Introduction
Hepatocellular carcinoma (HCC) is the second most
common cause of cancer-related death worldwide and nearly half of
all HCC cases occur in China (1).
Despite improvements in surgical techniques and the development of
novel therapies during the past few decades, the clinical prognosis
of HCC patients is still poor due to recurrence and metastasis. The
molecular mechanisms involved in HCC development remain obscure.
Therefore, it is of great clinical value to further identify
malignant factors in order to understand the molecular mechanisms
underlying the progression of HCC.
Tumor angiogenesis plays an important role in tumor
growth and metastasis (2). Vascular
endothelial growth factor (VEGF) has been implicated as an invasion
and tumor progression promoter molecule (3). VEGF is a potent mitogen that
contributes to both physiological and pathological angiogenesis
(4). VEGF is believed to secrete
homodimeric glycoprotein that stimulates proliferation and
migration of endothelial cells and enhances vascular permeability
(5). An increasing number of
studies have demonstrated a strong association between
overexpression of VEGF and advanced disease or poor prognosis in
various types of cancers (6–8). VEGF
was recently found to be upregulated in HCC, and it was also shown
to be associated with the carcinogenesis, metastasis, recurrence
and prognosis of HCC (9,10). However, further investigation is
needed to confirm the molecular mechanisms underlying the effects
of VEGF on the development of HCC.
Several mechanisms have been reported to participate
in the regulation of VEGF gene expression. Among these, several
cytokines or growth factors play a major role. VEGF mRNA expression
is rapidly and reversibly induced by epidermal growth factor (EGF),
transforming growth factor-β (TGF-β), or keratinocyte growth factor
(11). Ren et al (12) reported that macrophage migration
inhibitory factor (MIF) can stimulate the secretion of VEGF from
tumor cells. The cytokine MIF is regarded as a major regulator of
inflammation and a key mediator that operates as a cytokine and an
enzyme (13). Many studies have
confirmed the use of MIF as a biomarker for different diseases that
have an inflammatory component (14). Moreover, recent studies have
demonstrated a role of MIF in tumor growth, such as control of cell
proliferation and promotion of angiogenesis (15). MIF also plays an important role in
the invasion and metastasis of prostate cancer, lung adenocarcinoma
and neuroblastoma cells (15,16).
Ren et al (17) found that
MIF mRNA was upregulated in HCC tissues when compared with normal
liver tissues, suggesting that MIF acts as a regulator of tumor
progression in HCC.
The above studies suggest that both VEGF and MIF may
be involved in the tumorigenesis of HCC. An examination of whether
the aberrant expression of these two proteins is associated with
clinicopathological characteristics of HCC patients is therefore
warranted. However, to date there has been no report on the
clinical relevance of combined VEGF and MIF expression in HCC
tissues. To address this problem, the aim of the present study was
to further investigate the potential association of the
co-expression of VEGF and MIF in HCC tissues with clinicopathologic
findings.
Patients and methods
Patients and tissue specimens
One hundred and fifty pairs of matched HCC and
adjacent non-cancer liver tissues were histopathologically and
clinically diagnosed at The First Affiliated Hospital of Sun
Yat-Sen University from January 2004 to June 2006. Plasma samples
from a peripheral vein were also collected from the 150 HCC
patients. Plasma samples were obtained from healthy volunteers who
underwent physical examination at the First Affiliated Hospital of
Sun Yat-Sen University. The 150 patients included 95 males and 55
females. The mean age of the patients was 58 years (range, 20–78
years). Clinicopathological classification and staging were carried
out according to the 6th edition of the American Joint Committee on
Cancer (AJCC) TNM classification system. Another independent 48
patients with histologically proven HCC were included in this
study. These 48 pairs of tumor tissues from HCC patients and paired
adjacent non-cancer specimens were collected for real-time RT-PCR
analysis as previously described (18). The study protocol was approved by
the Ethics Committee of the First Affiliated Hospital of Sun
Yat-Sen University. Informed consent was obtained from all patients
prior to surgery. All patients were recruited into this study after
providing informed consent.
Enzyme-linked immunosorbent assay
All peripheral blood samples were acquired following
a standard collection protocol. Briefly, samples were collected and
anticoagulated by ethylene diamine tetraacetic acid (EDTA) and
centrifuged for 10 min at 3000 rpm. The serum fractions were
aliquoted and stored at −80°C until analysis. The concentrations of
serum MIF were measured by quantitative sandwich enzyme-linked
immunosorbent assay (ELISA) kits (Quantikine, R&D Systems,
Minneapolis, MN, USA) according to the manufacturer’s protocols.
The levels of serum VEGF were determined using ELISA kits (Genzyme
Corp., USA).
Tissue microarray construction
The representative areas of each HCC specimen or
paired adjacent non-cancer liver tissue were punched with a tissue
cylinder (1 mm in diameter) from formalin-fixed/paraffin-embedded
tumor tissues or paired adjacent non-cancer tissue blocks. The
selected tissue cores were precisely arrayed into a new recipient
microarray block using a tissue arrayer (Beecher Instrument, Silver
Spring, MD, USA). Each sample was arrayed in triplicate.
Immunohistochemistry
Immunohistochemical analysis was performed to study
MIF and VEGF expression in 150 human HCC tissues and paired
adjacent non-cancer tissues. Briefly, paraffin-embedded
tissue-microarray blocks of HCC tissues and paired adjacent
non-cancer tissues were consecutively cut into 4-μm sections.
Slides were baked at 60°C for 1–2 h and then deparaffinized and
rehydrated. Endogenous peroxidase activity was blocked by
incubation with 3% hydrogen peroxide for 20 min at room
temperature. Slides were incubated overnight at 4°C with primary
antibodies (Abcam, Cambridge, UK; catalog no. ad55445; 1:500
dilution) and VEGF (Santa Cruz Biotechnology, Santa Cruz, CA, USA;
1:200 dilution) diluted in phosphate-buffered saline (PBS). After
washing, the tissue slides were subsequently treated with the
secondary antibody (anti-Rb or mouse IgG/HRP; Zhongshan, 1:2000)
for 1 h at room temperature, and then with 3,3′-diaminobenzidine
(DAB) solution followed by counterstaining with hematoxylin.
Analysis was performed with a Zeiss Axioscope 2 microscope at a
×400 magnification, respectively. The degree of immunohistochemical
staining was semi-quantitatively assessed and scored independently
by two observers. For levels of MIF and VEGF expression, staining
intensity was scored according to the following criteria: no
staining, 0; weak staining, 1; moderate staining, 2; and strong
staining, 3.
RNA extraction and real-time polymerase
chain reaction (PCR)
Total RNA was extracted from the tissue samples
using Trizol (Invitrogen) according to the manufacturer’s
instructions. Real-time PCR amplifications were performed in ABI
PRISM 7900 Sequence Detection System (Applied Biosystems, Foster
City, CA, USA) using EvaGreen™ qPCR Master Mix (Biotium, Hayward,
CA, USA). The primers for human VEGF were
5′-TCGAGACCCTGGTGGACATC-3′ (forward) and 5′-TGTTGGACTCCTCAGTGGGC-3′
(reverse). MIF primers were 5′-CAGAACCGCTCCTACAGCAAG-3′ (forward)
and 5′-CGGCTCTTAGGCGAAGGTG-3′ (reverse) and β-actin primers were
5′-ACAATGTGGCCGAGGACTTT-3′ (forward) and 5′-GGAGAGGACTGGGCCATTCT-3′
(reverse). The optimal PCR amplification for VEGF and MIF was 95°C
for 30 sec followed by 40 cycles (95°C for 5 sec, 60°C for 30 sec).
The expression of β-actin was used as the internal control. The
relative expression levels of VEGF and MIF mRNA were calculated
according to the comparative Ct method, and the expression of
target genes was normalized to β-actin expression levels in each
sample.
Statistical analysis
Data are presented as means ± standard deviation
(SD). All statistical analyses were performed using SPSS 13.0 for
Windows (SPSS Inc., Chicago, IL, USA). χ2 test or
Fisher’s exact test was used for comparisons between
immunohistochemical and serum results and clinicopathological
parameters. Spearman’s bivariate correlation test was used to
evaluate the correlation between VEGF and MIF. Differences in VEGF
mRNA and MIF mRNA expression between the groups were analyzed by
the Student’s t-test. A P-value <0.05 was considered to indicate
a statistically significant result.
Results
Upregulation of VEGF and MIF in serum
samples of patients with HCC
Using ELISA, the levels of serum VEGF and MIF were
evaluated in 150 patients with HCC and 30 normal volunteers. The
serum VEGF and MIF levels were significantly higher in patients
with HCC when compared with their levels in the normal controls
(Table I). Overexpression of serum
VEGF and MIF was significantly associated with tumor size,
intrahepatic metastasis, vascular invasion and TNM stage (Table II). Furthermore, the high levels of
VEGF in the serum were positively related with serum MIF expression
in HCC (r=0.579, P<0.05).
 | Table IComparison of serum VEGF and MIF
levels between HCC patients and the control group. |
Table I
Comparison of serum VEGF and MIF
levels between HCC patients and the control group.
| n | Means ± SD | P-value |
|---|
| VEGF | | | 0.011 |
| Patients with
HCC | 150 | 414.71±41.92
(ng/l) | |
| Control | 30 | 176.52±32.14
(ng/l) | |
| MIF | | | 0.032 |
| Patients with
HCC | 150 | 123.71±18.34
(μg/l) | |
| Control | 30 | 11.53±5.47
(μg/l) | |
 | Table IICorrelation between serum VEGF and MIF
levels and the clinicopathological characteristics of the HCC
patients. |
Table II
Correlation between serum VEGF and MIF
levels and the clinicopathological characteristics of the HCC
patients.
| Variable feature | n | VEGF (ng/l) | P-value | MIF (μg/l) | P-value |
|---|
| Tumor size (cm) | | | 0.011 | | 0.027 |
| ≤5 | 46 | 295.9±26.9 | | 58.7±13.8 | |
| >5 | 104 | 368.7±34.8 | | 116.8±23.8 | |
| TNM stage | | | 0.032 | | 0.034 |
| I | 30 | 306.7±42.9 | | 65.3±16.9 | |
| II | 80 | 412.5±51.3 | | 118.7±24.2 | |
| III | 40 | 634.6±73.4 | | 143.5±26.3 | |
| Vascular
invasion | | | 0.028 | | 0.035 |
| Absence | 103 | 312.3±40.4 | | 85.9±14.7 | |
| Presence | 47 | 586.7±64.8 | | 118.7±21.3 | |
| Intrahepatic
metastasis | | | 0.031 | | 0.026 |
| Absence | 93 | 337.4±36.5 | | 91.8±25.9 | |
| Presence | 57 | 668.3±54.6 | | 129.7±34.6 | |
Overexpression of VEGF and MIF in
archived HCC tissues
In subsequent studies, we detected the role of VEGF
and MIF in the clinical progression of HCC. We examined 150
paraffin-embedded, archived HCC tissues, including 30 cases of
stage I, 80 cases of stage II and 40 cases of stage III tumors,
using immunohistochemical staining. High levels of VEGF were
present in the cytoplasm of the malignant cells in 75% (112/150) of
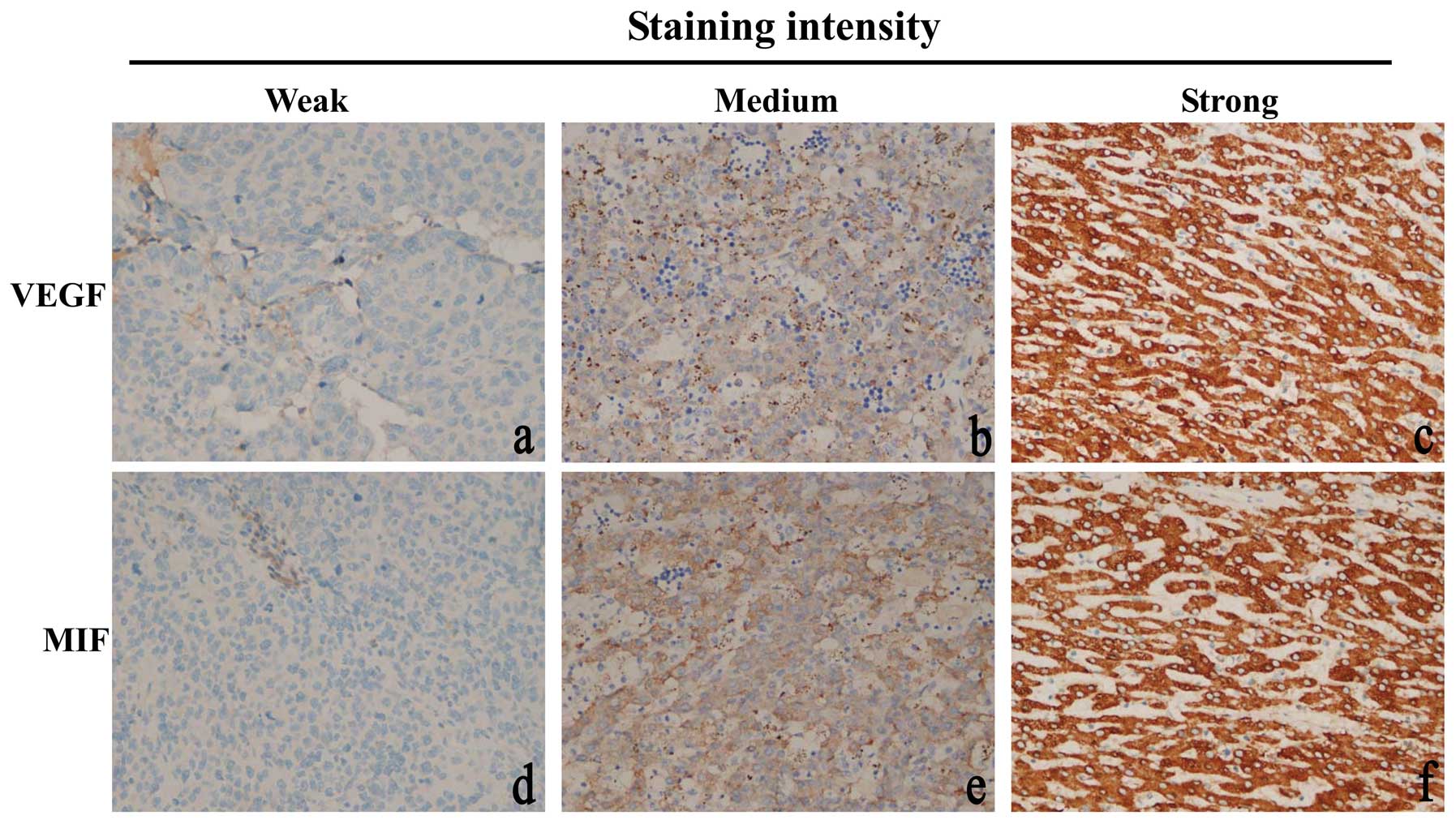
HCC tissues (Fig. 1b and c). In
contrast, VEGF was negatively or only weakly detectable in adjacent
non-cancer tissues (Fig. 1a). In
addition, the index values of VEGF staining were significantly
increased with the progression of tumor grades I to III (P=0.028).
Moreover, VEGF expression was strongly correlated with tumor size
(P=0.027), vascular invasion (P=0.032), and serum AFP levels
(P=0.043). However, our analyses did not show significant
associations between VEGF expression and other clinical features
including age, gender, history of hepatitis, liver cirrhosis and
tumor multiplicity (Table
III).
 | Table IIICorrelation between VEGF and MIF
expression and the clinicopathological characteristics of the HCC
patients. |
Table III
Correlation between VEGF and MIF
expression and the clinicopathological characteristics of the HCC
patients.
| | VEGF | | MIF | |
|---|
| |
| |
| |
|---|
| Variable
feature | n | 0 | 1 | 2 | 3 | P-value | 0 | 1 | 2 | 3 | P-value |
|---|
| Age (years) | | | | | | 0.834 | | | | | 0.675 |
| ≥50 | 95 | 22 | 17 | 22 | 34 | | 22 | 16 | 33 | 24 | |
| <50 | 55 | 16 | 8 | 12 | 19 | | 7 | 11 | 20 | 17 | |
| Gender | | | | | | 0.712 | | | | | 0.738 |
| Male | 125 | 35 | 24 | 32 | 34 | | 22 | 23 | 43 | 37 | |
| Female | 25 | 3 | 5 | 8 | 9 | | 7 | 3 | 6 | 9 | |
| Etiology | | | | | | 0.411 | | | | | 0.513 |
| Noninfection | 29 | 8 | 7 | 10 | 4 | | 12 | 3 | 8 | 6 | |
| Hepatitis B | 109 | 28 | 20 | 28 | 33 | | 15 | 23 | 36 | 35 | |
| Hepatitis C or
other | 12 | 2 | 2 | 3 | 5 | | 2 | 1 | 5 | 4 | |
| Liver
cirrhosis | | | | | | 0.038 | | | | | 0.041 |
| Absence | 44 | 15 | 11 | 10 | 8 | | 11 | 7 | 14 | 12 | |
| Presence | 106 | 23 | 14 | 30 | 39 | | 18 | 22 | 42 | 24 | |
| Tumor size
(cm) | | | | | | 0.027 | | | | | 0.022 |
| ≤5 | 46 | 10 | 8 | 18 | 10 | | 11 | 7 | 17 | 11 | |
| >5 | 104 | 28 | 21 | 23 | 32 | | 18 | 21 | 36 | 29 | |
| Serum AFP
(μg/l) | | | | | | 0.043 | | | | | 0.037 |
| ≤20 | 42 | 14 | 7 | 10 | 11 | | 6 | 12 | 14 | 10 | |
| >20 | 108 | 24 | 23 | 31 | 30 | | 23 | 15 | 39 | 31 | |
| TNM stage | | | | | | 0.0283 | | | | | 0.0134 |
| I | 30 | 19 | 3 | 5 | 4 | | 10 | 6 | 8 | 6 | |
| II | 80 | 9 | 14 | 26 | 31 | | 11 | 10 | 35 | 24 | |
| III | 40 | 10 | 8 | 12 | 10 | | 8 | 7 | 14 | 11 | |
| Vascular
invasion | | | | | | 0.0315 | | | | | 0.0267 |
| Absence | 103 | 31 | 20 | 23 | 29 | | 20 | 21 | 37 | 25 | |
| Presence | 47 | 7 | 8 | 19 | 13 | | 9 | 6 | 15 | 17 | |
| Intrahepatic
metastasis | | | | | | 0.0437 | | | | | 0.0391 |
| Absence | 93 | 30 | 18 | 23 | 22 | | 19 | 17 | 31 | 26 | |
| Presence | 57 | 8 | 10 | 18 | 21 | | 10 | 10 | 22 | 15 | |
MIF was localized in the cytoplasm of positive
staining HCC cells (Fig. 1d–f). MIF
was detected in 81% (121/150) of HCC cases (P<0.001). Our
studies showed that high levels of MIF expression were associated
with tumor size (P=0.022), tumor grade (P=0.013), presence of
intrahepatic metastasis (P=0.039) and vascular invasion (P=0.027)
and TNM stage (P=0.013). There were no further associations with
other clinicopathological parameters (Table III). Spearman correlation analysis
confirmed that VEGF expression was positively correlated with MIF
protein expression (r=0.619, P=0.022) in the HCC tissues.
VEGF and MIF mRNA expression in HCC and
correlations between VEGF and MIF mRNA expression
To confirm the effect of VEGF and MIF on the
progression of HCC and their correlation, we examined their mRNA
levels in 48 HCCs and paired adjacent non-tumor tissues by
real-time RT-PCR. The mRNA level of VEGF was significantly
increased in the HCC tissues when compared with the level in the
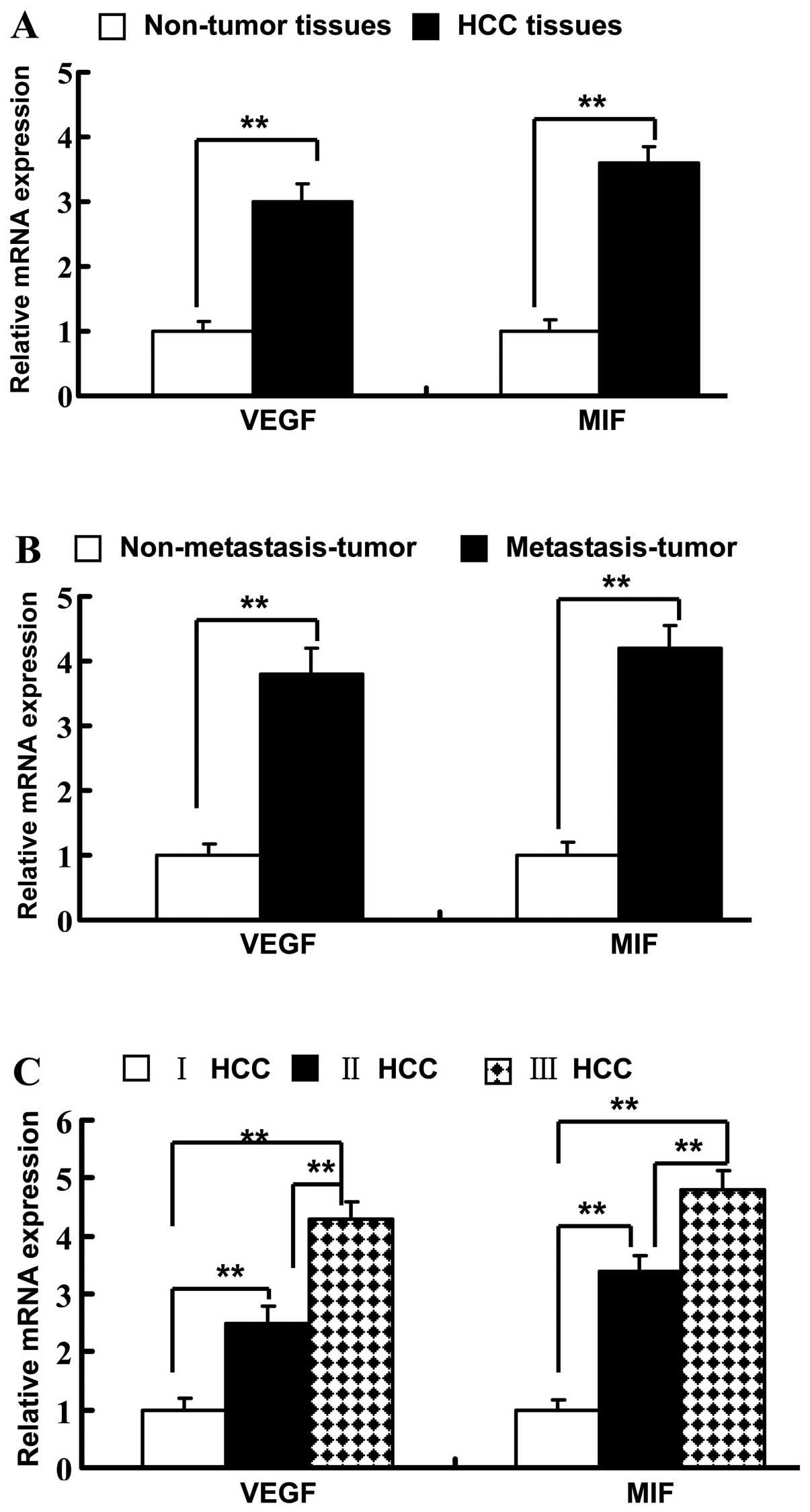
paired adjacent non-tumor tissues (P<0.01) (Fig. 2A). In HCC tissues, VEGF mRNA
expression increased according to increasing TNM stage (Fig. 2C). The mRNA level of VEGF was
significantly increased in metastatic HCC tissues when compared
with the level in the nonmetastatic tissues (Fig. 2B). Consistent with VEGF, the MIF
mRNA level was markedly higher in the HCC tissues when compared
with the level in the adjacent non-tumor tissues (P<0.001)
(Fig. 2A). MIF mRNA expression was
significantly elevated in later TNM stages (P<0.001) (Fig. 2C). MIF mRNA was higher in the
metastatic HCC tissues when compared with that in the nonmetastatic
tissues (Fig. 2B). A positively
correlation was noted between VEGF and MIF mRNA expression (r=0.72,
P=0.066).
Discussion
In the present study, we analyzed the expression of
VEGF and MIF in HCC and evaluated the levels of VEGF and MIF with
the clinicopathological parameters in 150 cases. We measured the
concentration of VEGF and MIF in a series of 150 serum samples from
HCC patients. Additionally, a series of 30 serum samples from
healthy volunteers was selected as controls. Moreover, we assessed
the relationship between the levels of VEGF and MIF and the
clinicopathological factors of the HCC cases. In the present study,
we found that the serum levels of VEGF and MIF were markedly
increased in the HCC group when compared to levels in the control
group. Overexpression of serum VEGF and MIF was significantly
associated with tumor size, tumor grade, intrahepatic metastasis,
vascular invasion and TNM stage. Furthermore, high levels of VEGF
in the serum were positively co-related with serum MIF expression
in HCC. These results were consistent with the expression of VEGF
and MIF in HCC tissue samples.
VEGF is known as one of the most potent
pro-angiogenic factors (19).
Several studies (20–23) have demonstrated that VEGF promotes
the growth of local foci of malignant tumors and facilitates
metastasis and invasion. VEGF, upregulated in various solid tumors,
is closely correlated with pathological characteristics, metastasis
and prognosis of tumors. Silencing of MMP-9 and VEGF decreases the
recurrence and metastasis of HCC after TACE (24,25).
Therefore, VEGF plays an important role in the tumorigenesis of
tumors. Our results showed that enhanced VEGF was associated with
intrahepatic metastasis, vascular invasion and later tumor stage.
In addition, VEGF expression was positively correlated with MIF
expression in the serum of patients with HCC. Furthermore,
quantitative PCR verified that VEGF mRNA was significantly
upregulated in HCC tissues when compared with that in adjacent
non-tumor tissues; there was a correlation between the upregulation
of VEGF mRNA with tumor TNM stage and metastasis in HCC.
MIF was initially found to contribute to the
inhibition of the random migration of macrophages (26). Recent studies have extablished that
MIF plays an important role in carcinogenesis by promoting cell
proliferation, tumor angiogenesis and metastasis (27). He et al (28) demonstrated that epithelial and serum
MIF expression was progressively increased in gastric cancer. Bando
et al (29) found that MIF
was overexpressed in 93 breast cancer tissues as detected by ELISA.
In esophageal squamous cell carcinoma, MIF expression was found to
be correlated with lymph node status (12). In the present study, the
immunohistochemical and ELISA results showed that MIF expression
was correlated with increasing tumor grade, intrahepatic metastasis
and vascular invasion. Moreover, MIF expression was positively
correlated with VEGF expression. Thus, these results suggest that
activated MIF/VEGF is involved in proliferation, invasion and
metastasis in HCC. Choudhary et al (30) reported that treatment with
inhibitors of MIF increased mRNA expression and protein secretion
of VEGF in bladder cancer. Bondza et al (31) indicated that MIF markedly stimulates
the secretion of VEGF, which is in accordance with the findings of
the present study.
MIF and VEGF were overexpressed in patients with HCC
in our study and their expression was correlated with tumor size,
intrahepatic metastasis and vascular invasion. MIF stimulation may
induce an increase in VEGF secretion, which contributes to
angiogenesis and tumor growth. Therefore, VEGF and MIF may be
markers of more aggressive HCC and they could be therapeutic
targets for patients with HCC.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81071871, 81108162, 81172079), the
Natural Science Foundation of Guangdong Province, China
(S2013010016831), the Science and Technology Planning Project of
Guangdong Province, China (2010b060500007; 2011B060300012), and the
Foundation for Youth Teachers by Sun Yat-Sen University
(11ykpy16).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amini A, Masoumi Moghaddam S, Morris DL
and Pourgholami MH: The critical role of vascular endothelial
growth factor in tumor angiogenesis. Curr Cancer Drug Targets.
12:23–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arcondéguy T, Lacazette E, Millevoi S,
Prats H and Touriol C: VEGF-A mRNA processing, stability and
translation: a paradigm for intricate regulation of gene expression
at the post-transcriptional level. Nucleic Acids Res. 41:7997–8010.
2013.PubMed/NCBI
|
|
5
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar
|
|
6
|
Koukourakis MI, Papazoglou D,
Giatromanolaki A, Bougioukas G, Maltezos E and Sivridis E: VEGF
gene sequence variation defines VEGF gene expression status and
angiogenic activity in non-small cell lung cancer. Lung Cancer.
46:293–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin Q, Hemminki K, Enquist K, Lenner P,
Grzybowska E, Klaes R, Henriksson R, Chen B, Pamula J, Pekala W,
Zientek H, Rogozinska-Szczepka J, Utracka-Hutka B, Hallmans G and
Försti A: Vascular endothelial growth factor polymorphisms in
relation to breast cancer development and prognosis. Clin Cancer
Res. 11:3647–3653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Celen O, Kahraman I, Yildirim E and
Berberoglu U: Correlation of vascular endothelial growth factor
(VEGF) and CEA with clinicopathological variables in colorectal
cancer patients. Neoplasma. 51:293–299. 2004.PubMed/NCBI
|
|
9
|
Shen YC, Hsu C and Cheng AL: Molecular
targeted therapy for advanced hepatocellular carcinoma: current
status and future perspectives. J Gastroenterol. 45:794–807. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L, Shi Y, Jiang CY, Wei LX, Lv YL,
Wang YL and Dai GH: Coexpression of PDGFR-alpha, PDGFR-beta and
VEGF as a prognostic factor in patients with hepatocellular
carcinoma. Int J Biol Markers. 26:108–116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ren Y, Law S, Huang X, Lee PY, Bacher M,
Srivastava G and Wong J: Macrophage migration inhibitory factor
stimulates angiogenic factor expression and correlates with
differentiation and lymph node status in patients with esophageal
squamous cell carcinoma. Ann Surg. 242:55–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Greven D, Leng L and Bucala R: Autoimmune
diseases: MIF as a therapeutic target. Expert Opin Ther Targets.
14:253–264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grieb G, Merk M, Bernhagen J and Bucala R:
Macrophage migration inhibitory factor (MIF): a promising
biomarker. Drug News Perspect. 23:257–264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meyer-Siegler KL, Iczkowski KA, Leng L,
Bucala R and Vera PL: Inhibition of macrophage migration inhibitory
factor or its receptor (CD74) attenuates growth and invasion of
DU-145 prostate cancer cells. J Immunol. 177:8730–8739. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ren Y, Chan HM, Fan J, Xie Y, Chen YX, Li
W, Jiang GP, Liu Q, Meinhardt A and Tam PK: Inhibition of tumor
growth and metastasis in vitro and in vivo by targeting macrophage
migration inhibitory factor in human neuroblastoma. Oncogene.
25:3501–3508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren Y, Tsui HT, Poon RT, Ng IO, Li Z, Chen
Y, Jiang G, Lau C, Yu WC, Bacher M and Fan ST: Macrophage migration
inhibitory factor: roles in regulating tumor cell migration and
expression of angiogenic factors in hepatocellular carcinoma. Int J
Cancer. 107:22–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang XW, Zhang LJ, Huang XH, Chen LZ, Su
Q, Zeng WT, Li W and Wang Q: miR-145 suppresses cell invasion in
hepatocellular carcinoma cells: miR-145 targets ADAM17. Hepatol
Res. Apr 28–2013.(Epub ahead of print). View Article : Google Scholar
|
|
19
|
Namisaki T, Yoshiji H, Noguchi R, Ikenaka
Y, Kitade M, Kaji K, Shirai Y, Aihara Y, Yoshii J, Yanase K,
Tsujimoto T, Kawaratani H and Fukui H: The vascular endothelial
growth factor (VEGF) receptor-2 is a major regulator of
VEGF-mediated salvage effect in murine acute hepatic failure. J
Angiogenes Res. 2:162010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu J, Chen C, Su Y, Du J, Qian X and Jin
Y: Vascular endothelial growth factor promotes the expression of
cyclooxygenase 2 and matrix metalloproteinases in Lewis lung
carcinoma cells. Exp Ther Med. 4:1045–1050. 2012.PubMed/NCBI
|
|
21
|
Li C, Liu B, Dai Z and Tao Y: Knockdown of
VEGF receptor-1 (VEGFR-1) impairs macrophage infiltration,
angiogenesis and growth of clear cell renal cell carcinoma (CRCC).
Cancer Biol Ther. 12:872–880. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu W, Chen L, Yang YQ, Falck JR, Guo AM,
Li Y and Yang J: Cytochrome P450 ω-hydroxylase promotes
angiogenesis and metastasis by upregulation of VEGF and MMP-9 in
non-small cell lung cancer. Cancer Chemother Pharmacol. 68:619–629.
2011.
|
|
23
|
Amano H, Ito Y, Suzuki T, Kato S, Matsui
Y, Ogawa F, Murata T, Sugimoto Y, Senior R, Kitasato H, Hayashi I,
Satoh Y, Narumiya S and Majima M: Roles of a prostaglandin E-type
receptor, EP3, in upregulation of matrix metalloproteinase-9 and
vascular endothelial growth factor during enhancement of tumor
metastasis. Cancer Sci. 100:2318–2324. 2009. View Article : Google Scholar
|
|
24
|
Deng G, Zhao DL, Li GC, Yu H and Teng GJ:
Combination therapy of transcatheter arterial chemoembolization and
arterial administration of antiangiogenesis on VX2 liver tumor.
Cardiovasc Intervent Radiol. 34:824–832. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Janani P, Sivakumari K, Geetha A, Yuvaraj
S and Parthasarathy C: Bacoside A downregulates matrix
metalloproteinases 2 and 9 in DEN-induced hepatocellular carcinoma.
Cell Biochem Funct. 28:164–169. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
David JR: Delayed hypersensitivity in
vitro: its mediation by cell-free substances formed by lymphoid
cell-antigen interaction. Proc Natl Acad Sci USA. 56:72–77. 1966.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chesney J, Metz C, Bacher M, Peng T,
Meinhardt A and Bucala R: An essential role for macrophage
migration inhibitory factor (MIF) in angiogenesis and the growth of
a murine lymphoma. Mol Med. 5:181–191. 1999.PubMed/NCBI
|
|
28
|
He XX, Yang J, Ding YW, Liu W, Shen QY and
Xia HH: Increased epithelial and serum expression of macrophage
migration inhibitory factor (MIF) in gastric cancer: potential role
of MIF in gastric carcinogenesis. Gut. 55:797–802. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bando H, Matsumoto G, Bando M, Muta M,
Ogawa T, Funata N, Nishihira J, Koike M and Toi M: Expression of
macrophage migration inhibitory factor in human breast cancer:
association with nodal spread. Jpn J Cancer Res. 93:389–396. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choudhary S, Hegde P, Pruitt JR, Sielecki
TM, Choudhary D, Scarpato K, Degraff DJ and Pilbeam CC: Macrophage
migratory inhibitory factor promotes bladder cancer progression via
increasing proliferation and angiogenesis. Carcinogenesis. Aug
2–2013.(Epub ahead of print).
|
|
31
|
Bondza PK, Metz CN and Akoum A: Macrophage
migration inhibitory factor up-regulates alpha(v)beta(3) integrin
and vascular endothelial growth factor expression in endometrial
adenocarcinoma cell line Ishikawa. J Reprod Immunol. 77:142–151.
2008. View Article : Google Scholar
|