Introduction
Liver cancer is a malignant tumour with high
morbidity and fatality rates that is common worldwide (1). New cases of liver cancer account for
4% of all malignant tumours in the world each year, and the
morbidity of liver cancer in China has been slowly increasing in
recent years, seriously affecting the life and health of its
citizens (2). With the morbidity of
liver cancer in China now higher than that of gastric cancer, liver
cancer currently has the highest fatality rate among malignant
tumours of the digestive tract. The number of liver cancer patients
in China accounts for 55% of all cases in the world, and the death
toll has reached 45% of the world total (3,4). The
occurrence of liver cancer is related to viral hepatitis, excessive
drinking and non-alcoholic cirrhosis, all of which may increase the
morbidity of liver cancer (5).
Additionally, because the onset of liver cancer is hidden, most
patients are already at the end-stage once a definite diagnosis is
made (5,6), depriving them of the chance for
radical treatment. Currently, the main clinical approaches to
treating primary liver cancer include partial hepatectomy, systemic
or local chemotherapy, radiotherapy, radiofrequency ablative
surgery and liver transplantation (7–11).
However, all of these approaches have shortcomings, including poor
prognosis and many side-effects. Thus, there is an urgent need to
search for new therapeutics with effective antitumour action for
liver cancer patients.
Due to the extremely high costs for developing
chemical synthetic drugs, it is quite difficult to discover an
anticancer drug from chemical compounds. Additionally, chemical
drugs can have many side-effects. Therefore, it has become an
important part of research on anticancer drugs both at home and
abroad to search for a single Chinese herb or effective ingredient
from plants. As one of the key approaches to tumour treatment,
traditional Chinese medicine plays an indispensable role. With
decades of effort, traditional Chinese medicine has achieved
breakthroughs in terms of enhancing the effect and reducing the
toxicity of chemotherapy and radiotherapy, preventing tumour
metastasis and relapse after surgery, alleviating the clinical
symptoms of advanced tumours, improving the quality of life of
patients with tumours and extending the patient’s long-term
survival. Traditional Chinese medicine has become a ‘hot’ topic in
cancer drug research due to its antitumour characteristics of
multi-target, multi-step and multi-effect, its action in various
steps in the occurrence and development of tumours, its lower
toxicity and fewer toxic effects, its ability to improve immunity
and its lower tendency toward drug resistance. The anticancer
mechanism of Chinese medicine is also a focus in today’s medical
research.
The occurrence of liver cancer is associated not
only with abnormal cell proliferation and differentiation but also
with abnormal apoptosis. The proliferation and apoptosis of tumour
cells consist of a precise process regulated by multiple genes.
Many studies both at home and abroad have demonstrated that
flavonoid compounds can inhibit the proliferation of tumour cells,
induce the apoptosis of tumour cells and regulate the expression of
relevant genes to block the occurrence and development of tumour
cells (12–15). Isoquercitrin, the effective monomer
from Bidens bipinnata L. extract, was used in the present
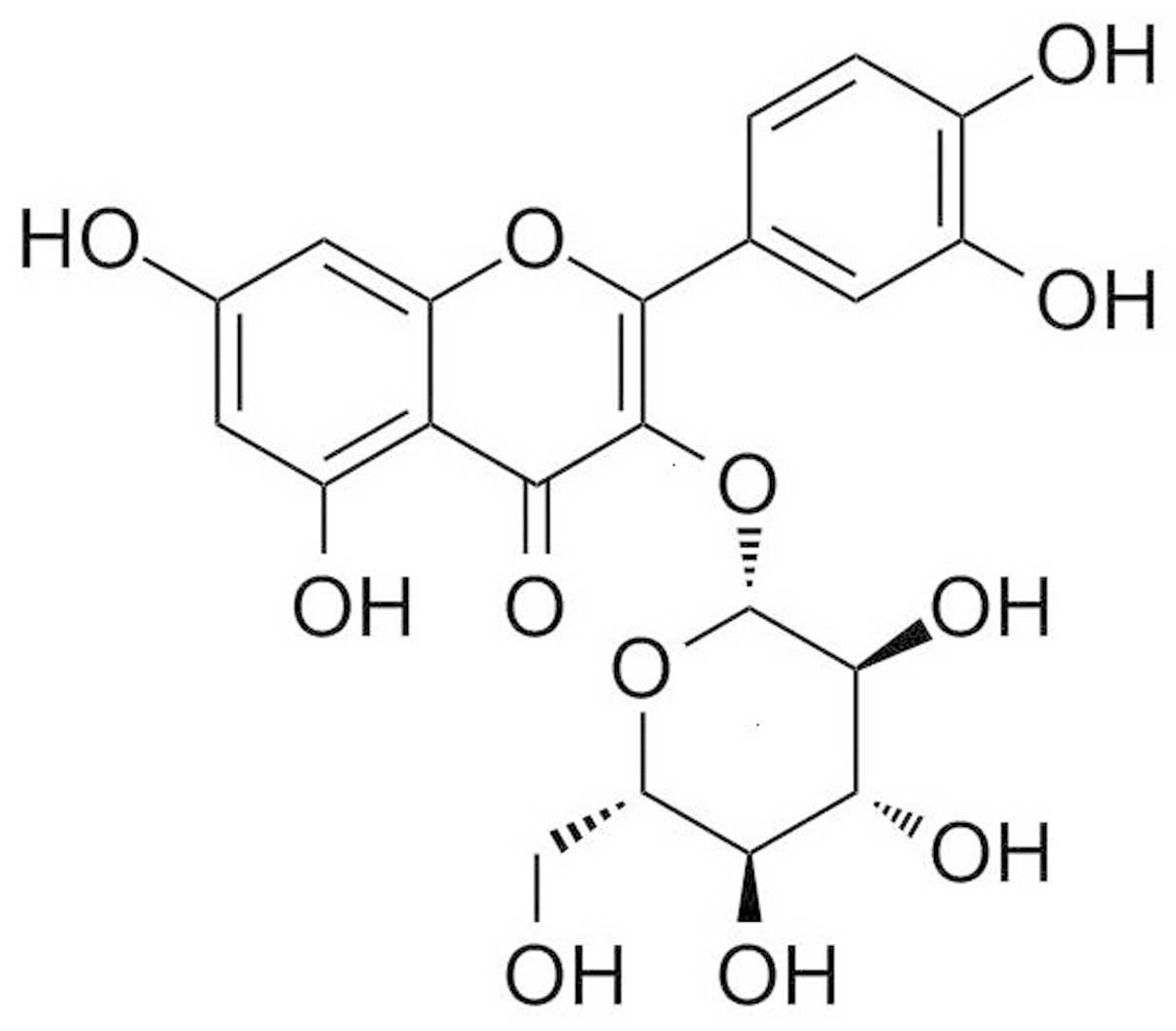
study. The molecular formula of isoquercitrin is
C20H21O12, with a molecular weight
of 464.38 (Fig. 1 illustrates its
structural formula). Isoquercitrin was used to treat human liver
cancer cells to study its role in regulating the proliferation,
apoptosis and cell cycle of liver cancer cells and to explore a
possible antitumour signalling pathway.
Materials and methods
Cell culture, antibodies and
reagents
Human liver cancer cell lines HepG2 and Hep3B were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The cells were inoculated in Dulbecco’s
modified Eagle’s medium (DMEM) containing 10% fetal bovine serum
(FBS) (both from Gibco, USA) in a 37°C incubator containing 5%
CO2 and saturated humidity. Isoquercitrin (≥98% purity)
was purchased from Sigma, USA. The Annexin V-FITC apoptosis
analysis kit was purchased from BD, USA. Antibodies for
phospho-JNK, phospho-ERK1/2, phospho-p38MAPK and PKC were purchased
from Santa Cruz Biotechnology, USA. The RT-PCR kit was purchased
from Takara Bio (Japan).
Cell viability analysis
Liver cancer cells in logarithmic growth phase were
digested with 0.25% trypsin, rinsed with phosphate-buffered saline
(PBS), and resuspended in DMEM culture medium containing 10% FBS as
single cells to be inoculated into a 96-well plate
(1×104 cells/well) for overnight culture in a 5%
CO2 incubator at 37°C. The following day, the medium was
replaced, and isoquercitrin was added to final concentrations of 0,
100, 200, 400 and 800 μM. A blank was established in one well with
culture medium only. There were 6 double-wells for each group that
was cultured for 24, 48 and 72 h. The medium was replaced 4 h prior
to the analysis, and MTT was added (20 μl at 0.5 mg/ml) into each
well. Cells were cultured for 4 h in the incubator at 37°C. DMSO
(150 μl) was added, and the OD values of each well were measured
with a microplate reader at the wavelength of 490 nm.
Annexin V-FITC/PI double stain flow
cytometry for apoptosis analysis
Cells in the logarithmic growth phase were
inoculated in a 6-well plate, and the cell density was adjusted to
1×106 cells/well. After adherence, cells were treated
with different concentrations of isoquercitrin added to the culture
medium. Cells were trypsinised 48 h after the isoquercitrin
treatment, and then, Annexin V-FITC (5 μl) and propidium iodide
(PI) (10 μl) were added to the cell suspension, which was mixed and
incubated for 15 min at room temperature in the dark before the
flow cytometric analysis. This procedure was repeated with each
experimental group three times.
Caspase activity assay
HepG2 cells were treated with different
concentrations of isoquercitrin (0, 100, 200, 400 and 800 μM) for
48 h, collected and tested for the viability of caspase-3, -8 and
-9 using a caspase activity assay kit according to the
manufacturer’s instructions (Beyotime, China). Fluorescence was
measured using a spectrophotometer at an excitation wavelength of
400 nm and an emission wavelength of 505 nm.
Cell cycle analysis
Cells in the logarithmic growth phase were
inoculated in a 6-well plate at a density of 1×106
cells/well. After adherence, cells were treated with different
concentrations of isoquercitrin added to the culture medium. Cells
were then trypsinised 48 h after the isoquercitrin treatment and
resuspended in solution. Cold absolute ethyl alcohol (75%) was
added, and cells were fixed for >18 h at 4°C. Cells were washed
twice with PBS, treated with RNase A (50 mg/l) for 30 min at 37°C,
and subsequently put in an ice-bath for 2 min. Cells were stained
with PI dye (50 mg/l) for 30 min at 4°C, then analysed using FACS.
CellQuest software was used to analyse the distribution of all
cells.
Western blot assays
Cells from all treatment conditions were collected
and washed twice with chilled PBS. Lysis buffer was added, and then
cells were placed on ice for 20 min and subsequently centrifuged at
13,000 rpm for 20 min at 4°C. The supernatant was collected, and
total cellular protein was extracted. Protein was quantified using
the BCA method. Samples were subjected to SDS-PAGE gel
electrophoresis at 80 V and 4°C. Samples were electrophoresed in
the stacking gel for 1 h, and then electrophoresed at 150 V for
~1.5 h until the bromophenol blue dye front reached the bottom of
the separation gel, and the power was turned off. Separated lysates
were then transferred to a membrane for 2 h at 100 V. Membranes
were stained with Ponceau S and blocked with 5% milk via shaking at
room temperature for 1 h. Primary antibodies were diluted in
Tris-buffered saline Tween-20 (TBST) and incubated overnight at
4°C. Antibody dilutions were as follows: phospho-ERK1/2, 1:500;
phospho-JNK, 1:800; phospho-p38, 1:800 and PKC, 1:600. Secondary
antibodies were incubated via shaking at 37°C for 1 h, washed three
times with TBST for 10 min each, and developed using
chemiluminescence. The target protein expression levels were
normalised using a ratio to β-actin levels.
RT-PCR
Total RNA was extracted from all cells and was
subjected to reverse transcription following determination of
purity and integrity. RNA concentration was calculated and RT-PCR
reactions were performed according to the manufacturer’s
instructions (Takara Bio). β-actin and PKC primers were synthesised
by Invitrogen using the following sequences: β-actin forward primer
5′-AAGGAAGGCTGGAAGAGTGC-3′ and reverse primer
5′-CTGGGACGACATGGAGAAAA-3′; PKC forward primer
5′-TGAATCCTCAGTGGAATGAGT-3′ and reverse primer
5′-GGTTGCTTTCTGTCTTCTGAA-3′. The PCR reaction volume was 50 μl
using the following reaction conditions: 94°C for 2 min and a 94°C
denaturation for 30 sec; a 60°C annealing for 30 sec; and a 72°C
extension for 30 sec for a total of 32 cycles. PCR products were
electrophoresed on a 1.0% agarose gel, then scanned and analysed
with a gel imaging system.
Vaccination of nude mice
The animal experimentation program was approved by
the Medical Ethics Committee of Guilin Medical University. Twenty
male nude mice were purchased from the Animal Experiment Center of
Guilin Medical University at the age of 6 to 8 weeks and a weight
of ~20 g. The mice were randomly divided into two groups of 10 mice
each: the model group and isoquercitrin group. Liver cancer cells
were collected in the logarithmic log phase. After washing with
PBS, the cells were suspended in serum-free medium. Subsequently,
200 μl of the cell suspension (containing 2×107 cells)
was injected subcutaneously into the right groin area of the mice.
After tumours formed, the mice were gavaged with isoquercitrin
every day and observed for the growth of tumours at 7, 14, 21 and
28 days. Mice were sacrificed by cervical vertebral dislocation 4
weeks later. Subcutaneous transplanted tumour tissue was surgically
removed under aseptic conditions for index analysis.
Statistical analysis
All the experimental data were analysed with SPSS
15.0 statistical software. The measurement data are expressed as
the means ± standard deviation, and the counting data are expressed
as percentages. The comparison among various groups was carried out
using one-way analysis of variance, and the comparison between
pairs within a group was conducted using the Q test. p<0.05 was
considered to indicate a statistically significant difference.
Results
Isoquercitrin inhibits the proliferation
of liver cancer cells
The human liver cancer cell lines HepG2 and Hep3B
were treated with various concentrations of isoquercitrin (0, 100,
200, 400 and 800 μM) for 24, 48 and 72 h. Cellular activity was
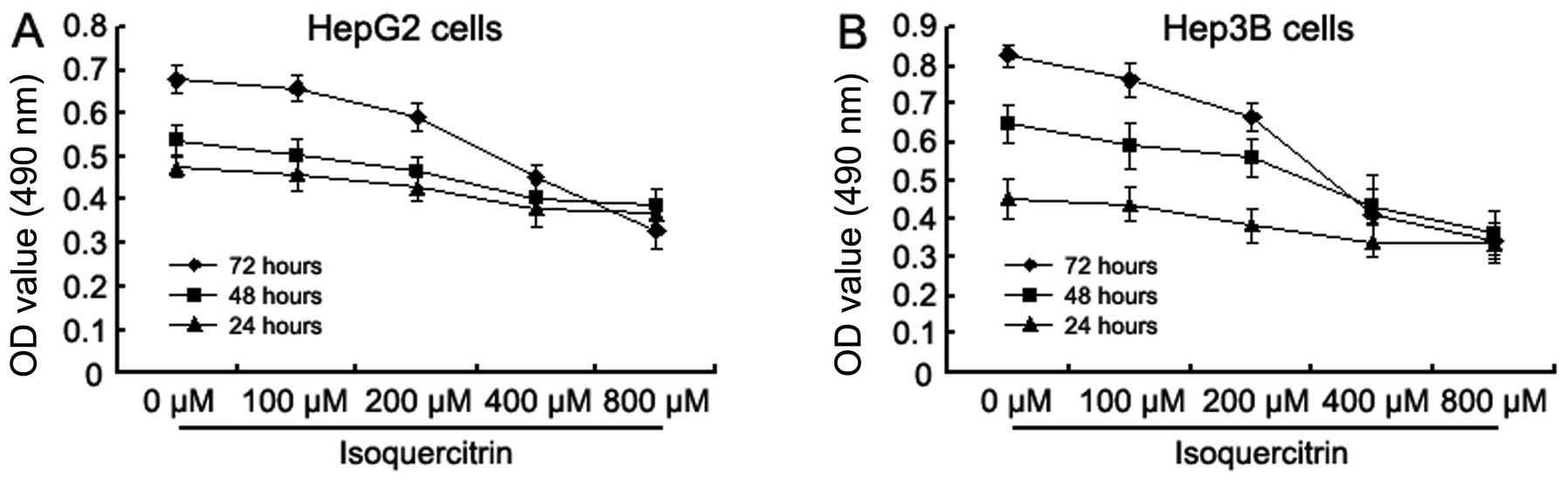
assayed with MTT. Isoquercitrin inhibited the proliferation of
HepG2 and Hep3B cells in a time- and concentration-dependent manner
(Fig. 2). When the concentration of
isoquercitrin increased from 0 to 800 μM, the A490 values in the
HepG2 and Hep3B cells decreased gradually; the most significant
decrease was observed at 400 μM.
Isoquercitrin induces apoptosis of liver
cancer cells
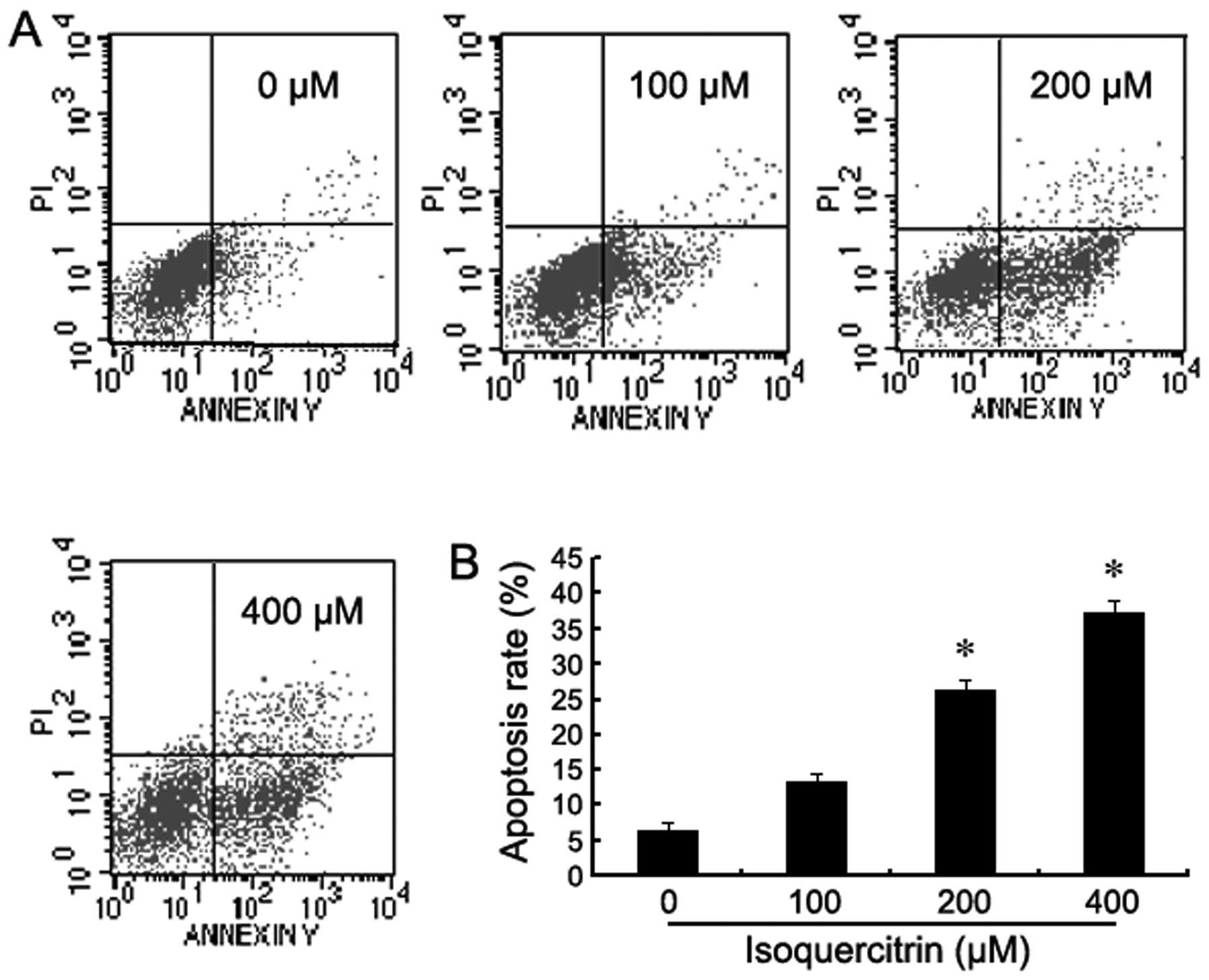
To confirm whether isoquercitrin induces the
apoptosis of liver cancer cells, Annexin V-FITC/PI double stain
flow cytometry was used to assay for the apoptosis of HepG2 cells
following treatment with various concentrations of isoquercitrin
for 48 h. As the concentration of isoquercitrin gradually
increased, the percentage of dead HepG2 cells increased in a
concentration-dependent manner when compared with the control group
(Fig. 3).
Isoquercitrin activates caspases in liver
cancer cells
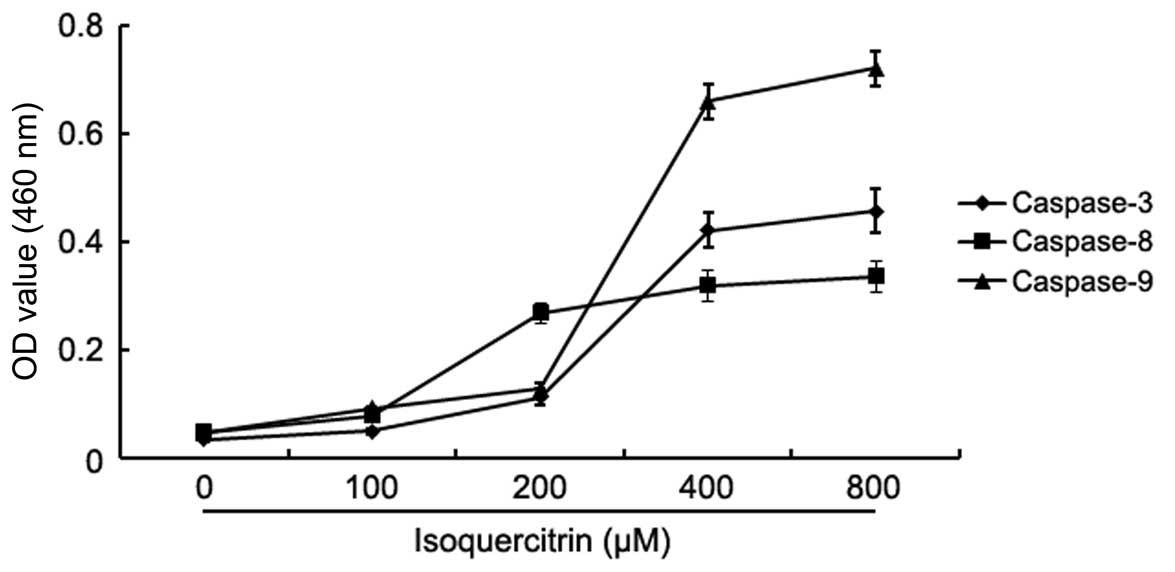
To confirm whether isoquercitrin induces the
apoptosis of liver cancer cells, the change in the activity of
caspase-3, -8 and -9 was analysed after HepG2 cells were treated
with various concentrations of isoquercitrin for 48 h. The activity
of caspase-3, -8 and -9 increased significantly after HepG2 cells
were treated with isoquercitrin (Fig.
4). Our findings showed that isoquercitrin induced the
apoptosis of HepG2 cells in a caspase family-dependent manner.
Isoquercitrin inhibits the proliferation
of liver cancer cells via the MAPK signalling pathway
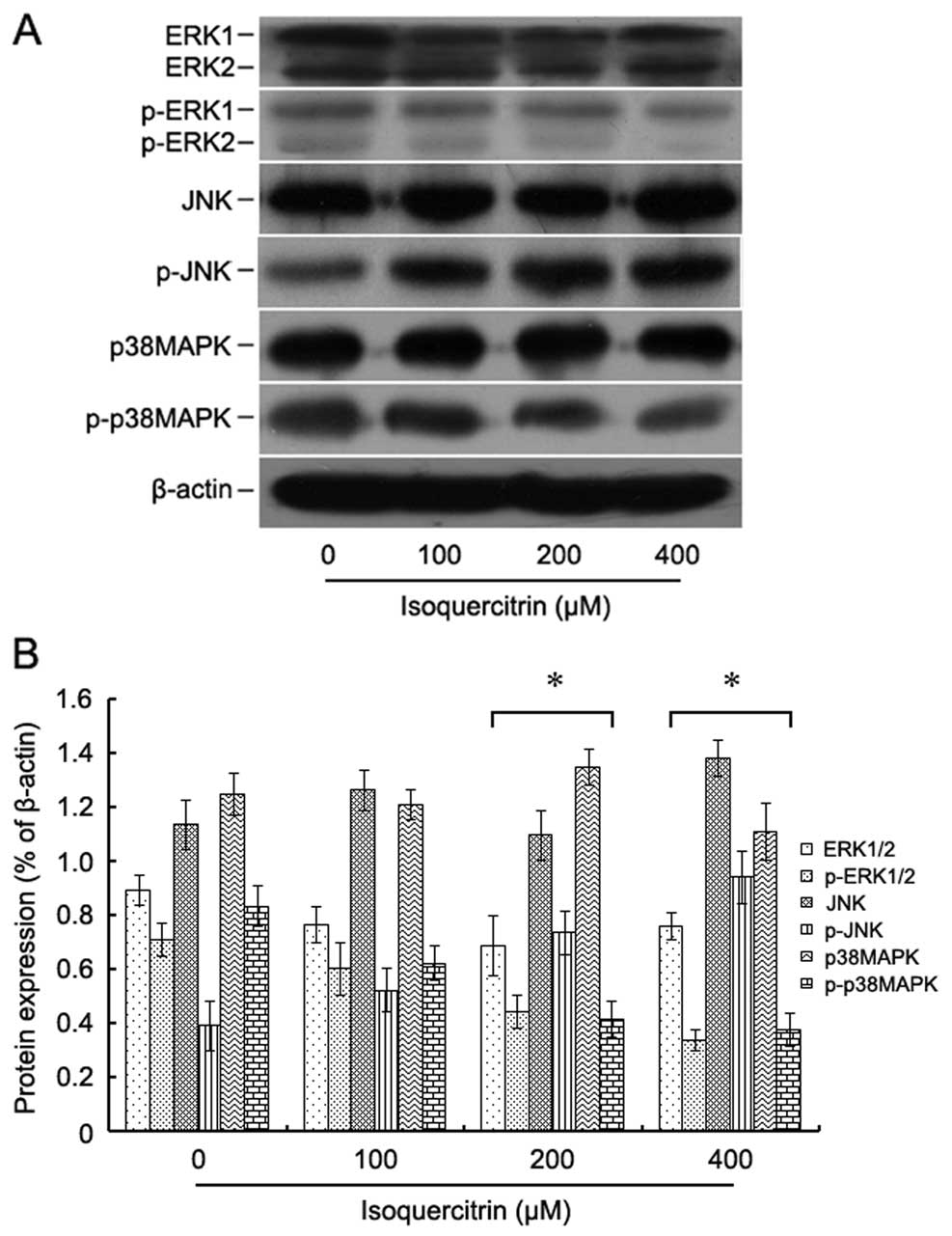
To explore the molecular mechanism of
isoquercitrin-induced inhibition of liver cancer cell
proliferation, HepG2 cells were treated with various concentrations
of isoquercitrin for 48 h, and then the expression and
phosphorylation levels of the MAPK pathway proteins ERK, JNK and
p38MAPK were assayed by western blotting. We found that as the
concentration of isoquercitrin gradually increased, the
phosphorylation levels of both ERK and p38MAPK decreased, and the
phosphorylation level of JNK increased (Fig. 5). Our findings showed that
isoquercitrin promoted the phosphorylation of JNK, which in turn
promoted the apoptosis of liver cancer cells.
Isoquercitrin inhibits the proliferation
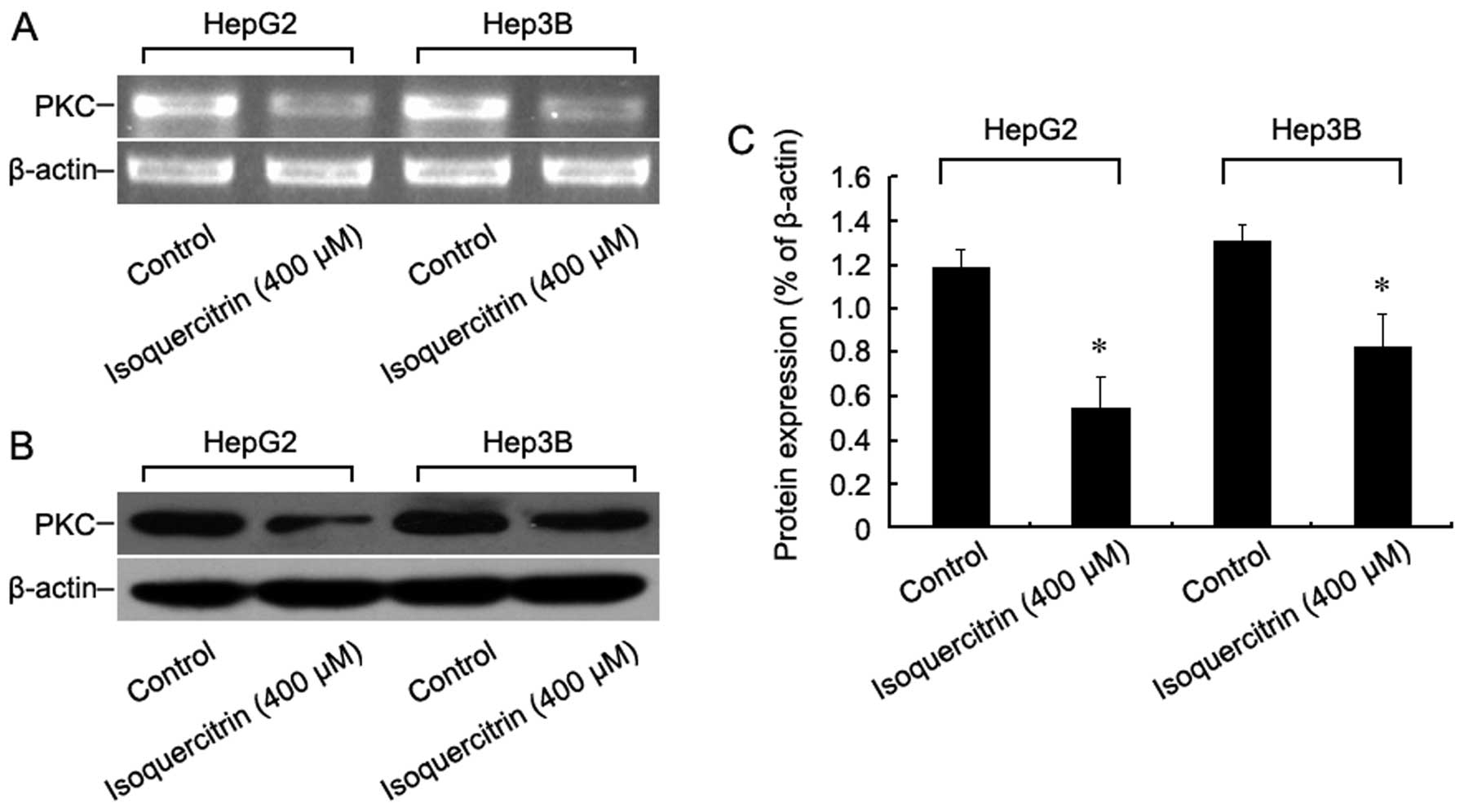
of liver cancer cells via the PKC signalling pathway
To further explore the molecular mechanism of
isoquercitrin-induced inhibition of liver cancer cell
proliferation, HepG2 and Hep3B cells were treated with
isoquercitrin for 48 h, and then changes in PKC mRNA and protein
expression were measured using RT-PCR and western blot assays,
respectively. We found that, following treatment with
isoquercitrin, both PKC mRNA and protein expression levels dropped
significantly (Fig. 6). Our
findings indicate that isoquercitrin inhibits the proliferation of
liver cancer cells by downregulating PKC.
Isoquercitrin blocks the liver cancer
cell cycle in the G1 phase
To investigate whether isoquercitrin regulates
changes in the liver cancer cell cycle, flow cytometry was used to
analyse changes in the cell cycle of HepG2 cells after they were
treated with various concentrations of isoquercitrin for 48 h. It
was found that as the concentration of isoquercitrin gradually
increased, the percentages of HepG2 cells entering the S and the
G2/M phases gradually decreased, while most cells were blocked in
the G1 phase (Fig. 7). Our findings
showed that the anticancer role that isoquercitrin plays in the
liver may be induced by the blockade of the cell cycle.
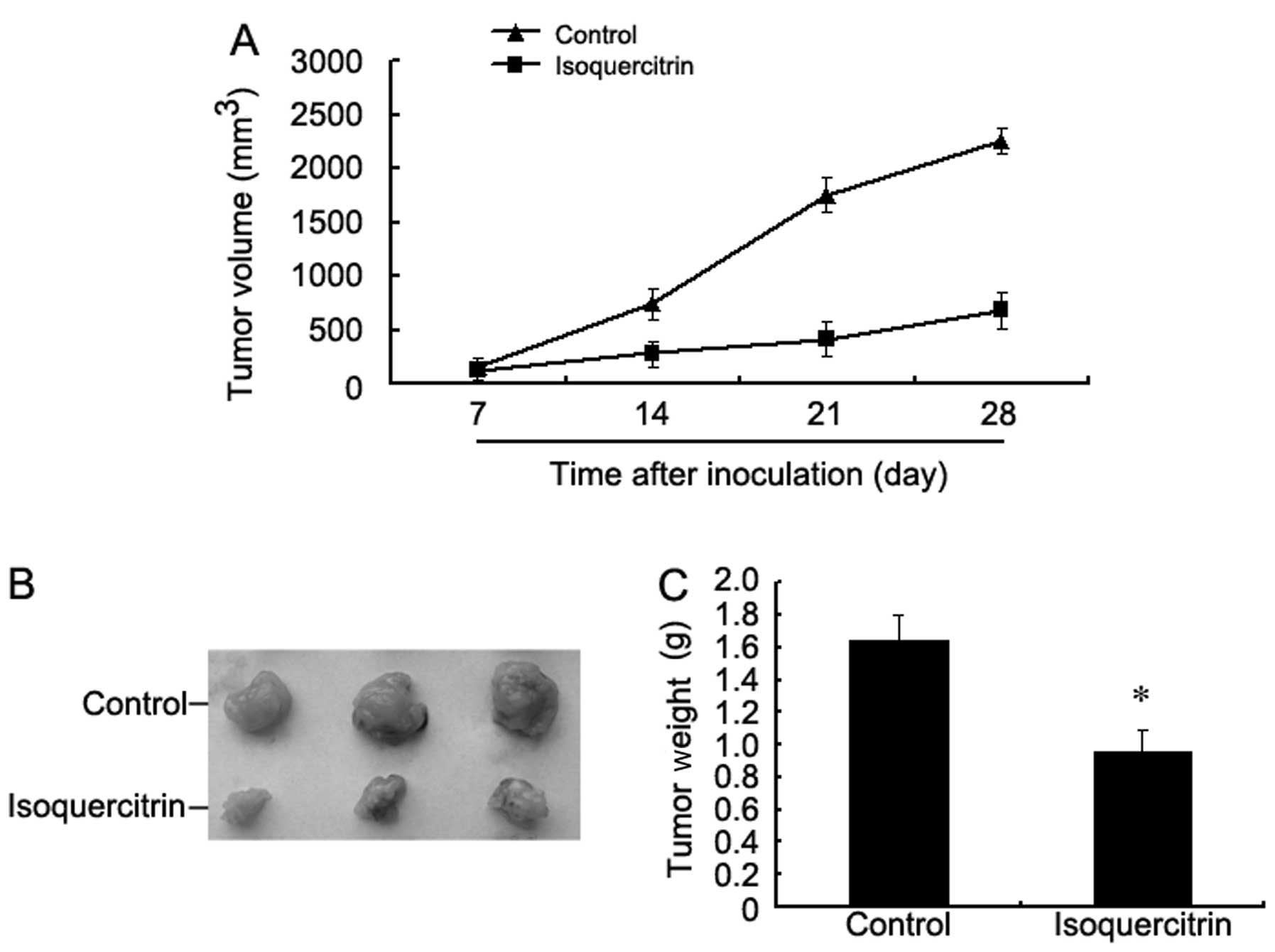
Isoquercitrin inhibits transplanted
tumour growth in nude mice
We found that the tumour volume of the
isoquercitrin-treated mouse group was consistently smaller than
that of the control group at every time point examined.
Additionally, the weight of the tumours obtained by surgery was
also significantly less than that of the control group (Fig. 8). These findings strongly indicate
that isoquercitrin obviously inhibits the progression of liver
cancer.
Discussion
Bidens bipinnata L. is a whole dry herb of
feverfew Bidens bipinnata L. that mainly grows in the warm
and humid environment of south China and is widely distributed in
most areas of Guangxi Province. It has historically been used as a
basic drug in the local area of Guangxi to treat such diseases as
malaria, diarrhoea, dysentery, hepatitis and acute nephritis,
having the effects of diaphoresis, clearing heat and toxicity, and
dissipating stasis. In recent years, Bidens bipinnata L. has
often been used by local people to treat hypertension,
hyperlipidaemia, diabetes, and anti-hepatic fibrosis and as an
antitumour agent with positive effects. Many studies have proven
that the flavonoid compounds of Bidens bipinnata L. have
antitumour effects (15–20). Isoquercitrin is a flavonoid compound
isolated from Bidens bipinnata L. Ma et al (21) and Zhong et al (22) demonstrated that the main components
of the total flavone of Bidens bipinnata L. are
isoquercitrin and hyperin via separation using macroporous
adsorption resin and high performance liquid chromatography. Zhong
et al (23) and Yuan et
al (24) also studied the role
that the Bidens bipinnata L. flavonoid plays in protecting
mice against acute liver injury using a mouse model of acute liver
injury induced by circulating tumour cells (CTCs). However, no
report is available on its role in liver cancer cells. The
formation of liver cancer is closely associated with abnormal
proliferation and apoptosis of liver cells. To understand the
effect of isoquercitrin on liver cancer cells, its role in liver
cancer cell inhibition was preliminarily explored in in vivo
and in vitro experiments. The possible signalling pathways
involved in the inhibition of liver cancer cell growth by
isoquercitrin were analysed by determining the expression levels of
relevant proteins and genes to provide an experimental basis for
its development and use.
There are many flavonoid drugs that can inhibit the
proliferation of tumour cells. Various concentrations of
isoquercitrin were administered to human liver cancer cells, and
the MTT method was used to analyse its impacts on cell
proliferation capacity. We found that when the concentration of
isoquercitrin was higher than 100 μM, the inhibition of human liver
cancer cell proliferation gradually increased as the concentration
of isoquercitrin increased. The inhibition of human liver cancer
cell proliferation was most obvious when the concentration of
isoquercitrin was 400 μM. This means that isoquercitrin can inhibit
the progression of human liver cancer in vitro in a
concentration-dependent manner. In addition, we found in the in
vivo experiment that the tumour formation rate of the nude mice
decreased and that tumour growth was restrained following treatment
with isoquercitrin. This means that isoquercitrin can inhibit the
progression of human liver cancer in vivo as well.
Liver cancer is caused by the proliferation of
tumour cells and the unbalanced regulation of apoptosis due to the
abnormal activation of cell proliferation signals and the abnormal
inhibition of cell apoptotic signals (25,26).
In these experiments, the apoptosis of liver cancer cells was
tested using the flow cytometry Annexin V/PI double stain method
following treatment with isoquercitrin for 48 h. We found that
isoquercitrin induced the apoptosis of liver cancer cells in a
concentration-dependent manner. The apoptosis of cells is achieved
mainly through two pathways, the death receptor pathway and the
mitochondrial pathway (27–29). Caspases are specific cysteine
aspartic acid proteases, and they are present in an inactive
zymogen state in normal cells. Once a caspase is activated, it
participates in initiating and executing cell apoptosis. Caspase-9
is at a comparatively upstream stage of the cell apoptotic signal
transduction process. If caspase-9 is activated, it activates the
downstream effector cysteine proteases caspase-3 and -8 to trigger
a caspase cascade reaction, which in turn promotes subsequent cell
apoptosis signals and the initiation of apoptosis. We found that
the expression of caspase-3, -8 and -9 all increased following
treatment with isoquercitrin. This means that isoquercitrin induces
cell apoptosis by activating caspase family proteins inside human
liver cancer cells.
Tumour growth is a disorder of cell cycle regulation
due to changes in multiple genes. Two key points in the cell cycle
regulatory mechanism are the G1 and the S phases. The blockade of
tumour cells in the G1 or the S phase is an important mechanism to
resist the growth of tumour cells (30,31).
In the present study, changes in the cell cycle of human liver
cells were analysed by flow cytometry after they were treated with
isoquercitrin to determine the mechanism by which isoquercitrin
inhibits the proliferation of human liver cancer cells. We found
that the percentage of tumour cells in the G1 phase increased after
these cells were treated with isoquercitrin for 48 h, meaning that
cells were blocked in the G1 phase. Therefore, isoquercitrin had
the effect of preventing human liver cancer cells from
transitioning from the G1 to the S phase and the G2/M phase.
The MAPK signalling transduction pathway is a key
information transfer pathway for signals to enter the cell nucleus
from the cell surface and an intersection for signal transfer
between cell proliferation and differentiation. It mainly consists
of extracellular regulating protein kinase (ERK), c-Jun N-terminal
kinase (JNK) and p38MAPK pathways. Three pathways, ERK, JNK and
p38MAPK, were found to be closely associated with many malignant
tumours, including breast, ovarian cancer and non-small cell lung
cancer. ERK mainly takes part in the proliferation and
differentiation of cells (32–35),
while JNK and p38MAPK mainly take part in the stress reaction and
induction of apoptosis. Additionally, JNK plays a role as a
proto-oncogene in hepatocellular carcinoma (36). When JNK is activated through
phosphorylation, it regulates the expression of downstream target
genes and the activity of target proteins to induce apoptosis
(37). When Nateri et al
knocked down the c-Jun gene or changed the locus of JNK
phosphorylation, intestine tumours in mice became smaller, the
number of tumour cells decreased, and the lifespan of the mice was
extended (38). The activation of
the p38MAPK pathway plays a key role in inflammation, apoptosis,
cell cycle regulation and cell differentiation. The abnormal
activation of the p38MAPK pathway is closely correlated to the
occurrence of tumours. Some studies indicate that p38MAPK is
extensively and continuously activated in many human tumours. In
addition, the p38MAPK pathway participates in all processes of
tumour development and metastasis. One study proved that the use of
a p38MAPK inhibitor inhibited the occurrence of some types of
tumours (39). Therefore, further
study concerning the key genes and proteins of the MAPK signalling
pathway is warranted. We found that isoquercitrin strongly
inhibited the phosphorylation of ERK and p38MAPK proteins while
promoting the phosphorylation of JNK, which indicates that
isoquercitrin may play a role in regulating the proliferation and
apoptosis of human liver cancer cells via the MAPK signalling
pathway.
Protein kinase C (PKC) plays an important role in
the transmembrane signal transfer process. By catalysing Ser/Thr
phosphorylation of various proteins, PKC regulates the metabolism,
growth, proliferation and differentiation of various types of
cells. Current studies indicate that PKC is closely related to the
proliferation and attack of liver cancer cells (40). We found that isoquercitrin
significantly lowered the expression level of phosphorylated PKC;
thus, isoquercitrin may affect the progression of liver cancer via
the PKC signalling pathway.
In summary, we found that isoquercitrin inhibited
the progression of human liver cancer cells. Therefore,
isoquercitrin may be a potential new antitumour drug for the
treatment of liver cancer.
Acknowledgements
This study was funded by the Project of The National
Natural Science Foundation of China (81260661), the Traditional
Chinese Medicine Project of The Health Department of Guangxi
(GZPT13-45), and The Self-initiated Project of the Health
Department of Guangxi (Z2010270).
References
|
1
|
van Meer S, de Man RA, Siersema PD and van
Erpecum KJ: Surveillance for hepatocellular carcinoma in chronic
liver disease: evidence and controversies. World J Gastroenterol.
19:6744–6756. 2013.PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
3
|
Liu Y, Chang CC, Marsh GM and Wu F:
Population attributable risk of aflatoxin-related liver cancer:
systematic review and meta-analysis. Eur J Cancer. 48:2125–2136.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li M, Qiao C, Qin L, Zhang J and Ling C:
Application of traditional Chinese medicine injection in treatment
of primary liver cancer: a review. J Tradit Chin Med. 32:299–307.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing, and treating hepatocellular carcinoma.
CA Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Belghiti J and Fuks D: Liver resection and
transplantation in hepatocellular carcinoma. Liver Cancer. 1:71–82.
2012. View Article : Google Scholar
|
|
7
|
Eguchi S, Kanematsu T, Arii S, Omata M,
Kudo M, Sakamoto M, Takayasu K, Makuuchi M, Matsuyama Y and Monden
M; Liver Cancer Study Group of Japan. Recurrence-free survival more
than 10 years after liver resection for hepatocellular carcinoma.
Br J Surg. 98:552–557. 2011.PubMed/NCBI
|
|
8
|
Huang J, Yan L, Cheng Z, Wu H, Du L, Wang
J, Xu Y and Zeng Y: A randomized trial comparing radiofrequency
ablation and surgical resection for HCC conforming to the Milan
criteria. Ann Surg. 252:903–912. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang W, Shi J and Xie WF: Transarterial
chemoembolization in combination with percutaneous ablation therapy
in unresectable hepatocellular carcinoma: a meta-analysis. Liver
Int. 30:741–749. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marelli L, Stigliano R, Triantos C,
Senzolo M, Cholongitas E, Davies N, Yu D, Meyer T, Patch DW and
Burroughs AK: Treatment outcomes for hepatocellular carcinoma using
chemoembolization in combination with other therapies. Cancer Treat
Rev. 32:594–606. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roomi MW, Roomi NW, Kalinovsky T,
Niedzwiecki A and Rath M: Micronutrient synergy in the fight
against hepatocellular carcinoma. Cancers. 4:323–339. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ghosh A, Ghosh D, Sarkar S, Mandal AK,
Thakur Choudhury S and Das N: Anticarcinogenic activity of
nanoencapsulated quercetin in combating diethylnitrosamine-induced
hepatocarcinoma in rats. Eur J Cancer Prev. 21:32–41. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khan MS, Halagowder D and Devaraj SN:
Methylated chrysin induces co-ordinated attenuation of the
canonical Wnt and NF-kB signaling pathway and upregulates apoptotic
gene expression in the early hepatocarcinogenesis rat model. Chem
Biol Interact. 193:12–21. 2011. View Article : Google Scholar
|
|
14
|
Liu H, Dong A, Gao C, Tan C, Xie Z, Zu X,
Qu L and Jiang Y: New synthetic flavone derivatives induce
apoptosis of hepatocarcinoma cells. Bioorg Med Chem. 18:6322–6328.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ullmannova V and Popescu NC: Inhibition of
cell proliferation, induction of apoptosis, reactivation of
DLC1, and modulation of other gene expression by dietary
flavone in breast cancer cell lines. Cancer Detect Prev.
31:110–118. 2007.PubMed/NCBI
|
|
16
|
Yang QH, Yang J, Liu GZ, Wang L, Zhu TC,
Gao HL and Kou XG: Study on in vitro anti-tumor activity of
Bidens bipinnata L. extract. Afr J Tradit Complement Altern
Med. 10:543–549. 2013.PubMed/NCBI
|
|
17
|
Wu J, Wan Z, Yi J, Wu Y, Peng W and Wu J:
Investigation of the extracts from Bidens pilosa Linn. var
radiata Sch Bip for antioxidant activities and cytotoxicity
against human tumor cells. J Nat Med. 67:17–26. 2013.
|
|
18
|
Kumari P, Misra K, Sisodia BS, Faridi U,
Srivastava S, Luqman S, Darokar MP, Negi AS, Gupta MM, Singh SC and
Kumar JK: A promising anticancer and antimalarial component from
the leaves of Bidens pilosa. Planta Med. 75:59–61. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kviecinski MR, Felipe KB, Schoenfelder T,
de Lemos Wiese LP, Rossi MH, Gonçalez E, Felicio JD, Filho DW and
Pedrosa RC: Study of the antitumor potential of Bidens
pilosa (Asteraceae) used in Brazilian folk medicine. J
Ethnopharmacol. 117:69–75. 2008.PubMed/NCBI
|
|
20
|
Ong PL, Weng BC, Lu FJ, Lin ML, Chang TT,
Hung RP and Chen CH: The anticancer effect of protein-extract from
Bidens alba in human colorectal carcinoma SW480 cells
via the reactive oxidative species- and glutathione
depletion-dependent apoptosis. Food Chem Toxicol. 46:1535–1547.
2008.PubMed/NCBI
|
|
21
|
Ma TT, Xie J, Zhang QL, Xu H, Li J and
Chen FH: Analysis of fingerprint and bioactive components of
Bidens biternata by HPLC. Zhong Yao Cai. 35:892–896.
2012.(In Chinese).
|
|
22
|
Zhong MM, Chen FH, Yuan LP, Wang XH and Wu
FR: Study on the property of adsorption and separation of the
macroporous resins for total flavonoids of Bidens bipinnata
L. Zhong Yao Cai. 30:338–341. 2007.(In Chinese).
|
|
23
|
Zhong MM, Chen FH, Yuan LP, Wang XH, Wu
FR, Yuan FL and Cheng WM: Protective effect of total flavonoids
from Bidens bipinnata L. against carbon
tetrachloride-induced liver injury in mice. J Pharm Pharmacol.
59:1017–1025. 2007.PubMed/NCBI
|
|
24
|
Yuan LP, Chen FH, Ling L, Bo H, Chen ZW,
Li F, Zhong MM and Xia LJ: Protective effects of total flavonoids
of Bidens bipinnata L. against carbon tetrachloride-induced
liver fibrosis in rats. J Pharm Pharmacol. 60:1393–1402.
2008.PubMed/NCBI
|
|
25
|
Fabregat I, Roncero C and Fernández M:
Survival and apoptosis: a dysregulated balance in liver cancer.
Liver Int. 27:155–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koschny R, Brost S, Hinz U, Sykora J,
Batke EM, Singer S, Breuhahn K, Stremmel W, Walczak H, Schemmer P,
Schirmacher P and Ganten TM: Cytosolic and nuclear caspase-8 have
opposite impact on survival after liver resection for
hepatocellular carcinoma. BMC Cancer. 13:5322013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar S and Vaux DL: Apoptosis. A
cinderella caspase takes center stage. Science. 297:1290–1291.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dewson G and Kluck RM: Mechanisms by which
Bak and Bax permeabilise mitochondria during apoptosis. J Cell Sci.
122:2801–2808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fombonne J, Bissey PA, Guix C, Sadoul R,
Thibert C and Mehlen P: Patched dependence receptor triggers
apoptosis through ubiquitination of caspase-9. Proc Natl Acad Sci
USA. 109:10510–10515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rew DA and Wilson GD: Cell production
rates in human tissues and tumours and their significance. Part II:
clinical data. Eur J Surg Oncol. 26:405–417. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kazi A and Dou QP: Cell cycle and drug
sensitivity. Methods Mol Med. 111:33–42. 2005.
|
|
32
|
Fecher LA, Amaravadi RK and Flaherty KT:
The MAPK pathway in melanoma. Curr Opin Oncol. 20:183–189. 2008.
View Article : Google Scholar
|
|
33
|
Junttila MR, Li SP and Westermarck J:
Phosphatase-mediated crosstalk between MAPK signaling pathways in
the regulation of cell survival. FASEB J. 22:954–965. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kohno M and Pouyssegur J: Targeting the
ERK signaling pathway in cancer therapy. Ann Med. 38:200–211. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Das M, Garlick DS, Greiner DL and Davis
RJ: The role of JNK in the development of hepatocellular carcinoma.
Genes Dev. 25:634–645. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dhanasekaran DN and Reddy EP: JNK
signaling in apoptosis. Oncogene. 27:6245–6251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nateri AS, Spencer-Dene B and Behrens A:
Interaction of phosphorylated c-Jun with TCF4 regulates intestinal
cancer development. Nature. 437:281–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marengo B, De Ciucis CG, Ricciarelli R,
Furfaro AL, Colla R, Canepa E, Traverso N, Marinari UM, Pronzato MA
and Domenicotti C: p38MAPK inhibition: a new combined approach to
reduce neuroblastoma resistance under etoposide treatment. Cell
Death Dis. 4:e5892013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guo K, Liu Y, Zhou H, Dai Z, Zhang J, Sun
R, Chen J, Sun Q, Lu W, Kang X and Chen P: Involvement of protein
kinase C β-extracellular signal-regulating kinase 1/2/p38
mitogen-activated protein kinase-heat shock protein 27 activation
in hepatocellular carcinoma cell motility and invasion. Cancer Sci.
99:486–496. 2008.
|