analysis comparing iC3-1 cells
and parental HTR8/SVneo/EGFP cells were performed as previously
reported (10). Briefly, total RNA
from those cells was extracted using the Isogen kit (Nippon Gene,
Tokyo, Japan) according to the manufacturer’s instructions. After
being amplified, labeled and hybridized by the Human Whole Genome
Oligo Microarray kit (G4112F; Agilent, Santa Clara, CA, USA)
following the Agilent user’s manual protocol, the arrays were
scanned using the Agilent Dual-Laser DNA Microarray Scanner
(G2565A; Agilent).
Lectin microarray analysis
Lectin microarray analysis was performed as
previously described (13).
Briefly, the membrane fractions of HTR8/SVneo and iC3-1
cells were obtained using the ProteoExtract Subcellular Proteome
Extraction kit (539790; Merck). Glycoproteins from formalin-fixed
paraffin-embedded tissue of normal villi and choriocarcinoma were
obtained as previously reported (14). The total protein content of each
sample was determined using a Micro BCA Protein Assay kit (23235;
Thermo Scientific, Hemel Hempstead, UK) and adjusted to 50 μg/ml
with phosphate-buffered saline. A portion of each sample (1 μg) was
added to 100 μg of Cy3 monoreactive dye pack (PA23001; GE
Healthcare) and incubated for 1 h at room temperature in the dark.
Cy3-labeled proteins were desalted using a Zeba Spin Desalting
Column (89882; Thermo Scientific) and diluted to 2 μg/ml with
probing solution (TBS containing 1% Triton X-100, 500 mM
glycine).
A sample of 100 μl was added to each well on a
lectin microarray glass slide (GP Biosciences, Yokohama, Japan) and
incubated in a chamber (>80% humidity) for 150 min at room
temperature in the dark until the binding reached equilibrium.
Fluorescent images of the lectin microarrays were acquired using an
evanescent-field fluorescence scanner (GlycoStation™ Reader 1200;
GP Biosciences). The net intensity for each spot was calculated by
subtracting the background value from the raw signal intensity,
with averaging of the results from three spots. Acquired data were
normalized by making the total fluorescence in each well (i.e. for
45 lectins) equivalent. All data were analyzed with Array-Pro
Analyzer v. 4.5 (Media Cybernetics, Bethesda, MD, USA).
Results
Differences in glycogene expression in
the iC3-1 and HTR8/SVneo cells
Gene expression profiles of iC3-1 cells
and a parental trophoblast cell line, HTR8/SVneo, derived from
first trimester placental tissue, were determined by microarray
analysis to examine alterations in glycogenes in choriocarcinoma
tumorigenesis. As previously reported, in iC3-1 cells
compared to HTR8/SVneo/EGFP cells, 3,094 genes were upregulated and
3,126 genes were downregulated among the 44,000 genes examined
(10). To search the differences in
glycol-gene expression, we referred to the GlycoGene DataBase
(http://riodb.ibase.aist.go.jp/rcmg/ggdb/) for the
upregulated or downregulated genes. This analysis showed that 20
glycogenes were significantly upregulated and 15 were significantly
downregulated in iC3-1 cells as compared to the
HTR8/SVneo/EGFP cells (Table I).
The upregulated genes included hyaluronan synthase (HAS2), which we
previously found to show immunoreactivity in choriocarcinoma
samples, but not in normal first trimester villi and term placenta
(11).
 | Table IGlycogenes with altered expression in
iC3-1 cells as compared to HTR8/SVneo/EGFP cells. |
Table I
Glycogenes with altered expression in
iC3-1 cells as compared to HTR8/SVneo/EGFP cells.
| Gene symbol | Family | Fold-change | P-value |
|---|
| Upregulated |
| HAS2 |
Glucuronyltransferases
N-acetylglucosaminyltransferases | 664.90 | 1.22.E-04 |
| HS6ST2 |
Sulfotransferases | 14.56 | 4.94.E-03 |
| MGAT4A |
N-acetylglucosaminyltransferases | 9.18 | 1.01.E-03 |
| XYLT1 |
Xylosyltransferases | 9.18 | 1.05.E-03 |
| GALNT14 |
UDP-GalNAc:polypeptide
N-acetylgalactosaminyltransferase | 8.15 | 1.13.E-03 |
| POFUT1 |
Fucosyltransferases | 6.62 | 3.61.E-03 |
| ST6GALNAC3 |
Sialyltransferases | 5.92 | 1.58.E-03 |
| UST |
Sulfotransferases | 4.27 | 2.38.E-03 |
| GALNT3 |
UDP-GalNAc:polypeptide
N-acetylgalactosaminyltransferase | 3.87 | 2.89.E-03 |
| ST3GAL4 |
Sialyltransferases | 3.40 | 2.64.E-03 |
| GCNT2 |
N-acetylglucosaminyltransferases | 3.33 | 2.42.E-03 |
| ST8SIA5 |
Sialyltransferases | 3.11 | 3.04.E-03 |
| CHST2 |
Sulfotransferases | 2.70 | 5.01.E-03 |
| GALNT6 |
UDP-GalNAc:polypeptide
N-acetylgalactosaminyltransferase | 2.65 | 5.22.E-03 |
| SLC35D1 | Nucleotide sugar
transporters | 2.48 | 6.01.E-03 |
| HS3ST3A1 |
Sulfotransferases | 2.47 | 6.02.E-03 |
| MGAT5B |
N-acetylglucosaminyltransferases | 2.26 | 1.20.E-02 |
| GALNT10 |
UDP-GalNAc:polypeptide
N-acetylgalactosaminyltransferase | 2.20 | 7.98.E-03 |
| FUT8 |
Fucosyltransferases | 2.12 | 8.67.E-03 |
| HAS3 |
Glucuronyltransferases
N-acetylglucosaminyltransferases | 2.03 | 5.27.E-03 |
| Downregulated |
| B4GALNT3 |
N-acetylgalactosaminyltransferases | 0.18 | 2.32.E-03 |
| ST6GALNAC2 |
Sialyltransferases | 0.19 | 6.96.E-03 |
| B3GALT5 |
Galactosyltransferases | 0.24 | 7.09.E-03 |
| GAL3ST1 |
Sulfotransferases | 0.27 | 4.03.E-03 |
| GALNT9 |
UDP-GalNAc:polypeptide
N-acetylgalactosaminyltransferase | 0.27 | 1.06.E-02 |
| B3GAT1 |
Glucuronyltransferases | 0.28 | 3.79.E-02 |
| LARGE |
Glycosyltransferase-like | 0.30 | 9.68.E-03 |
| MFNG |
N-acetylglucosaminyltransferases | 0.31 | 3.78.E-03 |
| CHST1 |
Sulfotransferases | 0.36 | 1.58.E-03 |
| ST3GAL5 |
Sialyltransferases | 0.36 | 1.69.E-03 |
| NDST1 |
Sulfotransferases | 0.38 | 5.41.E-03 |
| POMT2 |
Mannosyltransferases | 0.43 | 6.87.E-03 |
| CHST3 |
Sulfotransferases | 0.44 | 7.25.E-03 |
| B3GALT2 |
Galactosyltransferases | 0.49 | 2.45.E-03 |
| GALNT2 |
UDP-GalNAc:polypeptide
N-acetylgalactosaminyltransferase | 0.50 | 1.00.E-02 |
Glycan profiling analysis in
choriocarcinoma
Glycan profiling analysis of normal villi,
choriocarcinoma, HTR8/SVneo and iC3-1 cells was used to
investigate changes in glycan structure in the development of
choriocarcinoma, using a lectin microarray. Signals for Cy3-labeled
glycoproteins on the LecChip, which includes 45 different lectins,
were scanned using GlycoStation Reader 1200 independently four
times (Fig. 1A). The net intensity
of each lectin signal was quantified using Glycostation Tools Pro
and Array-Pro Analyzer (Fig. 1B).
Lectins with increased levels in choriocarcinoma samples (>20%
increase in signal intensity compared to villi) were Sambucus
nigra agglutinin (SNA), Sambucus sieboldiana agglutinin
(SSA), Trichosanthes japonica agglutinin I (TJA-I),
Ricinus communis agglutinin I (RCA120), erythroagglutinating
isolectin of phytohemagglutinin (PHAE), Datura stramonium
agglutinin (DSA), Agrocybe cylindracea galectin (ACG),
TxLC-I, Urtica dioica agglutinin (UDA), Jacalin, and wheat
germ agglutinin (WGA) (Fig. 1B and
Table II). In contrast, those with
decreased levels in choriocarcinoma samples (>20% decrease in
signal intensity compared to villi) were Pisum sativum
agglutinin (PSA), Lens culinaris agglutinin (LCA),
Aspergillus oryzae lectin (AOL), Narcissus
pseudonarcissus agglutinin (NPA), Galanthus nivalis
agglutinin (GNA), Bauhinia purpurea lectin (BPL),
Wisteria floribunda agglutinin (WFA), Amaranthus
caudatus agglutinin (ACA), Helix pomatia agglutinin
(HPA) and Maackia amurensis hemagglutinin (MAH) (Fig. 1B and Table II). A comparison of
iC3-1 and parental HTR8/SVneo cells showed quite similar
glycan profiles, suggesting that activation of the MAPK/PI3K
pathway does not contribute to the alteration of glycan structures
(Fig. 1C).
 | Table IILectins and their glycan-binding
specificity. |
Table II
Lectins and their glycan-binding
specificity.
| Lectin | Signal ratio | Specificity |
|---|
| TxLC-I | 3.010 |
Manα1-3(Manα1-6)Man, bi- and tri-antennary
complex-type N-glycan, GalNAc |
| Jacalin | 2.116 | Galβ1-3GalNAc,
GalNAc |
| ACG | 1.973 |
Siaα2-3Galβ1-4GlcNAc |
| WGA | 1.806 | Chitin oligomers,
Sia |
| SSA | 1.729 |
Siaα2-6Gal/GalNAc |
| PHAE | 1.666 | Bi-antennary
complex-type N-glycan with outer Gal and bisecting GlcNAc |
| TJA-I | 1.533 |
Siaα2-6Gal/GalNAc |
| RCA120 | 1.455 | Galβ1-4GlcNAc |
| UDA | 1.408 | GlcNAcβ1-4GlcNAc,
mixture of Man5 to Man9 |
| SNA | 1.379 |
Siaα2-6Gal/GalNAc |
| DSA | 1.206 | (GlcNAcβ1-4)n,
Galβ1-4GlcNAc |
| NPA | 0.572 | High-Mannose,
Manα1-6Man |
| ACA | 0.608 | Galβ1-3GalNAc |
| BPL | 0.717 | Galβ1-3GalNAc,
GalNAc |
| AOL | 0.724 | Fucα1-6GlcNAc (core
fucose) |
| MAH | 0.731 |
Siaα2-3Galβ1-3(Siaα2-6)GalNAc |
| GNA | 0.744 | High-Mannose,
Manα1-3Man |
| LCA | 0.774 | Fucα1-6GlcNAc,
α-D-Glc, α-D-Man |
| PSA | 0.783 | Fucα1-6GlcNAc,
α-D-Glc, α-D-Man |
| HPA | 0.793 | α-linked terminal
GalNAc |
Based on the glycan-binding specificity of these
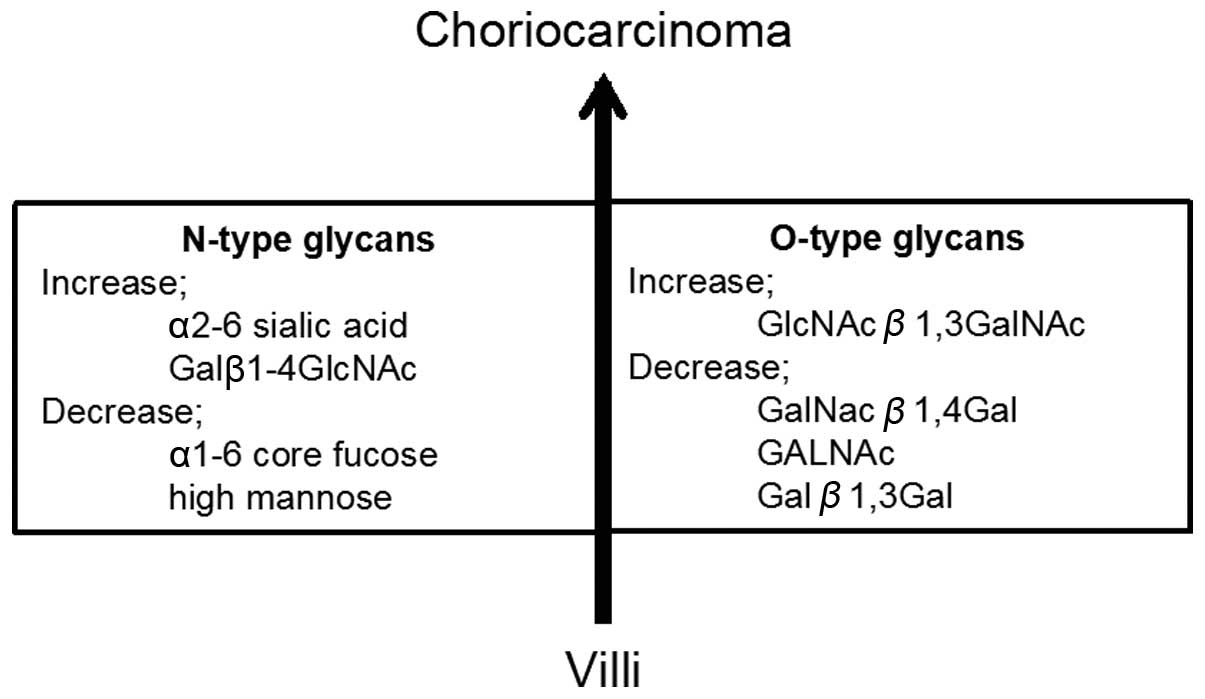
lectins, among the N-type glycans, choriocarcinoma had higher
levels of α-2-6 sialic acids (SNA, SSA, TJA-I and WGA), reduced
levels of α-1-6 core fucoses (PSA, LCA and AOL), increases in
Galβ1-4GlcNAc structures (RCA120, PHAE and DSA), and decreases in
high mannose (NPA and GNA), compared to normal villi (Fig. 2 and Table II). In regards to O-type glycans,
choriocarcinoma had increased GlcNAcβ1-3GalNAc structures (Jacalin)
and decreases in GalNacβ1-4Gal (WFA), GALNAc (Tn antigen) (HPA) and
Galβ1-3Gal (ACA), when compared to normal villi (Fig. 2 and Table II).
Discussion
The function of most proteins depends on
post-translational modifications such as glycosylation,
phosphorylation, methylation and sulfation (15). In glycosylation, glycan attachment
modifies the function of proteins in areas such as cell
recognition, cell-cell interactions and immune response, and is
also important in carcinogenesis, including proliferation,
invasion, metastasis and tumor progression (16,17).
Therefore, glycan analysis is a key to the understanding of
physiological function and pathogenesis. However, progress in
glycobiology has been slow due to the complexity of glycans and a
lack of systematic methods with which to analyze their structures.
This difficulty has been addressed by the introduction of
technology for rapid, simple and high-throughput evaluation
(18). In particular, the lectin
microarray, based on an evanescent-field fluorescence detection
principle, permits sensitive and quantitative real-time observation
of multiple lectin-carbohydrate interactions unlike other
conventional methods, e.g., mass spectrometry and chromatography
(19). Even though a lectin
microarray has emerged only in recent years, it has already been
applied for the development of disease-related glycoprotein
markers, the evaluation of stem cells and the investigation of
pluripotent cell markers (19).
Glycan analysis in choriocarcinoma has not
previously been performed, in contrast to other cancers. Therefore,
in the present study, we used microarrays for glycogenes and
lectins in our established choriocarcinoma cell line and clinical
samples to investigate glycan alterations in choriocarcinoma
tumorigenesis. Conventional microarray comparison of
iC3-1 and parental HTR8/SVneo cells indicated changes in
several significant glycogenes. These include: HAS2, which we have
shown to be significantly overexpressed in choriocarcinoma samples
(10); HAS3, which is correlated
with tumor growth in colon cancer and esophageal squamous cell
carcinoma (20,21); HS6ST2, which plays an important role
in cell growth, invasion, migration and tumorigenicity in several
types of cancers (22–24); FUT8, the expression of which is
upregulated in lung, liver, ovarian, thyroid and colorectal cancer
and correlates with tumor metastasis, disease recurrence and poor
survival (25); POFUT1, which has
higher expression in colon cancer and glioblastoma compared to
normal tissue (26,27); GalNT14, a biomarker that is
predictive of the response to PARA/extrinsic pathway-targeted
therapy in non-small cell lung cancer, since high levels of GalNT14
mRNA and protein in tumor cell lines are associated with
Apo2L/TRAIL sensitivity (28);
MGAT5B, which is upregulated in prostate cancer cells and is a
marker of tumor progression (29);
CHST2, a clinical molecular marker for the outcome of osteosarcoma
(30); and HS3ST3A1, expression of
which is higher in cancer tissue compared to normal lung tissue
(31).
The lectin microarray also produced novel findings
for glycans in choriocarcinoma. Among the lectins with increased or
decreased levels in choriocarcinoma as compared to normal villi,
SSA, TJA-I, RCA120, SNA, ACA, BPL and HPA were found to exhibit the
same binding pattern in colon cancer when compared to normal tissue
(14). Thus, these lectins may be
useful as diagnostic and therapeutic markers in choriocarcinoma. In
particular, the increased levels of SNA, SSA and TJA-I indicate the
presence of a higher level of α-2-6 sialic acid, and also indicate
the aggressive behavior of choriocarcinoma as these lectins were
found to be highly expressed in poorly differentiated endometrial
cancer cells compared to well-differentiated cells (32). In addition, SSA is a potential
marker for isolation of cancer stem cells (CSCs) (33). iC3-1 cells may contain
components of choriocarcinoma CSCs and CD44 may be a marker for
these CSCs (10). Thus, it will be
of interest to attempt to isolate choriocarcinoma CSCs using CD44
and SSA.
The emerging technology of the lectin microarray is
likely to permit elucidation of the precise glycan characteristics
of proteins of importance in choriocarcinoma. Our data revealed
that there are differences in glycogenes and lectin expression that
represent potential therapeutic targets for choriocarcinoma.
Acknowledgements
We thank Charles H. Graham (Queen’s University,
Kingston, ON, Canada) for kindly providing the HTR8/SVneo cells;
Yasunori Yoshimura (Keio University, Tokyo, Japan) for kindly
donating the villi and placenta tissue; Ikuyo Ishimatsu for
technical assistance in histological analyses; and Akiyoshi Noguchi
for the fundamental support.
References
|
1
|
Shih IeM: Gestational trophoblastic
neoplasia - pathogenesis and potential therapeutic targets. Lancet
Oncol. 8:642–650. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cavaliere A, Ermito S, Dinatale A and
Pedata R: Management of molar pregnancy. J Prenat Med. 3:15–17.
2009.
|
|
3
|
Cole LA: hCG, the wonder of today’s
science. Reprod Biol Endocrinol. 10:242012.PubMed/NCBI
|
|
4
|
Morelle W, Canis K, Chirat F, Faid V and
Michalski JC: The use of mass spectrometry for the proteomic
analysis of glycosylation. Proteomics. 6:3993–4015. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohtsubo K and Marth JD: Glycosylation in
cellular mechanisms of health and disease. Cell. 126:855–867. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goetz JA, Mechref Y, Kang P, Jeng MH and
Novotny MV: Glycomic profiling of invasive and non-invasive breast
cancer cells. Glycoconj J. 26:117–131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Satomaa T, Heiskanen A, Leonardsson I, et
al: Analysis of the human cancer glycome identifies a novel group
of tumor-associated N-acetylglucosamine glycan antigens. Cancer
Res. 69:5811–5819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patwa T, Li C, Simeone DM and Lubman DM:
Glycoprotein analysis using protein microarrays and mass
spectrometry. Mass Spectrom Rev. 29:830–844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fry SA, Afrough B, Lomax-Browne HJ, Timms
JF, Velentzis LS and Leathem AJ: Lectin microarray profiling of
metastatic breast cancers. Glycobiology. 21:1060–1070. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kobayashi Y, Shimizu T, Naoe H, et al:
Establishment of a choriocarcinoma model from immortalized normal
extravillous trophoblast cells transduced with HRASV12. Am J
Pathol. 179:1471–1482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kobayashi Y, Banno K, Shimizu T, et al:
Gene expression profile of a newly established choriocarcinoma cell
line, iC3-1, compared to existing choriocarcinoma cell
lines and normal placenta. Placenta. 34:110–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Graham CH, Hawley TS, Hawley RG, et al:
Establishment and characterization of first trimester human
trophoblast cells with extended lifespan. Exp Cell Res.
206:204–211. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuno A, Uchiyama N, Koseki-Kuno S, et al:
Evanescent-field fluorescence-assisted lectin microarray: a new
strategy for glycan profiling. Nat Methods. 2:851–856. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuda A, Kuno A, Ishida H, Kawamoto T,
Shoda J and Hirabayashi J: Development of an all-in-one technology
for glycan profiling targeting formalin-embedded tissue sections.
Biochem Biophys Res Commun. 370:259–263. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Muthana SM, Campbell CT and Gildersleeve
JC: Modifications of glycans: biological significance and
therapeutic opportunities. ACS Chem Biol. 7:31–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sacchettini JC, Baum LG and Brewer CF:
Multivalent protein-carbohydrate interactions. A new paradigm for
supermolecular assembly and signal transduction. Biochemistry.
40:3009–3015. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stanley P: Biological consequences of
overexpressing or eliminating N-acetylglucosaminyltransferase-TIII
in the mouse. Biochim Biophys Acta. 1573:363–368. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao J, Patwa TH, Lubman DM and Simeone
DM: Protein biomarkers in cancer: natural glycoprotein microarray
approaches. Curr Opin Mol Ther. 10:602–610. 2008.PubMed/NCBI
|
|
19
|
Hirabayashi J, Yamada M, Kuno A and Tateno
H: Lectin microarrays: concept, principle and applications. Chem
Soc Rev. 42:4443–4458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Teng BP, Heffler MD, Lai EC, et al:
Inhibition of hyaluronan synthase-3 decreases subcutaneous colon
cancer growth by increasing apoptosis. Anticancer Agents Med Chem.
11:620–628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Twarock S, Freudenberger T, Poscher E, et
al: Inhibition of oesophageal squamous cell carcinoma progression
by in vivo targeting of hyaluronan synthesis. Mol Cancer.
10:302011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song K, Li Q, Peng YB, et al: Silencing of
hHS6ST2 inhibits progression of pancreatic cancer through
inhibition of Notch signalling. Biochem J. 436:271–282. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Waaijer CJ, de Andrea CE, Hamilton A, van
Oosterwijk JG, Stringer SE and Bovée JV: Cartilage tumour
progression is characterized by an increased expression of heparan
sulphate 6O-sulphation-modifying enzymes. Virchows Arch.
461:475–481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferreras C, Rushton G, Cole CL, et al:
Endothelial heparan sulfate 6-O-sulfation levels regulate
angiogenic responses of endothelial cells to fibroblast growth
factor 2 and vascular endothelial growth factor. J Biol Chem.
287:36132–36146. 2012.PubMed/NCBI
|
|
25
|
Chen CY, Jan YH, Juan YH, et al:
Fucosyltransferase 8 as a functional regulator of nonsmall cell
lung cancer. Proc Natl Acad Sci USA. 110:630–635. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kroes RA, Dawson G and Moskal JR: Focused
microarray analysis of glyco-gene expression in human
glioblastomas. J Neurochem. 103(Suppl 1): S14–S24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Loo LW, Tiirikainen M, Cheng I, et al:
Integrated analysis of genome-wide copy number alterations and gene
expression in microsatellite stable, CpG island methylator
phenotype-negative colon cancer. Genes Chromosomes Cancer.
52:450–466. 2013. View Article : Google Scholar
|
|
28
|
Soria JC, Márk Z, Zatloukal P, et al:
Randomized phase II study of dulanermin in combination with
paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell
lung cancer. J Clin Oncol. 29:4442–4451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lange T, Ullrich S, Müller I, et al: Human
prostate cancer in a clinically relevant xenograft mouse model:
identification of β(1,6)-branched oligosaccharides as a marker of
tumor progression. Clin Cancer Res. 18:1364–1373. 2012.PubMed/NCBI
|
|
30
|
Chen X, Yang TT, Qiu XC, et al: Gene
expression profiles of human osteosarcoma cell sublines with
different pulmonary metastatic potentials. Cancer Biol Ther.
11:287–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakano T, Shimizu K, Kawashima O, et al:
Establishment of a human lung cancer cell line with high metastatic
potential to multiple organs: Gene expression associated with
metastatic potential in human lung cancer. Oncol Rep. 28:1727–1735.
2012.PubMed/NCBI
|
|
32
|
Nishijima Y, Toyoda M, Yamazaki-Inoue M,
et al: Glycan profiling of endometrial cancers using lectin
microarray. Genes Cells. 17:826–836. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moriwaki K, Okudo K, Haraguchi N, et al:
Combination use of anti-CD133 antibody and SSA lectin can
effectively enrich cells with high tumorigenicity. Cancer Sci.
102:1164–1170. 2011. View Article : Google Scholar : PubMed/NCBI
|